Abstract
Electrochemical reactions via carbocation intermediates remain fundamental transformations that build up molecular functionality and complexity in a sustainable manner. Enantioselective control of such processes is a great challenge in a highly ionic electrolyte solution. Here, we report an anodic generation of chiral α-imino carbocation intermediates by enamine catalysis. The chiral carbocation intermediates can be intercepted by a variety of nucleophiles such as alcohols, water and thiols with high stereoselectivity. The key SN1 step proceeds via a tertiary amine-mediated proton shuttle that facilitates facial selection in reacting with carbocation.
Subject terms: Synthetic chemistry methodology, Electrocatalysis
Carbocations are useful synthetic intermediates which enable direct functionalization of carbon centers, but their generation in a manner that uses mild conditions and enables stereoselective interception remains a longstanding goal of organic chemists. Here, the authors use electrochemical enamine oxidation to synthesize chiral scaffolds from racemic α-branched aldehydes.
Introduction
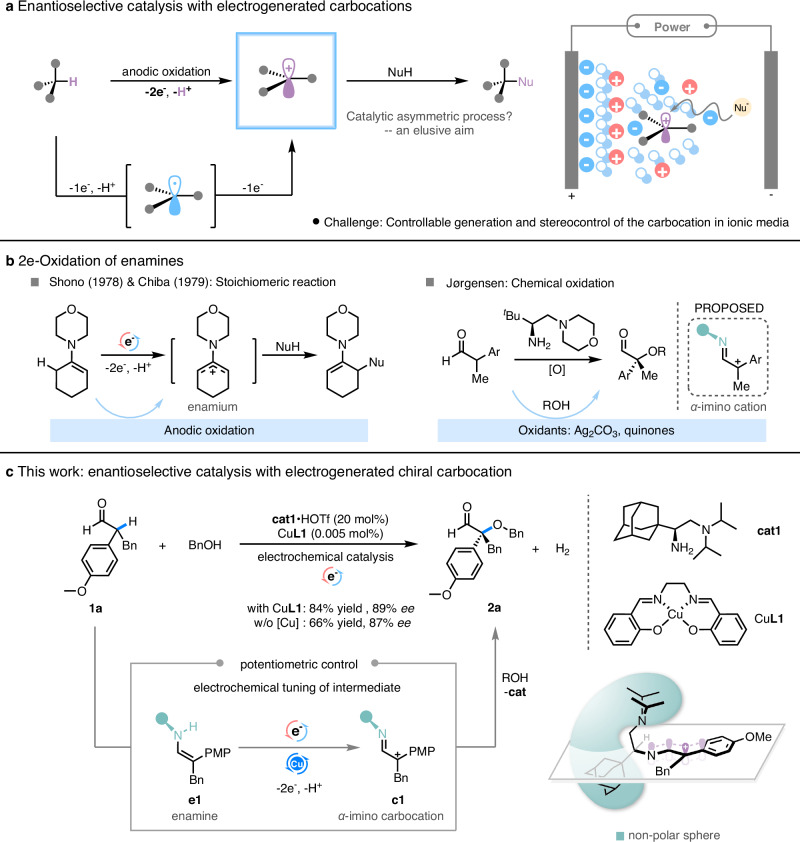
Carbocations are versatile reactive intermediates for building up molecular skeletons and functionalization in chemical and biological processes1,2. Among various approaches for their generation, electrochemical C–H oxidation is the most straightforward and atom-economical strategy as it employs unfunctionalized starting materials that are readily available and avoids the use of stoichiometric oxidants3. To ensure controllable carbocation reactivity, electrochemical strategies such as mediated electron transfer4, stepwise cation-pool5 or electroauxiliary6–8 have been developed to facilitate a sequential 2e-H transfer and nucleophilic trap on anodes (Fig. 1a). Despite of these advances, to achieve enantioselective control with electrogenerated carbocations remains a formidable challenge9–13. The most difficulty can be understood by considering the fact that chiral ion pairing, a prevalent strategy in stereoselective reactions with carbocation9–24, may not function well in highly ionic media that is essential for the electrochemical process.
Fig. 1. Strategies on electrogeneration of carbocations.
a Enantioselective catalysis with electrogenerated carbocations. b 2e-Oxidation of enamines. c This work: enantioselective catalysis with electrogenerated chiral α-imino carbocation. aConditions: 1a (0.1 mmol, 1.0 eq.), nucleophile (0.3 mmol, 3.0 eq.), cat·acid (pre-mixed, 20 mol%), CuL1 (0.005 mol%) and 0.1 M nBu4NPF6 in DCM (3 mL), RVC as the anode and Pt plate as the cathode, electrolysis at r.t. under Ar and a constant Ucell for 20 h, the magnetic stirrer was set at 1200 rpm. bYields: detected by GC. cEnantiomeric excess: determined by HPLC with a chiral column. dPMP: para-methoxyphenyl group.
