Abstract
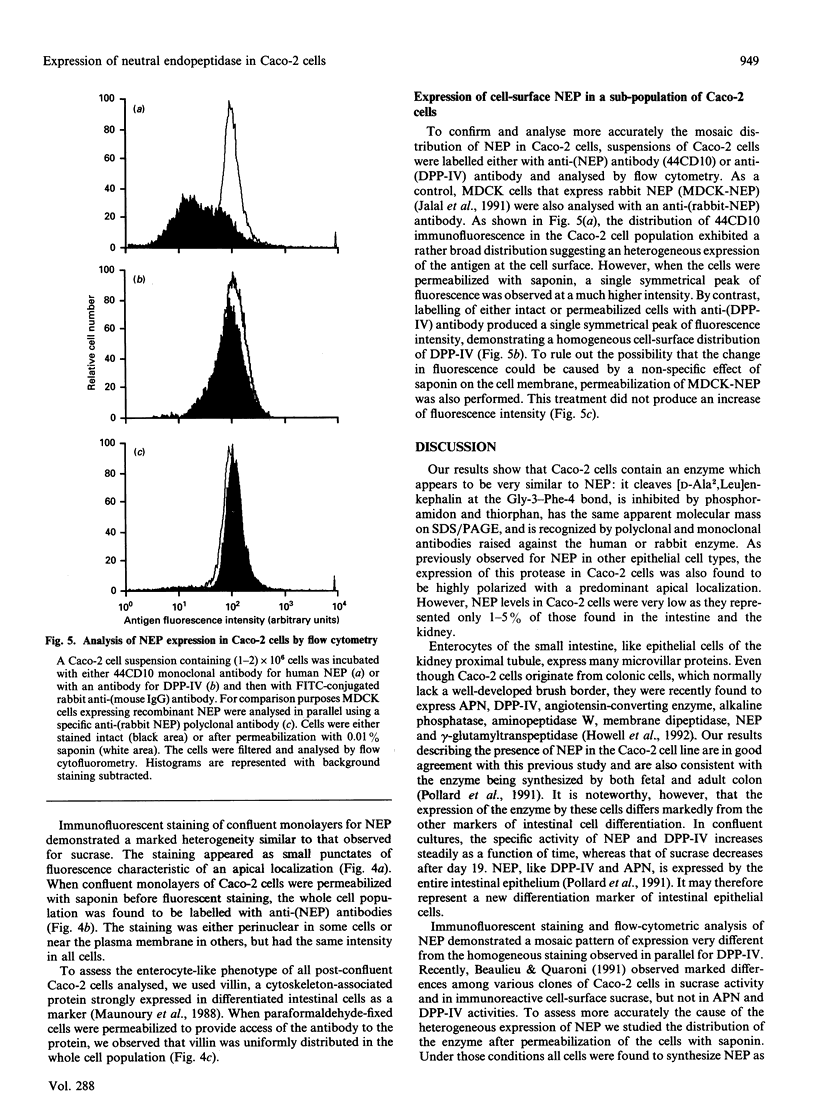
The human colon cancer cell line Caco-2 undergoes spontaneous enterocytic differentiation during growth, and expresses a number of brush-border-membrane-associated hydrolases typical of a differentiated phenotype. Among these are alkaline phosphatase, dipeptidyl peptidase IV and sucrase-isomaltase (sucrase, EC 3.2.1.48). Neutral endopeptidase 24.11 [EC 3.4.24.11, neprilysin (NEP)] is another abundant protease of normal enterocytes but its presence in Caco-2 cells has not been fully documented yet. In this paper, we show that Caco-2 cell extracts hydrolyse tritiated [D-Ala2Leu5]enkephalin with a Km of 180 microM, very close to the value obtained for the NEP present in the rabbit kidney (118 microM). Western-blot analysis of brush-border membranes purified from post-confluent cells revealed a protein with an apparent molecular mass of 94000 Da similar to that of the rabbit kidney NEP. The amount of enzyme in cell extracts increased as a function of the age of the culture, indicating that NEP expression is correlated with the degree of cell differentiation as is also the case for sucrase and dipeptidylpeptidase IV (DPP-IV). Binding of a radiolabelled antibody to Caco-2 cell monolayers grown on semi-permeable filters indicated that 95% of NEP molecules present at the cell surface are on the apical side. Immunocytochemical and flow cytometric analysis of intact and permeabilized cells were also used to investigate the presence of NEP and DPP-IV at the surface of Caco-2 cells. Whereas DPP-IV staining appeared to be homogeneous throughout the entire cell population, NEP-related fluorescence exhibited a bimodal distribution which indicates an uneven expression of the protein at the cell surface. Permeabilization of monolayers with saponin before staining restored a labelling pattern for NEP similar to the one obtained for DPP-IV. This suggests that although DPP-IV and NEP follow similar patterns of expression when enzymic activities are measured on whole-cell extracts, targeting of these brush-border proteins to the cell surface appears to be regulated in different ways.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almenoff J., Wilk S., Orlowski M. Membrane bound pituitary metalloendopeptidase: apparent identity to enkephalinase. Biochem Biophys Res Commun. 1981 Sep 16;102(1):206–214. doi: 10.1016/0006-291x(81)91508-4. [DOI] [PubMed] [Google Scholar]
- Bartles J. R., Feracci H. M., Stieger B., Hubbard A. L. Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathways taken by apical and basolateral proteins using subcellular fractionation. J Cell Biol. 1987 Sep;105(3):1241–1251. doi: 10.1083/jcb.105.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J. F., Quaroni A. Clonal analysis of sucrase-isomaltase expression in the human colon adenocarcinoma Caco-2 cells. Biochem J. 1991 Dec 15;280(Pt 3):599–608. doi: 10.1042/bj2800599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais A., Bissonnette P., Berteloot A. Common characteristics for Na+-dependent sugar transport in Caco-2 cells and human fetal colon. J Membr Biol. 1987;99(2):113–125. doi: 10.1007/BF01871231. [DOI] [PubMed] [Google Scholar]
- Blais A., Jalal F., Crine P., Paiement J., Berteloot A. Increased functional differentiation of rabbit proximal tubule cells cultured in glucose-free media. Am J Physiol. 1992 Jul;263(1 Pt 2):F152–F162. doi: 10.1152/ajprenal.1992.263.1.F152. [DOI] [PubMed] [Google Scholar]
