Abstract
Stool has multiple components, which include undigested food material, plant, animal products, normal intestinal microbiome, and parasites. Due to the existence of all the elements, stool parasite examination is cumbersome, especially with identification of the eggs of Ascaris lumbricoides. We examined 650 stool samples of pregnant women before anti-helminthic treatment. We found that the prevalence of Ascaris lumbricoides was 5.4% (95% CI 3.8–7.4, n = 35) by a single observer in microscopy, and the majority (33/35) were identified as decorticated fertilized eggs. The prevalence of Ascaris by molecular methods was 2.6% (95% CI 1.5–4.2%, n = 17). Five samples were positive by both methods. The prevalence of structures resembling Ascaris was 4.6% (95% CI 3.1–6.5, n = 30). Three of the positive samples were confirmed with sequencing. With the subjective nature of microscopy along with the naked eye examination, errors can happen. Hence adequate training and confirmation with molecular techniques for identification of Ascaris lumbricoides are advisable.
Keywords: Ascaris, Artefact, Microscopy, Misclassification
Background
Soil-transmitted helminths (STH) are a group of neglected tropical diseases that are highly prevalent in low- and middle-income countries. Over five billion people worldwide are at risk of these infections, with approximately 1.5 million individuals were currently infected (Mogaji et al. 2020). STH includes parasites such as Ascaris lumbricoides, Necator americanus, Ancylostoma duodenale, and Trichuris trichiura, which require soil for their eggs to develop and mature. In India, recent data from 2010 indicates that one in five individuals, (258 million people) is infected with STH, with 148 million specifically infected with Ascaris (Ajjampur et al. 2021).
The World Health Organisation (WHO) has identified pre-school children, school-going children, and women of reproductive age, including pregnant and lactating women, as high-risk groups for STH infections (World Health Organization 2012). In 2001, the WHO passed a resolution to control STH morbidity through large-scale deworming programs, with a target of achieving 75% coverage in school-going children. India successfully reached this target in 2015. The WHO's 2030 targets for STH control programs aim to eliminate STH morbidity in preschool-age children and school-age children (Drake et al. 2015).
Microscopy is the preferred method for identifying STH infections in stool samples, including Ascaris lumbricoides, in low- and middle-income countries. The WHO recommends the Kato-Katz egg counting method for this purpose. However, identifying STH eggs in stool samples requires trained personnel (Khurana et al. 2021). Stool microscopy identifies all the forms intestinal parasites which includes tropozoites, cyst, egg and larva. Eggs of A. lumbricoides exist in three forms: fertilized, unfertilized, and decorticated. Unfertilized eggs are elongated and measure 85–95 × 43–47 µm. They contain a small, unsegmented, atrophied ovum and a thin outer albuminous coat. Fertilized eggs are round to oval, measuring 45–75 × 35–50 µm, and have a thick, mamillated albuminous coat. They also contain a large unsegmented ovum with clear space on both ends (Garcia and Microbiology 2016).
During microscopy, various artefacts such as pollen grains and plant cells can resemble parasite eggs, particularly the decorticated fertilized eggs of Ascaris lumbricoides (Szwabe and Kurnatowski 2012). These artefacts can lead to misdiagnosis and contribute to the burden of A. lumbricoides. Technicians must be trained to differentiate these artefacts from actual parasite eggs, highlighting the crucial role of microscopy in STH screening. Stool concentration macroscopic techniques enhance the likelihood of identifying parasites by eliminating undigested food particles and debris. However, these methods may not eliminate all extraneous elements, such as plant cells, from the sample. In this study, we present the prevalence of Ascaris like structures in stool samples from pregnant women, which could be possibly misclassified as Ascaris lumbricoides.
Methods
We conducted a study involving 650 stool samples obtained from pregnant women to investigate the presence of soil-transmitted helminth (STH) infections. Each woman provided a single stool sample, which was promptly transported to the laboratory within 4 h of collection. A trained observer with expertise in microscopic techniques for parasite identification screened all the samples. Additionally, all positive slides and a random selection of 10% of negative slides were cross-checked by a microbiologist. Saline and iodine wet mount preparations, as well as concentration techniques, were employed to examine the slides on the same day, using 10X and confirmed with 40X magnifications.