When properly coupled with proton transfer, enamines may undergo a sequential 1e-1e oxidation to give a carbocationic species. As early as in 1970s, Shono and Chiba independently reported anodic oxidation of performed tertiary enamines derived from morpholine in the coupling with nucleophiles25,26. The reactions were proposed to proceed through an enaminyl cation (enamium) intermediate (Fig. 1b). The extension to an aminocatalytic version is easily conceived; however, this was not further pursued for half a century, even in the golden rush of aminocatalysis. Recently, Jørgensen reported oxidative coupling of catalytic secondary enamine with oxygen-centered nucleophiles such as alcohols in the presence of chemical oxidants such as silver carbonate or quinones27–30. An α-imino carbocation intermediate was proposed27, while this proposal with 2e-oxidation via the intermediacy of α-imino radical31 was disproved in the presence of quinones32, and the reaction prefers a canonical enamine-electrophile coupling pathway, attesting to the challenges for in-situ generation of catalytic carbocation intermediate29,32. To achieve asymmetric catalysis via carbocation intermediates such as enamium ion or α-imino cation remains largely unfulfilled, particularly under electrochemical conditions. The attempts in transferring MacMillan’s SOMO system33 into an electrochemical setting34,35 only met with rather limited successes. The same catalytic system showed a serious reduction of both stereoselectivity and reactivity when applied to the electrochemical conditions.
We sought to address the pitfalls by capitalizing on classical tenets in chemists’ historical quest on carbocation chemistry1,2. Here we report an electrochemical enamine oxidation strategy that overcomes the difficulties associated with oxidative carbocation transformations. Accordingly, sterically bulky primary-tertiary diamine catalysts were designed to facilitate electrochemical generation and transformation of the enamium intermediate. The ethylene diamine provides a proton shuttle to aid the 2e-oxidation of enamine to α-imino carbocation31,36, meanwhile the bulkiness of the out-sphere alkyl groups provides non-polar sphere that is favorable for the carbocation species (Fig. 1c)37. In this circumstance, the then interfering electrolyte can be harnessed to stabilize the formed cationic species by tuning the non-coordinating nature of the electrolyte anions. This, together with the flexibility of potentiometric control in electrochemistry, ensures a smooth asymmetric electrochemical carbocation transformation by chiral primary amine catalysis (Fig.1c, for the proposed mechanism, see SI, Fig. 22).
Results and discussion
Optimization
Racemic α-branched aldehyde 1a bearing a large α-benzyl group was selected as a model substrate, and its oxidative coupling with benzyl alcohol was investigated under electrochemical conditions (for full data of optimization, see SI, Tables 7–12). A bulky chiral primary amine catalyst cat1·HOTf with an adamantanyl side chain and di-isopropylamino group was identified to give the expected oxidative adduct 2a in 84% isolated yield and 89% ee (Fig. 1c). In contrast, less bulky primary amine catalysts with smaller side alkyl group such as tert-butyl or bearing smaller tertiary amino moieties such as dialkyl amine or morpholine all led to lower yields and enantioselectivity (SI, Table 11). Further increasing the size to di-3-pentylamino group resulted in depletion of activity. In addition, no reaction was observed in the absence of primary amine catalysts (SI, Table 11, entry 23). These results pinpoint the critical role of aminocatalysis in the process. As expected, the selection of non-coordinating electrolyte anions such as PF6- was found to be beneficial for both activity and stereoselectivity (SI, Table 12). During optimization, it was noted that the addition of a trace amount of copper salts CuL1 (50 ppm) facilitated higher productivity (84% vs 66% yield) with slightly improved enantioselectivity (89% ee vs 87% ee) (Fig. 1c). Considering its fully coordinated nature, the optimal additive CuL1 may mainly mediate electron-transfer with anode without participating directly in the key stereogenic step (vide infra for discussions).
Scope for O-centered nucleophiles (alcohols)
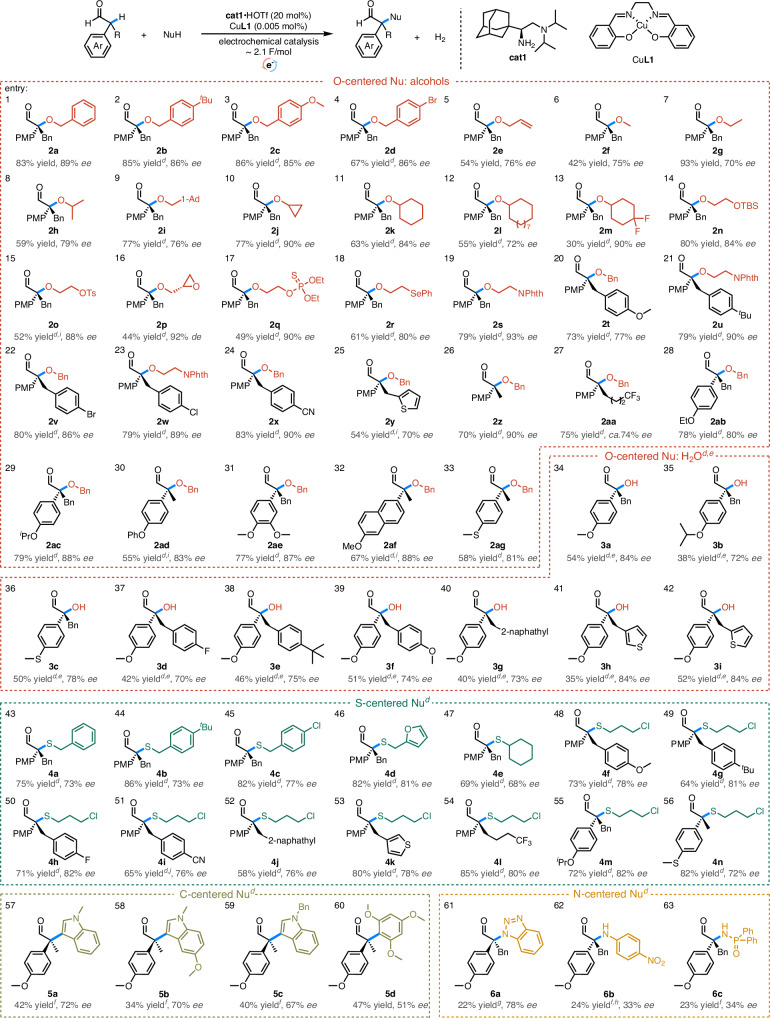
