Abstract
The virion host shutoff (vhs) protein encoded by herpes simplex virus type 1 (HSV-1) destabilizes both viral and host mRNAs. An HSV-1 strain with a mutation in vhs is attenuated in virulence and induces immune responses in mice that are protective against corneal infection with virulent HSV-1, but it has the capacity to establish latency. Similarly, a replication-incompetent HSV-1 strain with a mutation in ICP8 elicits an immune response protective against corneal challenge, but it may be limited in viral antigen production. We hypothesized therefore that inactivation of vhs in an ICP8− virus would yield a replication-incompetent mutant with enhanced immunogenicity and protective capacity. In this study, a vhs−/ICP8− HSV-1 mutant was engineered. BALB/c mice were immunized with incremental doses of the vhs−/ICP8− double mutant or vhs− or ICP8− single mutants, or the mice were mock immunized, and protective immunity against corneal challenge with virulent HSV-1 was assessed. Mice immunized with the vhs−/ICP8− mutant showed prechallenge serum immunoglobulin G titers comparable to those immunized with replication-competent vhs− virus and exceed those of mice immunized with the ICP8− single mutant. Following corneal challenge, the degrees of protection against ocular disease, weight loss, encephalitis, and establishment of latency were similar for vhs−/ICP8− and vhs− virus-vaccinated mice. Moreover, the double deleted vhs−/ICP8− virus protected mice better in all respects than the single deleted ICP8− mutant virus. The data indicate that inactivation of vhs in a replication-incompetent virus significantly enhances its protective efficacy while retaining its safety for potential human vaccination. Possible mechanisms of enhanced immunogenicity are discussed.
Herpes simplex virus type 1 (HSV-1) is a common human pathogen, infecting approximately 80% of individuals by adulthood (49). The virus typically enters the body at epithelial and mucosal surfaces, where lytic infection of epithelial cells and fibroblasts leads to infection of sensory neurons innervating the mucosa and to the rapid establishment of latent infection in the neuronal cell bodies. In this latent reservoir, HSV infection is maintained for the life of the host. Either initial infection or reactivation can result in serious human disease, including rare but devastating encephalitis and keratitis, which is the second most common cause of nontraumatic corneal blindness (49). A vaccine to obviate or therapeutically alleviate these HSV-1-mediated diseases is a desirable goal.
Development of an antiviral vaccine requires consideration of both safety and immunogenicity. An effective balance between these can be difficult to achieve, especially when faced with HSV that has a complex and persistent lifestyle. Immunization with live attenuated virus has the potential advantages of generating immune responses to a broad spectrum of viral proteins and induction of type 1 T-cell as well as humoral responses. In the development of prototypic live virus vaccines, several viral proteins that regulate host cell and viral synthetic processes have been manipulated to advantage. During infection, one of the earliest viral activities is that mediated by the virion host shutoff (vhs) protein, a product of the UL41 gene. This viral tegument component exerts its effects immediately upon entry into the cell, prior to viral gene expression (13, 39). The vhs protein is associated with degradation of both cellular and viral mRNAs (24–26, 36, 39, 43) and endoribonucleolytic activity (9, 52), and the destabilization of viral messages mediated by vhs has been theorized to promote the switch from transcription of one kinetic class of viral genes to the next (43). We have previously shown that mice immunized with an HSV-1 strain that is deficient in vhs activity, UL41NHB, are significantly protected against corneal challenge with virulent HSV-1 in a model of HSV-1-induced ocular disease (47). Replication of challenge virus in the cornea and acute and latent infection of the trigeminal ganglia all are reduced in mice immunized with UL41NHB compared with mice immunized with UV-inactivated virus. Protection against shedding of HSV-1 from the cornea after UVB radiation-induced reactivation can also be achieved by therapeutic immunization of latently infected mice (46). A second viral gene that has been modified in vaccine approaches is UL29, which encodes ICP8. Numerous viral gene products are expressed by cells infected with ICP8− virus, including the major viral glycoproteins gB and gD, but because ICP8 is essential for virus DNA replication (6, 27, 48, 50), progeny virions are not produced. We have shown that prophylactic immunization of mice with a replication-incompetent HSV-1 strain deficient in ICP8, d301, similarly reduces acute infection of the cornea and trigeminal ganglia and latent infection in the nervous system compared to that in mice immunized with UV-inactivated virus (29). Immunization with d301 elicits humoral (29) and cytolytic T-cell (3) responses. We have demonstrated that protection against acute infection and disease after corneal challenge is long-lived (30) and is dependent on both HSV-immune antibodies and T cells (31).