- Breitfeld P. P., Casanova J. E., Simister N. E., Ross S. A., McKinnon W. C., Mostov K. E. Sorting signals. Curr Opin Cell Biol. 1989 Aug;1(4):617–623. doi: 10.1016/0955-0674(89)90024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan M. J., Anderson H. C., Palade G. E., Jamieson J. D. Intracellular sorting and polarized cell surface delivery of (Na+,K+)ATPase, an endogenous component of MDCK cell basolateral plasma membranes. Cell. 1986 Aug 15;46(4):623–631. doi: 10.1016/0092-8674(86)90888-3. [DOI] [PubMed] [Google Scholar]
- Casanova J. E., Mishumi Y., Ikehara Y., Hubbard A. L., Mostov K. E. Direct apical sorting of rat liver dipeptidylpeptidase IV expressed in Madin-Darby canine kidney cells. J Biol Chem. 1991 Dec 25;266(36):24428–24432. [PubMed] [Google Scholar]
- Crine P., LeGrimellec C., Lemieux E., Labonté L., Fortin S., Blachier A., Aubry M. The production and characterization of a monoclonal antibody specific for the 94,000 dalton enkephalin-degrading peptidase from rabbit kidney brush border. Biochem Biophys Res Commun. 1985 Aug 30;131(1):255–261. doi: 10.1016/0006-291x(85)91796-6. [DOI] [PubMed] [Google Scholar]
- De Mey J., Moeremans M., Geuens G., Nuydens R., De Brabander M. High resolution light and electron microscopic localization of tubulin with the IGS (immuno gold staining) method. Cell Biol Int Rep. 1981 Sep;5(9):889–899. doi: 10.1016/0309-1651(81)90204-6. [DOI] [PubMed] [Google Scholar]
- Devault A., Nault C., Zollinger M., Fournie-Zaluski M. C., Roques B. P., Crine P., Boileau G. Expression of neutral endopeptidase (enkephalinase) in heterologous COS-1 cells. Characterization of the recombinant enzyme and evidence for a glutamic acid residue at the active site. J Biol Chem. 1988 Mar 15;263(8):4033–4040. [PubMed] [Google Scholar]
- Gilbert T., Rodriguez-Boulan E. Induction of vacuolar apical compartments in the Caco-2 intestinal epithelial cell line. J Cell Sci. 1991 Nov;100(Pt 3):451–458. doi: 10.1242/jcs.100.3.451. [DOI] [PubMed] [Google Scholar]
- Gorvel J. P., Ferrero A., Chambraud L., Rigal A., Bonicel J., Maroux S. Expression of sucrase-isomaltase and dipeptidylpeptidase IV in human small intestine and colon. Gastroenterology. 1991 Sep;101(3):618–625. doi: 10.1016/0016-5085(91)90517-o. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Sterchi E. E., Bienz D., Fransen J. A., Marxer A. Expression and intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J Cell Biol. 1985 Sep;101(3):838–851. doi: 10.1083/jcb.101.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S., Kenny A. J., Turner A. J. A survey of membrane peptidases in two human colonic cell lines, Caco-2 and HT-29. Biochem J. 1992 Jun 1;284(Pt 2):595–601. doi: 10.1042/bj2840595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal F., Lemay G., Zollinger M., Berteloot A., Boileau G., Crine P. Neutral endopeptidase, a major brush border protein of the kidney proximal nephron, is directly targeted to the apical domain when expressed in Madin-Darby canine kidney cells. J Biol Chem. 1991 Oct 15;266(29):19826–19832. [PubMed] [Google Scholar]
- Jumarie C., Malo C. Caco-2 cells cultured in serum-free medium as a model for the study of enterocytic differentiation in vitro. J Cell Physiol. 1991 Oct;149(1):24–33. doi: 10.1002/jcp.1041490105. [DOI] [PubMed] [Google Scholar]