The scope of this electrochemical catalysis was next investigated (Fig. 2). Benzyl alcohols, bearing electron donating or withdrawing groups gave moderate to good yields and high enantioselectivities (2a–d). Allyl alcohol (2e) and simple alkyl alcohols (2f–h), including methanol (2f), ethanol (2g), isopropanol (2h) and 1-adamantyl methanol (2i) worked well under the present conditions. It is noted that secondary alcohol such as isopropanol (2h) led to lower yields probably due to the steric effect. In the case with methanol, decomposition of the methoxylated product (2f) was observed, giving a lower yield. Cyclic alcohols with varied ring sizes including three- (2j), six- (2k) and twelve- (2l) membered rings all worked well and 4-gem-difluoro cyclohexanol can be applied to give the desired ether with 90% ee (2m). Functional groups such as silyl ether (2n), tosylate (2o), glycidol (2p), phosphorothioate (2q), selane (2r) and imide (2s) can be well tolerated with good activity and high enantioselectivity. Variations on the aldehydes cover electronic tuning of the benzyl group including both electron-donating groups (2t and 2u), and electron-withdrawing groups including halogens (2v and 2w) and cyanogroup (2x), and the reactions worked well to give the chiral ether adducts with 77–90% ee. Thiophene was also workable, albeit with slightly reduced yield and enantioselectivity (2y). The change of α-benzyl group to simple alkyl groups such as methyl (2z) or a larger n-trifluorobutyl (2aa) led to maintaining activity in both cases, indicating different sizes of the α-substitutions are well tolerable in these instances. An electron-rich α-aryl moiety is essential as the replacement of p-methoxyphenyl to phenyl resulted in only a trace product (SI, Fig. 4). Electron-rich aryl rings with different substituents (2ab–ag) worked well to deliver the desired chiral ethers.
Fig. 2. Substrate scope.
aConditions: as described in Fig.1. bIsolated yield. cEnantiomeric excess: determined by HPLC with a chiral column. dGraphite felt as the anode. eWith PhCOOH as an additive (20 mol%), and the loading of water was 1.5 eq. fUsing 0.1 M LiClO4 in CH3CN instead. gUsing 0.1 M LiClO4 in THF instead. hAldehyde (0.2 mmol), nucleophile (0.1 mmol), with 50 mol% PhCOOH, the loading of additives and the yield was based on the nucleophile.icat1·HOTf (30 mol%). jUcell = 2.2 V. kPMP: para-methoxyphenyl group, 1-Ad: 1-adamantyl group, TBS: tert-butyldimethylsilyl group, Phth: phthalimidyl group. lFor complete catalog of substrates and products, see SI, Table 1–5. mFor a full list of failed substrates, see SI, Table 6.
Scope for O-centered nucleophiles (H2O)
Enantioselective C–H hydroxylation with water as the terminal oxygen donors represents an ideal process in terms of reaction economy. There are reports on hydroxylation at the α-postion achieved by several oxygen sources38–40, however, such process with water has not been achieved in asymmetric catalysis. In our case, water can be successfully applied in this electrochemical SN1-type process to give the expected chiral tertiary alcohols with 35–54% yield and up to 84% ee (3a–i) with benzoic acid as an additive.
Scope for S-, C- and N-centered nucleophiles
Aliphatic thiols can be also successfully applied to give chiral thioethers with good yields and 72–82% ee (4a–n). In these cases, the oxidative side-reactions normally associated with free thiols can be largely suppressed under the present anodic conditions and the reactions proceeded chemoselectively via the oxidative enamine pathway. To the best of our knowledge, this represents the first oxidative sulfuration process with aliphatic thiols. CV analysis indicated the reaction proceed through a preferential oxidation of enamine in the presence of aliphatic thiols, supporting a carbocationic mechanism (SI, Fig 20).
Electro-rich indoles (5a–c), trimethoxybenzene (5d) as well as nitrogen-centered nucleophiles such as benzotriazole (6a), aniline (6b) and phosphamide (6c) also worked in this reaction with 21–24% yields and 45–78% ee. Dehydrogenation to α, β-unsaturated enals were detected in the presence of nitrogen-centered nucleophiles, suggesting basicity of these nucleophiles favors elimination instead of substitution. We noticed in the cases of 5a–d the chiral induction was inverted, presumably due to the absence of H-bonding sites in these nucleophiles (SI, Fig. 2).