In microscopy, the fertilised eggs are round in shape with a thick outer shell with an external mammillated layer. But sometimes, the mammillated outer layer is absent known as decorticated eggs. Unfertilised eggs are elongated and their outer shell is thin with or without protuberances. The presence of eggs or larvae was considered positive for Ascaris lumbricoides.
Furthermore, a portion of each collected stool sample was stored at −80 °C for molecular analysis. DNA extraction was performed using the Qiagen stool DNA-mini kit according to the manufacturer's instructions. Prior to extraction, one gram of stool sample was mixed with one ml of lysis buffer and subjected to two cycles of 30 s each in a bead beater (MP bio) using the human faeces program. Conventional PCR was conducted for all 650 samples using the primers designed for internal transcribed spacer region of 5.8 s rRNA forward (5'-ATTTGCGCGTATACGTGAGC-3') and reverse (5'-CCACCGCTAAGATTTGTTCA-3'). The PCR reaction mixture consisted of 25 µl, comprising 12.5 µl of Amplicon Red dye master mix, 0.5 µl of forward and reverse primers, 8.5 µl of nuclease-free water, and 3 µl of extracted DNA template. The resulting PCR products (420 bp) were visualized using 1.5% agarose gels under ultraviolet radiation. Detailed procedure was given elsewhere (Ulaganeethi et al. 2023).
The prevalence of Ascaris infection, as identified through PCR analysis, along with the presence of Ascaris-like structures, was reported as proportions with a 95% confidence interval (CI).
Ethics and informed consent
The study protocol was reviewed and approved by scientific advisory committee and Institute ethics committee (for human studies) of a tertiary teaching hospital, Puducherry, India. A written informed consent has been obtained from each participant before enrolment. Ethical approval has been taken with the number JIP/IEC/2019/137, dated 02/07/2019.
Results
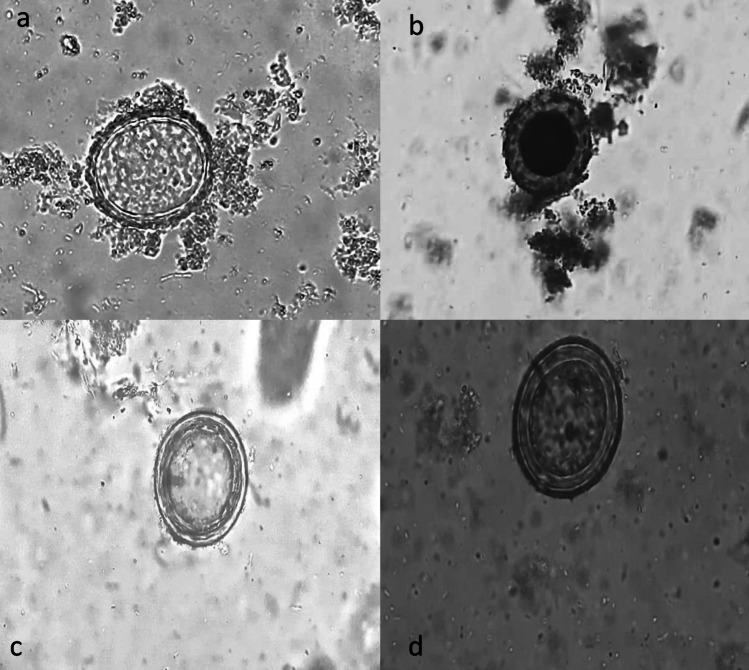
Among the 650 stool samples examined, microscopy identified 35 samples (5.4%, 95% CI 3.8–7.4) as positive for Ascaris. Out of these positive samples, 33 (95% CI 80.8–99.3) were fertilized decorticated eggs, while two (5.7%, 95% CI 0.7–19.2) were fertilized corticated eggs of Ascaris. Microscopic pictures of the parasite and artefacts are shown in Fig. 1.
Fig. 1.