- Kenny A. J., Maroux S. Topology of microvillar membrance hydrolases of kidney and intestine. Physiol Rev. 1982 Jan;62(1):91–128. doi: 10.1152/physrev.1982.62.1.91. [DOI] [PubMed] [Google Scholar]
- Kenny A. J. Regulatory peptide metabolism at cell surfaces: the key role of endopeptidase-24.11. Biomed Biochim Acta. 1986;45(11-12):1503–1513. [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):477–488. doi: 10.1042/bj1370477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J., Boekestijn J. C., Mulder A. M., Fransen J. A., Ginsel L. A. Intracellular localization and endocytosis of brush border enzymes in the enterocyte-like cell line Caco-2. Eur J Cell Biol. 1991 Feb;54(1):76–84. [PubMed] [Google Scholar]
- Kojima K., Hama T., Kato T., Nagatsu T. Rapid chromatographic purification of dipeptidyl peptidase IV in human submaxillary gland. J Chromatogr. 1980 Feb 29;189(2):233–240. doi: 10.1016/s0021-9673(00)81523-x. [DOI] [PubMed] [Google Scholar]
- Kunst F., Pascal M., Lefesant J. A., Walle J., Dedonder R. Purification and some properties of an endocellular sucrase from a constitutive mutant of Bacillus subtilis Marburg 168. Eur J Biochem. 1974 Mar 1;42(2):611–620. doi: 10.1111/j.1432-1033.1974.tb03376.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Quaroni A., Nichols B., Rodriguez-Boulan E. Biogenetic pathways of plasma membrane proteins in Caco-2, a human intestinal epithelial cell line. J Cell Biol. 1990 Oct;111(4):1351–1361. doi: 10.1083/jcb.111.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A., Sambuy Y., Mostov K., Rodriguez-Boulan E. Vectorial targeting of an endogenous apical membrane sialoglycoprotein and uvomorulin in MDCK cells. J Cell Biol. 1990 May;110(5):1533–1539. doi: 10.1083/jcb.110.5.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemay G., Zollinger M., Waksman G., Roques B. P., Crine P., Boileau G. Recombinant neutral endopeptidase-24.11 expressed in mouse neuroblastoma cells is associated with neurite membranes. Biochem J. 1990 Apr 15;267(2):447–452. doi: 10.1042/bj2670447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Le Bivic A., Sargiacomo M., Rodriguez-Boulan E. Steady-state distribution and biogenesis of endogenous Madin-Darby canine kidney glycoproteins: evidence for intracellular sorting and polarized cell surface delivery. J Cell Biol. 1989 Nov;109(5):2117–2127. doi: 10.1083/jcb.109.5.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfroy B., Swerts J. P., Guyon A., Roques B. P., Schwartz J. C. High-affinity enkephalin-degrading peptidase in brain is increased after morphine. Nature. 1978 Nov 30;276(5687):523–526. doi: 10.1038/276523a0. [DOI] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem J. 1984 Oct 15;223(2):433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K., Brauchbar M., Bucher K., Hauri H. P. Sorting of endogenous plasma membrane proteins occurs from two sites in cultured human intestinal epithelial cells (Caco-2). Cell. 1990 Feb 9;60(3):429–437. doi: 10.1016/0092-8674(90)90594-5. [DOI] [PubMed] [Google Scholar]
- Matter K., Hauri H. P. Intracellular transport and conformational maturation of intestinal brush border hydrolases. Biochemistry. 1991 Feb 19;30(7):1916–1923. doi: 10.1021/bi00221a026. [DOI] [PubMed] [Google Scholar]