Synthetic applications
To extend synthetic application of this methodology, late-stage modification was conducted (Fig. 3a). Naturally available alcohols such as geraniol (2ah), protected galactose (2ai), and pharmaceutical alcohols derived from rosuvastatin (2aj), nordazepam (2ak), sofosbuvir precursor (2al) can also be incorporated with high levels of stereocontrol, demonstrating the applicability. α-Branched aldehydes derived from indoprofen (2am) and indomethacin (2an) worked extremely well to give the desired adducts with high enantioselectivity. In the latter case, the adapalene derivative as the alcohol could be applied to 97% ee. The current process can be scaled up to 7.5 mmol scale (gram-scale), maintaining similar outcomes (Fig. 3bvs Fig. 2, 2a) and the current efficiency η(2a) is determined to be 78%. We set up a solar-driven electrochemical apparatus with parallel circuits for magnetic stirring and electrolysis (SI, Fig. 3). By exposing the whole device to sunlight for three full days (ca. 30 h irradiation), the desired product could be isolated with 72% yield and 80% ee, indicating the current asymmetric electrolysis can be easily adapted to green energy source.
Fig. 3. Synthetic applications.
a Late-stage modification. aConditions: as described in Fig. 1 & Fig. 2. bIsolated yield. cEnantiomeric excess: determined by HPLC with a chiral column. dGraphite felt as the anode. b Large scale synthesis.
Control reactions
The role of copper additive was further explored. The detection of the initial reaction rate clearly indicated the addition of copper additive Cu(II)L1 at 50 ppm could enhance the reaction rate, leading to improved productivity but with similar enantioselectivity (Fig. 4d, i). Further increasing the loading of copper salts or switching to different copper complexes showed only a marginal effect on the reactivity and enantioselectivity (Fig. 4a). Particularly, there appears no obvious difference between a chiral ligand (R, R)-L2 and its enantiomer (Fig. 4a, entries 3 and 4). These observations suggest that Cu(II) additive mainly functions as a redox mediator to facilitate outer-sphere electron transfer with the anode in generating chiral carbocation intermediate (e.g. c1). The direct participation of copper in the form of an inner-sphere Cu(III) species such as complex I (Fig. 4a), though it cannot be completely excluded41, should be neglectable in the present electrochemical setting42–44. The possible involvement of an SN2 process via a triflate intermediate II, similar to those proposed in Jørgensen’s study27, can also be discounted by considering the virtually unchanged reaction outcomes under triflate-free conditions (Fig. 4a, entry 5).
Fig. 4. Mechanistic investigations.
a Control experiment. b Stoichiometric experiment. c Exclusion of a SOMO and α-imino radical mechanism: (i) tests of chiral secondary amine catalysts, (ii) radical-clock experiment. d Kinetic studies. See SI for details. From left to right: (i) initial rates on CuL1, and plots of initial rates against (ii) [BnOH] (from 50 to 70 mM), (iii) [1a] (from 5 to 25 mM). (iv) [cat1·HOTf] (from 1 to 5 mM). e Detection of the carbocation by nano-ESI mass spectrometry.
A stoichiometric experiment with preformed imine i1 proceeded smoothly to give the expected adduct 2a in 65% yield and 87% ee, verifying the aminocatalytic nature (SI, Figs. 7, 8). In this instance, the acceleration effect of copper salt was clearly noted and the stoichiometric reaction in the absence of Cu(OTf)2 gave only 26% yield with 85% ee (Fig.4b).
The SOMO process33–35 could be excluded by examination with typical secondary aminocatalysts, showing no reactivity (Fig. 4c, i;and SI, Table 11, entries 24–27). On the other hand, the radical cations formed by primary amine in our case is endowed with a rather acidic free N–H (pKa ca. 8–10 in DMSO37; for comparison, pKa of benzoic acid is 10.6), hence the proton-transfer of this radical cation is quite facile, followed by a barrier-less anodic electron transfer, with time scale much faster than its coupling with another nucleophiles. This provides a rational basis for the proposed carbocation mechanism. Control experiment on a radical-clock type aldehyde (1y) gave no rearrange products but the expected chiral ether (2ah), ruling out a possible radical-pathway (Fig. 4c, ii). Last but notleast, the substituent electronic effects showed the demand of electron donating groups, in consistence with a carbocation mechanism (SI, Fig. 4).
Kinetic studies
To study the kinetics, a fixed surface of electrode is necessary for consistent and reproduceable results (SI, Fig. 9). The reaction kinetics were determined by measuring the initial rates with varying concentrations of alcohol, aldehyde or aminocatalyst, respectively (SI, Figs. 10–15, Tables 13–18). It was found that the reaction is zero-order on benzyl alcohol (Fig. 4d, ii), and positive correlation to aldehyde 1a and aminocatalyst cat1·HOTf (Fig. 4d, iii and iv). Theoretically, the net reaction rate would increase as the electrode surface area increases. With finite surface area, the determined kinetic slope is generally less than 1.0. When cat1·HOTf was fixed on the anode by Nafion, no reaction was observed (SI, Table 11, entry 28). This observation indicates that the key bond formation step is most likely homogenous, adding credibility to the proposed kinetic experiments. Other cathodic materials were also examined, showing neglectable impacts (SI, Table 10, entries 4 and 5), indicating the cathodic counter reaction, hydrogen evolution (SI, Figs. 20, 21) is not a rate limiting step. This kinetic behavior suggests a rate-limiting step precedes the C–O bond formation, which is in accordance with the SN1-type process involving α-imino carbocation c1 derived from aminocatalyst and aldehyde 1a.