Microscopic pictures of the parasite and artefacts identified in the study. a, b Fertilised corticated eggs of Ascaris; c, d Artefacts resembling fertilised decorticated eggs of Ascaris
PCR detected a total of 17 samples as Ascaris out of the 650 samples. The prevalence of Ascaris infection determined by PCR was 2.6% (95% CI 1.5–4.2%). Among the 35 samples identified as positive by microscopy, only 5 were positive by PCR. The remaining 30 samples mimicked Ascaris in microscopy but did not show PCR confirmation. The prevalence of structures resembling Ascaris eggs was 30 (4.6%, 95% CI 3.1–6.5). Additionally, microscopy failed to detect 12 samples, which were mistakenly classified as negatives (Table 1).
Table 1.
Number of samples identified as Ascaris lumbricoides by microscopy and PCR (N = 650)
| Microscopy | PCR | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 5 | 30 | 35 |
| Negative | 12 | 603 | 615 |
| Total | 17 | 633 | 650 |
Sequences obtained in the study were checked with NCBI database for confirmation. The sequences identified in our study were submitted to Genbank with accession numbers OQ 778745–47.
Discussion
Microscopy serves as the preferred diagnostic method in many low and middle-income countries for the detection of soil-transmitted helminths (STH). However, its effectiveness is reliant on the worm load and requires at least three stool samples due to the intermittent shedding of eggs (Van Gool et al. 2003). Moreover, the presence of artefacts that resemble parasite eggs contributes to the potential for false burden estimation (Maurelli et al. 2021).
Given that these infections are typically asymptomatic, clinical diagnosis is not feasible. Therefore, it is essential to establish a robust methodology that can accurately differentiate true parasites from spurious ones. By doing so, we can effectively work towards achieving and maintaining the elimination of STH-related morbidity.
In our study, the prevalence of structures resembling Ascaris was found to be 4.6%, while the actual presence of Ascaris determined by PCR was 2.6%. It was observed that the prevalence of these Ascaris-like structures was twice as high as the true Ascaris infection rate. A study conducted by Maurelli et al. reported similar findings. In this study, the stool samples were collected from primary school children in Delhi and immigrants from Naples and Italy. The findings showed that the Kato-Katz showed the Ascaris prevalence of 22.4% but out of these 25 (39.1%) were reported as elements ascribable to Ascaris by Mini-Flotac. The Mini-Flotac identified the prevalence was 13.6% (Cringoli et al. 2017).Additionally, elements that resemble Ascaris, such as pollen grains, plant cells, diatoms, mushroom spores, epithelial cells of the human intestine, or free-living cells, have been identified in previous studies (Colmer-Hamood 2001; Szwabe and Kurnatowski 2012).
Another study specifically highlighted that many collected pollen grains, such as those from the Lilium variety 'Wiener Blut,' Iris sibirica, and Lilium candidum, might resemble to the eggs of A. lumbricoides (Szwabe and Kurnatowski 2012). Hence, the chances of misclassification might be more with the subjective nature of microscopy.
In the current study microscopy missed 12 cases which are picked up by PCR. A study was done in Venezuela reported that PCR diagnosis increases the sensitivity of detecting Ascaris lumbricoides than microscopy (Incani et al. 2017). This could be due to reduced prevalence with low intensity of infection. Hence, multiple stool sample collection over several days might help to improve the diagnosis. In addition to this training of manpower in differentiating parasite eggs from artefacts help in the reduction of false positive errors.
With the limitations of microscopy, molecular techniques evolved in the diagnosis of the STH infections. Even though, PCR is a highly sensitive and specific tool for identifying these infections, it requires an established laboratory with trained manpower in molecular techniques and it is not applicable in field based diagnosis. PCR involves amplifying specific fragments of parasitic DNA using two primers, and it is possible that DNA from non-living parasites could be detected. Therefore, this method is not currently employed in studies assessing treatment efficacy (Rijsman et al. 2016). Inhibitors in stool like bile salts, urea, phenol, and some proteins like collagen and myoglobin, hemoglobin, and immunoglobin G should be kept in mind before processing. It might produce false negative results (Hedman and Rådström 2013).
Some of the samples identified positive by PCR in this study were confirmed with sequencing. This conventional PCR is comparatively less costly than real-time assays and it is suitable for poor resource settings in routine diagnosis in the era of STH elimination.