- Matter K., McDowell W., Schwartz R. T., Hauri H. P. Asynchronous transport to the cell surface of intestinal brush border hydrolases is not due to differential trimming of N-linked oligosaccharides. J Biol Chem. 1989 Aug 5;264(22):13131–13139. [PubMed] [Google Scholar]
- Maunoury R., Robine S., Pringault E., Huet C., Guénet J. L., Gaillard J. A., Louvard D. Villin expression in the visceral endoderm and in the gut anlage during early mouse embryogenesis. EMBO J. 1988 Nov;7(11):3321–3329. doi: 10.1002/j.1460-2075.1988.tb03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim H. Y., Roth J., Sterchi E. E., Lentze M., Milla P., Schmitz J., Hauri H. P. Sucrase-isomaltase deficiency in humans. Different mutations disrupt intracellular transport, processing, and function of an intestinal brush border enzyme. J Clin Invest. 1988 Aug;82(2):667–679. doi: 10.1172/JCI113646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard H., Moreau J., Ronco P., Verroust P., Schwartz J. C. Immunoautoradiographic localisation of enkephalinase (EC 3.4.24.11) in rat gastrointestinal tract. Neuropeptides. 1991 Jul;19(3):169–178. doi: 10.1016/0143-4179(91)90115-y. [DOI] [PubMed] [Google Scholar]
- Quackenbush E. J., Letarte M. Identification of several cell surface proteins of non-T, non-B acute lymphoblastic leukemia by using monoclonal antibodies. J Immunol. 1985 Feb;134(2):1276–1285. [PubMed] [Google Scholar]
- Quaroni A. Crypt cell development in newborn rat small intestine. J Cell Biol. 1985 May;100(5):1601–1610. doi: 10.1083/jcb.100.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. Applications of immunocolloids in light microscopy. Preparation of protein A-silver and protein A-gold complexes and their application for localization of single and multiple antigens in paraffin sections. J Histochem Cytochem. 1982 Jul;30(7):691–696. doi: 10.1177/30.7.7050239. [DOI] [PubMed] [Google Scholar]
- Rousset M., Laburthe M., Pinto M., Chevalier G., Rouyer-Fessard C., Dussaulx E., Trugnan G., Boige N., Brun J. L., Zweibaum A. Enterocytic differentiation and glucose utilization in the human colon tumor cell line Caco-2: modulation by forskolin. J Cell Physiol. 1985 Jun;123(3):377–385. doi: 10.1002/jcp.1041230313. [DOI] [PubMed] [Google Scholar]
- Simons K., Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990 Jul 27;62(2):207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Stieger B., Matter K., Baur B., Bucher K., Höchli M., Hauri H. P. Dissection of the asynchronous transport of intestinal microvillar hydrolases to the cell surface. J Cell Biol. 1988 Jun;106(6):1853–1861. doi: 10.1083/jcb.106.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Salas D. E., Salas P. J., Rodriguez-Boulan E. Modulation of the expression of an apical plasma membrane protein of Madin-Darby canine kidney epithelial cells: cell-cell interactions control the appearance of a novel intracellular storage compartment. J Cell Biol. 1987 May;104(5):1249–1259. doi: 10.1083/jcb.104.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels H. P., Hansen G. H., Fuhrer C., Look A. T., Sjöström H., Norén O., Spiess M. Aminopeptidase N is directly sorted to the apical domain in MDCK cells. J Cell Biol. 1990 Dec;111(6 Pt 2):2923–2930. doi: 10.1083/jcb.111.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]