The nano-ESI apparatus, equipped a Pt wire for electrospray, was employed to in-situ detect the oxidized species (Fig. 4e)45–47. The expected α-imino carbocation c1 was identified (SI, Figs. 16–19 & Tables 19–22). Similar carbocation species such as c2 and c3 derived from other aldehydes were also characterized. When 1 vol% of methanol was added into the solvent, the signal of ether adduct c2-OMe was successfully identified and its structure was further established by collision-induced dissociation analysis (SI, Fig. 19), adding supports to the α-imino carbocation mechanism.
DFT Calculations
The origin of the stereoselectivity on this carbocation process was next investigated by DFT calculations (Fig. 5a; SI, Figs. 23–27 & Table 23, 24). As known, chiral primary aminocatalysts such as cat1·HOTf would form two geometrically varied E- and Z-enamines, e3-E and e3-Z, of which the E-isomer is favored sterically. The two enamine isomers exhibit similar Eox (Fig. 5a) and hence both would undergo anodic oxidation48, leading to α-imino carbocations c3-E and c3-Z, respectively. The former is favored by 3.9 kcal/mol due to its highly conjugated π-structure. In contrast, carbocation c3-Z is slightly de-conjugated with the aryl ring twisted about 28° relative to the π-allylic plane, second-order perturbation analysis of NBOs also showed that the interaction between the filled C = N bond orbitals and the C-C anti-bond orbitals of the carbocation center was significantly weakened in c3-Z (Fig. 5b). In c3-E, the positive charge is highly delocalized, contributing its preferential formation and reaction. In the SN1-type reaction with c3-E, the Re-facial attack, facilitated via a tertiary amine-assisted proton transfer, is favored by 1.94 kcal/mol over Si-facial attack (Fig. 6, TSE-Re and TSE-Si) where the proton-shuttle is absent. Interestingly, for the disfavored carbocation intermediate c3-Z, the Re-facial transition state TSZ-Re contains a more conjugated cationic structure than that of Si-facial TSZ-Si (Fig. 6, dihedral angle 10.2° vs 43.1°). The bias toward the proton-shuttle in the Si-facial attack is significantly compromised by the large distortion of the conjugated π-system. Hence, a Re-facial selection is plausible even with disfavored carbocation intermediate as a result of the conjugation effect. This suggests that the current carbocation strategy overrides the stereochemical constraints normally associated with enamine geometry in typical aminocatalysis. The NCI analysis shows significant attractive interactions between the tertiary amine and O-H in TSE-Re and TSZ-Si (Fig. 6, blue cycle), indicating the amine-assisted H-transfer is critical for these transition states. the distortion-interaction analysis also showed significant H-bonding interactions, which was found to be the major contribution to the stereo-control. In addition, steric repulsion between iPr substituents and the phenyl ring could also be observed in these two TSs (Fig. 6, red dashed cycle), but such interaction could not surpass the dispersion and strong H-bonding attraction. Symmetric-adapted perturbation theory (SAPT) analysis also showed significant large electrostatic attraction (H-bonding) and dispersion interaction between iPr substituents and the phenyl ring in TSE-Re and TSZ-Si (SI, Table 24). E- and Z- carbocation intermediate with cis-configurations of the C-N single bond were also considered, the tertiary amine-assisted proton transfer process with these two s-cis-conformers showed much higher activation energy (Supplementary Fig S6.1.5, ΔΔG° = 3.8 and 4.8 kcal/mol respectively), thus the contribution of these reaction routes was negligible. On this scenario, our DFT calculation gave 87% ee for 2z, in good consistency with the experimental 90% ee. It is anticipated that the electrochemical α-imino carbocation process can be generally applied to other nucleophiles, significantly expanding the reaction spaces of enamine catalysis.
Fig. 5. DFT studies.
a Enantio-control model. b 2nd-order perturbation theory analysis. Computational method: M06-2X/def2-TZVPP (SMD, DCM).
Fig. 6. DFT studies for the key transition states.
Computational method: M06-2X/def2-TZVPP(SMD, DCM) //M06-2X/def2-SVP(SMD, DCM). Carbocations are useful synthetic intermediates that enable direct functionalization of carbon centers, but their generation in a manner that uses mild conditions and enables stereoselective interception remains a longstanding goal of organic chemists. Here, the authors use electrochemical enamine oxidation to synthesize chiral scaffolds from racemic α-branched aldehydes.
In summary, we have developed asymmetric electrochemical catalysis for oxidative functionalization of α-branched aldehydes by chiral primary aminocatalyst. The reaction proceeds through SN1-type process with anodic chiral α-imino carbocation intermediate and encompasses a range of readily available O- and S- center nucleophiles including weak nucleophilic water with good yields and high enantioselectivity. Mechanistic studies verified the carbocationic nature, wherein a tertiary amine mediated N–H–X proton shuttle plays a critical role in dictating the stereoselectivty of the C–X bond formation. It is anticipated that the electrochemical α-imino carbocation process can be generally applied to polar reactions, significantly expanding the reaction spaces of enamine catalysis, meanwhile enriching the carbocation chemistry in general.