Evidence of safety and immunogenicity in potential vaccine strains must be carefully extrapolated from mice to humans. UL41NHB is profoundly attenuated in mice, showing decreased capacity to replicate when inoculated intracranially or onto the scarified cornea. UL41NHB also establishes latency with reduced frequency and reactivates poorly upon explant of infected trigeminal ganglia (42). Despite these properties, UL41NHB theoretically retains the potential to cause disease in vaccinated humans because it is replication competent. d301, in contrast, is avirulent even in immunocompromised mice (L. A. Morrison and D. Knipe, unpublished observation). In addition, there is no amplification of viral DNA in the vaccinated host or latent infection in the nervous system (8). The adequacy of the immune response to a replication-incompetent virus remains a concern, however, because production of immunogenic viral proteins is limited to cells initially infected by the vaccine virions. We hypothesized that a vaccine strain defective in both vhs and ICP8 functions would be as immunogenic as vhs− virus, with improved immunogenicity and protective capacity over an ICP8− virus. A vhs−/ICP8− double mutant HSV-1 strain therefore was constructed and compared, using a mouse model of corneal infection with HSV-1, to vhs−, replication-incompetent and ICP8−, replication-competent viruses for immunogenicity and protection against disease and latent and fatal infection.
MATERIALS AND METHODS
Cells and viruses.
Vero and S2 cells were cultured as previously described (15). S2 cells stably express the ICP8 (single-stranded DNA-binding protein; product of UL29) of HSV-1 (15). Replication-incompetent ICP8− mutants d301 (15) and HD-2 (lacZ+ [15]), derived from KOS1.1 (21), were propagated on this cell line. Replication-competent vhs− mutants UL41NHB (42) and BGS41 (lacZ+) (42), derived from KOS, and wild-type HSV-1 strains KOS, KOS1.1, and microplaque (mP) (19) were propagated on Vero cells. Cell lysate stocks of all viruses were prepared by infection of S2 or Vero monolayers as previously described (30) and were used for in vitro assays. Partially purified, cell-free virus stocks were prepared as previously described (30) and were used for immunization studies. Uninfected control lysates and supernatants were prepared in the same manner as infected stocks. Titers of virus stocks were determined by standard plaque assay (22).
Generation of Δ41Δ29.
vhs/ICP8-deficient virus was constructed by insertional inactivation of the UL29 gene in the vhs mutant virus UL41NHB. Briefly, 1 μg of pICP8-LacZ plasmid DNA, containing the UL29 open reading frame (ORF) disrupted by a human cytomegalovirus (HCMV) IE:β-galactosidase (β-Gal cassette (7), and 1 μg of infectious UL41NHB DNA were cotransfected into S2 cells using Lipofectamine (Gibco BRL). Cultures were collected when they reached 100% cytopathic effect (CPE), then frozen, thawed, sonicated, and serially diluted onto S2 cell monolayers. Blue plaques were picked 72 h after the addition of agarose overlay supplemented with X-Gal (160 μg/ml). Isolates were plaque purified three times and analyzed for replication deficiency by comparing plaque formation on the complementing S2 cell line and noncomplementing Vero cells.
Southern blot analysis.
To confirm disruption of UL29 and UL41, 1 μg of viral DNA from each plaque isolate was digested with HpaI or EcoRV (New England Biolabs), electrophoresed on a 1% agarose gel, and transferred to nitrocellulose membranes for Southern hybridization. A 2-kb PstI/EcoRI fragment from pUL41 (42) was used to probe for the presence of an HpaI restriction site in the UL41 locus. A 2-kb NotI fragment of p8BS (15) was used to probe for the presence of a lacZ insertion in the UL29 locus. Southern blotting was performed as described elsewhere (38, 40), using the Alk Phos Direct Southern hybridization kit (Amersham Life Science), according to the manufacturer's directions. Images were obtained using a Storm PhosphorImager (Molecular Dynamics).
Northern blot analysis and mRNA degradation assay.
Total cytoplasmic RNA was prepared from monolayer cultures of infected or mock-infected Vero cells as described previously (42). Monolayer cultures of 5 × 105 to 5 × 106 cells were mock infected or infected at a multiplicity of infection (MOI) of 20 with KOS, KOS1.1, HD-2, Δ41Δ29, or BGS41 in the presence of actinomycin D (10 μg/ml). Mock-infected plates received Vero cell lysate only. Cytoplasmic RNAs were harvested at 8 h postinfection and analyzed for mRNA degradation by Northern blot analysis probing for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (14, 42). Filters were first probed for GAPDH, stripped, and then reprobed for the 28S ribosomal subunit as a loading control. Phosphorimages were scanned on a Storm 860 PhosphorImager (Molecular Dynamics) and quantified. The level of GAPDH for mock-infected cells was set at 100% and compared with the 28S-normalized GAPDH values of virus-infected cells.
Multistep growth assay.
S2 cell monolayers in 12-well plates were infected with KOS, KOS1.1, BGS41, HD-2, or Δ41Δ29 virus at approximately 100 PFU/well and were incubated at 37°C for 0 to 36 h. At each time point, monolayers were scraped, collected, and frozen at −80°C. Titers were determined by standard plaque assay.
Animals and inoculations.
Female BALB/c mice (6 weeks of age), purchased from the National Cancer Institute, were housed in accordance with Public Health Service (1) and institutional guidelines and were rested for 1 week before use. Mice were immunized subcutaneously (s.c.) in each rear flank with 20 μl of partially purified virus suspended in low-endotoxin normal saline. Twenty-four days after immunization, mice were anesthetized with pentobarbital sodium (Nembutal) and challenged with HSV-1 mP in a 5-μl volume after bilateral scarification of the corneas (5). Doses of immunizing and challenge viruses are indicated in the text.