Conclusion
Even though microscopy is considered as a gold standard for diagnosing STH infections, their limitations should be kept in mind to ensure the robustness of results. With the development of low cost molecular techniques, misclassification can be reduced compared to the traditional microscopy.
Funding
Jawaharlal Institute of Postgraduate Medical Education and Research (JIP/Res/Intramural/Phs2/2020–21).
Declarations
Conflict of interest
The authors declared no conflict of interest with respect to research, authorship or publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ajjampur SSR et al (2021) Epidemiology of soil transmitted helminths and risk analysis of hookworm infections in the community: results from the DeWorm3 Trial in southern India. PLoS Negl Trop Dis 15(4):e0009338–e0009338. 10.1371/journal.pntd.0009338 10.1371/journal.pntd.0009338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer-Hamood JA (2001) Fecal microscopy: artifacts mimicking ova and parasites. Lab Med 32(2):80–84. 10.1309/0VTA-YUXK-F20G-T20E 10.1309/0VTA-YUXK-F20G-T20E [DOI] [Google Scholar]
- Cringoli G et al (2017) The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nat Protoc 12(9):1723–1732. 10.1038/nprot.2017.067 10.1038/nprot.2017.067 [DOI] [PubMed] [Google Scholar]
- Drake LJ et al (2015) Bihar’s pioneering school-based deworming programme: lessons learned in deworming over 17 million Indian school-age children in one sustainable campaign. PLoS Negl Trop Dis 9(11):e0004106. 10.1371/journal.pntd.0004106 10.1371/journal.pntd.0004106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman J, Rådström P (2013) Overcoming inhibition in real-time diagnostic PCR. Methods Mol Biol (Clifton NJ) 943:17–48. 10.1007/978-1-60327-353-4_2 10.1007/978-1-60327-353-4_2 [DOI] [PubMed] [Google Scholar]
- Incani RN et al (2017) Diagnosis of intestinal parasites in a rural community of Venezuela: advantages and disadvantages of using microscopy or RT-PCR. Acta Trop 167:64–70. 10.1016/j.actatropica.2016.12.014 10.1016/j.actatropica.2016.12.014 [DOI] [PubMed] [Google Scholar]
- Khurana S, Singh S, Mewara A (2021) Diagnostic techniques for soil-transmitted helminths–recent advances. Res Rep Trop Med 12:181–196. 10.2147/RRTM.S278140 10.2147/RRTM.S278140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli MP et al (2021) Ascaris lumbricoides eggs or artefacts? A diagnostic conundrum. Parasitology 148(13):1554–1559. 10.1017/S0031182021001256 10.1017/S0031182021001256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogaji HO et al (2020) Distribution of ascariasis, trichuriasis and hookworm infections in Ogun State, Southwestern Nigeria. PLoS ONE 15(6):e0233423. 10.1371/journal.pone.0233423 10.1371/journal.pone.0233423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijsman LH, Monkelbaan JF, Kusters JG (2016) Clinical consequences of polymerase chain reaction-based diagnosis of intestinal parasitic infections. J Gastroenterol Hepatol 31(11):1808–1815. 10.1111/jgh.13412 10.1111/jgh.13412 [DOI] [PubMed] [Google Scholar]
- Szwabe K, Kurnatowski P (2012) ‘Comparative analysis of morphometric features of the eggs of selected alimentary tract parasites and of the plant pollens. Ann Parasitol 58(2):87–96 [PubMed] [Google Scholar]
- Ulaganeethi R et al (2023) Performance of microscopy compared to conventional PCR in identification of soil-transmitted helminth infections among antenatal women in a low-prevalence setting. Indian J Med Microbiol 46:100427. 10.1016/j.ijmmb.2023.100427 10.1016/j.ijmmb.2023.100427 [DOI] [PubMed] [Google Scholar]
- Van Gool T et al (2003) Triple faeces test: an effective tool for detection of intestinal parasites in routine clinical practice. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 22(5):284–290. 10.1007/s10096-003-0919-1 10.1007/s10096-003-0919-1 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2012) Soil-transmitted helminthiases: eliminating as public health problem soil-transmitted helminthiases in children: progress report 2001–2010 and strategic plan 2011–2020. World Health Organization, Geneva [Google Scholar]