Methods
The general experimental procedure
Add 5 μL CuL1 (dissolved in acetone, 1 mM, 0.005 mol%) to the electrolysis cell with a magnetic stirrer bar, and gently warm the cell to evaporate the solvent. Aldehyde 1a (24.30 mg, 0.1 mmol, 1.0 eq.), cat1·HOTf (8.57 mg, 0.02 mmol, 20 mol%), BnOH (32.44 mg, 0.3 mmol, 3.0 eq.) were separately added to three centrifuge tubes. Quickly take 1 mL the electrolyte (0.1 M nBu4NPF6 in DCM) to dissolve 1a, cat1·HOTf and BnOH, and the solution was transferred to the electrolysis cell (repeat 3 times). After addition, the plug was installed, and the valve was closed. The sealed electrolysis cell was freeze-thawing three times and purged with argon. The electrolysis cell was then connected to a DC power supply with a constant voltage set at 2.0 V. The stirring speed was set at 1200 rpm. The power supply was shut down after 20 h. The solution was purified by silica gel column chromatography (100–200 mesh, PE: EA = 20:1) to obtain the pure product ether 2a. After collecting the characterization data, a sample of 2a is sealed in an ampule with argon purged. The ampule was stored at –78 °C (SI, Fig. 1 for details).
Supplementary information
Source data
Acknowledgements
We thank J. Cheng and M. Wang from Tsinghua University for helpful discussions and comments, and H. Xu from Xiamen University for the share of electrode material. This work is supported by the Natural Science Foundation of China (22031006, 91956000 and 22074075), and Haihe Laboratory of Sustainable Chemical Transformations. We thank the Tsinghua Xuetang Talents Program for computational support. L.Z. is supported by the National Program of Top-notch Young Professionals.
Author contributions
S.L. directed the project. Q.L. and S.L. conceived the ideas and designed the experiments. Q.L. conducted synthetic and kinetic experiments with the help of Y.D., Y.L., K.Y. and Z.J. Q.L. and R.J. conducted mass spectrometry experiments with the directions of Y.X. and S.L. L.Z. performed the computational studies. S.L. and Q.L. wrote the manuscript with contributions from all authors.
Peer review
Peer review information
Nature Communications thanks Da-Gang Yu, Tong Zhu, Guo-Qiang Xu, Xuefeng Tan and the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data are available from the corresponding author upon request. Supplementary Information is available and includes general information, substrate and reagent synthesis, optimization details, general experimental procedures, and compound characterization, determination of the absolute configuration, mechanistic studies, HPLC, NMR spectra, and source data on DFT calculations. Source data are provided with this paper.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Long Zhang, Email: zhanglong@tsinghua.edu.cn.
Sanzhong Luo, Email: luosz@tsinghua.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-50945-2.
References
- 1.Olah, G. A. 100 years of carbocations and their significance in chemistry. J. Org. Chem.66, 5943–5957 (2001). 10.1021/jo010438x [DOI] [PubMed] [Google Scholar]
- 2.Naredla, R. R. & Klumpp, D. A. Contemporary carbocation chemistry: applications in organic synthesis. Chem. Rev.113, 6905–6948 (2013). 10.1021/cr4001385 [DOI] [PubMed] [Google Scholar]
- 3.Wang, H. et al. Electrochemical oxidation-induced etherification via C(sp3)─H/O─H cross-coupling. Sci. Adv.6, eaaz0590 (2020). 10.1126/sciadv.aaz0590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen, T. & Lambert, T. H. Electrophotocatalytic diamination of vicinal C–H bonds. Science371, 620–626 (2021). 10.1126/science.abf2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida, J. & Suga, S. Basic concepts of “cation pool” and “cation flow” methods and their applications in conventional and combinatorial organic synthesis. Chem. Eur. J.8, 2650 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Suga, S., Watanabe, M. & Yoshida, J. Electroauxiliary-assisted sequential introduction of two carbon nucleophiles on the same α-carbon of nitrogen: application to the synthesis of spiro compounds. J. Am. Chem. Soc.124, 14824–14825 (2002). 10.1021/ja028663z [DOI] [PubMed] [Google Scholar]
- 7.Xiang, J. et al. Hindered dialkyl ether synthesis with electrogenerated carbocations. Nature573, 398–402 (2019). 10.1038/s41586-019-1539-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Go, S. Y. et al. A unified synthetic strategy to introduce heteroatoms via electrochemical functionalization of alkyl organoboron reagents. J. Am. Chem. Soc.144, 9149–9160 (2022). 10.1021/jacs.2c03213 [DOI] [PubMed] [Google Scholar]
- 9.Lin, Q., Li, L. & Luo, S. Asymmetric electrochemical catalysis. Chem. Eur. J.25, 10033–10044 (2019). 10.1002/chem.201901284 [DOI] [PubMed] [Google Scholar]
- 10.Chang, X., Zhang, Q. & Guo, C. Asymmetric electrochemical transformations. Angew. Chem. Int. Ed.59, 12612–12622 (2020). 10.1002/anie.202000016 [DOI] [PubMed] [Google Scholar]
- 11.Ghosh, M., Shinde, V. S. & Rueping, M. A review of asymmetric synthetic organic electrochemistry and electrocatalysis: concepts, applications, recent developments and future directions. Beilstein J. Org. Chem.15, 2710–2746 (2019). 10.3762/bjoc.15.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin, Q. & Luo, S. Tailoring radicals by asymmetric electrochemical catalysis. Org. Chem. Front.7, 2997–3000 (2020). 10.1039/D0QO00803F [DOI] [Google Scholar]
- 13.Jiao, K.-J. et al. The applications of electrochemical synthesis in asymmetric catalysis. Chem. Catal.2, 3019–3047 (2022). 10.1016/j.checat.2022.09.039 [DOI] [Google Scholar]
- 14.Mahlau, M. & List, B. Asymmetric counteranion-directed catalysis: concept, definition, and applications. Angew. Chem. Int. Ed.52, 518–533 (2013). 10.1002/anie.201205343 [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee, S. & List, B. Chiral counteranions in asymmetric transition-metal catalysis: highly enantioselective Pd/brønsted acid-catalyzed direct α-allylation of aldehydes. J. Am. Chem. Soc.129, 11336–11337 (2007). 10.1021/ja074678r [DOI] [PubMed] [Google Scholar]
- 16.Brak, K. & Jacobsen, E. N. Asymmetric ion-pairing catalysis. Angew. Chem. Int. Ed.52, 534–561 (2013). 10.1002/anie.201205449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wendlandt, A. E., Vangal, P. & Jacobsen, E. N. Quaternary stereocentres via an enantioconvergent catalytic SN1 reaction. Nature556, 447–451 (2018). 10.1038/s41586-018-0042-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Properzi, R. et al. Catalytic enantiocontrol over a non-classical carbocation. Nat. Chem.12, 1174–1179 (2020). 10.1038/s41557-020-00558-1 [DOI] [PubMed] [Google Scholar]
- 19.Tan, X. et al. Enantioselective synthesis of tetraarylmethanes through meta-hydroxyl-directed benzylic substitution. Nat. Synth.2, 275–285 (2023). 10.1038/s44160-022-00211-4 [DOI] [Google Scholar]
- 20.Singh, V. K. et al. Taming secondary benzylic cations in catalytic asymmetric SN1 reactions. Science382, 325–329 (2023). 10.1126/science.adj7007 [DOI] [PubMed] [Google Scholar]
- 21.Zheng, Y. et al. Application of organocatalysis in asymmetric construction of nitrogen-containing heterocyclic compounds. Chin. J. Org. Chem.41, 1–11 (2021). 10.6023/cjoc202008037 [DOI] [Google Scholar]
- 22.Gao, F. et al. Asymmetric synthesis of bedaquiline based on bimetallic activation and non-covalent interaction promotion strategies. Sci. China Chem.65, 1968–1977 (2022). 10.1007/s11426-022-1387-7 [DOI] [Google Scholar]
- 23.He, Y.-M. et al. Recent progress of asymmetric catalysis from a Chinese perspective. CCS Chem. 5, 2685–2716 (2023). 10.31635/ccschem.023.202303347 [DOI] [Google Scholar]
- 24.Cai, M. et al. Enantioselective transformations by “1 + x” synergistic catalysis with chiral primary amines. Acc. Chem. Res.57, 1523–1537 (2024). 10.1021/acs.accounts.4c00128 [DOI] [PubMed] [Google Scholar]
- 25.Shono, T. et al. Electrooxidation of enamines, haloolefins, and enol ethers. Bull. Chem. Soc. Jpn.51, 2179–2180 (1978). 10.1246/bcsj.51.2179 [DOI] [Google Scholar]
- 26.Chiba, T., Okimoto, M., Nagai, H. & Takata, Y. Electrochemical oxidation of enamines in the presence of organic anions. J. Org. Chem.44, 3519–3523 (1979). 10.1021/jo01334a016 [DOI] [Google Scholar]
- 27.Leth, L. A. et al. Enantioselective oxidative coupling of carboxylic acids to α-branched aldehydes. J. Am. Chem. Soc.140, 12687–12690 (2018). 10.1021/jacs.8b07394 [DOI] [PubMed] [Google Scholar]
- 28.Lamhauge, J. N., Corti, V., Liu, Y. & Jørgensen, K. A. Enantioselective α‐etherification of branched aldehydes via an oxidative umpolung strategy. Angew. Chem. Int. Ed.60, 18728–18733 (2021). 10.1002/anie.202105721 [DOI] [PubMed] [Google Scholar]
- 29.Rezayee, N. M., Lamhauge, J. N. & Jørgensen, K. A. Organocatalyzed cross-nucleophile couplings: umpolung of catalytic enamines. Acc. Chem. Res.55, 1703–1717 (2022). 10.1021/acs.accounts.2c00149 [DOI] [PubMed] [Google Scholar]
- 30.Rezayee, N. M. et al. Metal-free, oxidative α-coupling of aldehydes with amine nucleophiles for the preparation of congested C(sp3)–N bonds. J. Org. Chem.87, 1756–1766 (2022). 10.1021/acs.joc.1c01937 [DOI] [PubMed] [Google Scholar]
- 31.Wang, D., Zhang, L. & Luo, S. Enantioselective decarboxylative α-alkynylation of β-ketocarbonyls via a catalytic α-imino radical intermediate. Org. Lett.19, 4924–4927 (2017). 10.1021/acs.orglett.7b02386 [DOI] [PubMed] [Google Scholar]
- 32.Rezayee, N. M. et al. An asymmetric SN2 dynamic kinetic resolution. J. Am. Chem. Soc.143, 7509–7520 (2021). 10.1021/jacs.1c02193 [DOI] [PubMed] [Google Scholar]
- 33.Beeson, T. D., Mastracchio, A., Hong, J.-B., Ashton, K. & MacMillan, D. W. C. Enantioselective organocatalysis using SOMO activation. Science316, 582–585 (2007). 10.1126/science.1142696 [DOI] [PubMed] [Google Scholar]
- 34.Bui, N.-N., Ho, X.-H., Mho, S.-I. & Jang, H.-Y. Organocatalyzed α-oxyamination of aldehydes using anodic oxidation. Eur. J. Org. Chem.2009, 5309–5312 (2009). 10.1002/ejoc.200900871 [DOI] [Google Scholar]
- 35.Ho, X.-H., Mho, S., Kang, H. & Jang, H.-Y. Electro-organocatalysis: enantioselective α-alkylation of aldehydes. Eur. J. Org. Chem.2010, 4436–4441 (2010). 10.1002/ejoc.201000453 [DOI] [Google Scholar]
- 36.Wang, Y. et al. Steric effect of protonated tertiary amine in primary–tertiary diamine catalysis: a double-layered sterimol model. Org. Lett.21, 407–411 (2019). 10.1021/acs.orglett.8b03584 [DOI] [PubMed] [Google Scholar]
- 37.Whittington, D. A. et al. Bornyl diphosphate synthase: structure and strategy for carbocation manipulation by a terpenoid cyclase. Proc. Natl Acad. Sci. USA.99, 15375–15380 (2002). 10.1073/pnas.232591099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witten, M. R. & Jacobsen, E. N. A simple primary amine catalyst for enantioselective α-hydroxylations and α-fluorinations of branched aldehydes. Org. Lett.17, 2772–2775 (2015). 10.1021/acs.orglett.5b01193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai, M. et al. Chiral primary amine/ketone cooperative catalysis for asymmetric α-hydroxylation with hydrogen peroxide. J. Am. Chem. Soc.143, 1078–1087 (2021). 10.1021/jacs.0c11787 [DOI] [PubMed] [Google Scholar]
- 40.Cai, M. et al. Visible light-promoted enantioselective aerobic hydroxylation of β-ketocarbonyls by chiral primary amine catalysis. ACS Catal.13, 7538–7543 (2023). 10.1021/acscatal.3c01477 [DOI] [Google Scholar]
- 41.Wang, X. et al. Flexible electrode for rapid glucose detection based on CuO nanoflowers/stereo‐graphene coated on carbon cloth. ChemElectroChem9, e202200529 (2022). 10.1002/celc.202200529 [DOI] [Google Scholar]
- 42.Bacha, J. D. & Kochi, J. K. Oxidation of alkyl radicals from decarboxylation of acids by lead(IV) and copper(II). J. Org. Chem.33, 83–93 (1968). 10.1021/jo01265a016 [DOI] [Google Scholar]
- 43.Kochi, J. K., Bemis, A. & Jenkins, C. L. Mechanism of electron transfer oxidation of alkyl radicals by copper(II) complexes. J. Am. Chem. Soc.90, 4616–4625 (1968). 10.1021/ja01019a018 [DOI] [Google Scholar]
- 44.Li, Q. Y. et al. Decarboxylative cross-nucleophile coupling via ligand-to-metal charge transfer photoexcitation of Cu(II) carboxylates. Nat. Chem.14, 94–99 (2022). 10.1038/s41557-021-00834-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, Y., Zhang, L. & Luo, S. Bond energies of enamines. ACS Omega7, 6354–6374 (2022). 10.1021/acsomega.1c06945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang, S. J. & Lessard, M. V. Stable halo enamines. possible SN1-type solvolysis. J. Am. Chem. Soc.90, 2432–2434 (1968). 10.1021/ja01011a048 [DOI] [Google Scholar]
- 47.Oberacher, H., Pitterl, F., Erb, R. & Plattner, S. Mass spectrometric methods for monitoring redox processes in electrochemical cells. Mass. Spectrom. Rev.34, 64–92 (2015). 10.1002/mas.21409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li, Y., Wang, D., Zhang, L. & Luo, S. Redox property of enamines. J. Org. Chem.84, 12071–12090 (2019). 10.1021/acs.joc.9b02003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the corresponding author upon request. Supplementary Information is available and includes general information, substrate and reagent synthesis, optimization details, general experimental procedures, and compound characterization, determination of the absolute configuration, mechanistic studies, HPLC, NMR spectra, and source data on DFT calculations. Source data are provided with this paper.