ELISA.
Blood was collected from the tail veins of immunized mice 5 days prior to challenge. HSV-1-specific immunoglobulin G (IgG) titers in sera were determined by enzyme-linked immunosorbent assay (ELISA) as described previously (28). Briefly, Immulon-2 96-well microtiter plates (Dynex Technologies) were incubated with lectin-purified HSV-1 KOS glycoprotein (37) for 48 h at 4°C and blocked with phosphate-buffered saline (PBS) containing 5% goat serum (GIBCO) for 2 h at 20°C. Serial twofold dilutions of sera in PBS plus 0.1% Tween 20 were added in duplicate to plates and were incubated for 2 h at 20°C. Wells were washed and incubated for 2 h with biotinylated goat anti-mouse IgG (Caltag), followed by 30-min incubation with streptavidin-HRP (Zymed). Wells were developed by the addition of 0.4 mg of o-phenylenediamine (OPD; Sigma)/ml plus 0.05% hydrogen peroxide and read at 490 and 630 nm with an EL340 microplate reader (BIOTEK). HSV-specific serum IgG concentrations were calculated based on comparison to a standard curve generated from serum for which the HSV-specific IgG concentration is known. HSV-specific IgG concentration in the standard serum had been previously determined by comparison to dilutions of purified IgG captured on an anti-kappa-coated plate (28). Geometric mean titers plus or minus the standard error of the mean (SEM) were calculated for each immunizing dose.
Acute replication.
Acute replication of mP in the cornea was assayed on days 0 through 4 postchallenge as previously described (30).
Clinical disease.
Blepharitis and weight change were monitored as clinical signs of HSV-induced disease. Blepharitis was scored in a masked manner to avoid bias. Disease was estimated on a scale of 0 to 4: 0, no apparent disease; 1, slight swelling and erythema of the eyelid; 2, moderate swelling and crusty exudate; 3, periocular lesions, severe swelling, and depilation; and 4, severe lesions, swelling, and depilation. Weight change was assessed daily from days 0 through 8. The mean weight change plus or minus the standard error of the mean compared with initial body weight was calculated daily for each group.
Latent and lethal infections.
Thirty days after challenge, surviving mice were sacrificed, and trigeminal ganglia were explanted to Vero cell monolayers as previously described (31). Reactivation was scored by the presence of CPE at 10 days postexplant. Monolayers showing no CPE after 10 days were scraped and homogenized using a minibead beater (BioSpec Products). Homogenized samples were incubated with fresh Vero monolayers and observed for 3 days for CPE. The proportion of mice in each group surviving challenge infection was recorded at 30 days postchallenge.
Statistics.
The difference in means for antibody titers and weight loss on individual days was determined by the Student t test (BGS41 or Δ41Δ29 versus HD-2). The severity of blepharitis was compared between groups using the nonparametric Kruskal-Wallis test; the parametric general linear models test yielded similar results. Differences in keratitis incidence, reactivation frequencies, and survival were compared by chi-square analysis of 2 by 2 contingency tables.
RESULTS
Construction and in vitro characterization of a vhs−/ICP8− HSV-1 mutant.
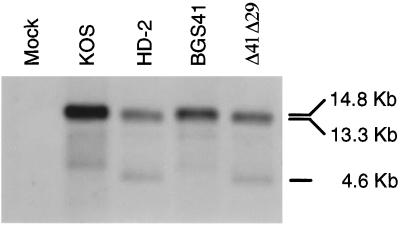
To test the hypothesis that ablation of the UL41 gene (vhs) in a replication-incompetent HSV-1 strain would enhance the immune response to vaccination, a virus was constructed that contained disrupted UL41 and UL29 genes. Infectious DNA from the replication-competent, vhs− strain, UL41NHB, was cotransfected with a plasmid, p8BS, containing a portion of the UL29 gene in which a 2-kb NotI fragment was replaced with a cytomegalovirus IE1 promoter:β-galactosidase cassette. This insertion disrupts the open reading frame of the essential UL29 gene, as previously reported (15). Recombinant plaques that developed on an ICP8-complementing cell line were screened by blue-white selection in the presence of X-Gal. Isolates that exhibited blue staining and replication incompetence were subjected to Southern blot analysis to verify disruption of UL29 and UL41. A probe spanning a portion of the UL41 locus confirmed the presence of a stop codon containing a unique HpaI restriction site (42; data not shown). EcoRV digestion of the wild-type genome yields a 14.8-kb fragment that encompasses the UL29 locus. Insertion of lacZ into the UL29 locus would increase its size by 3.1 kb but would also introduce an additional EcoRV site in the middle of lacZ. Thus, a probe spanning the lacZ insertion point in the UL29 locus would yield 2 bands of 4.6 and 13.3 kb and confirm disruption of UL29. One isolate that exhibited the appropriate banding pattern by Southern analysis (Fig. 1) was designated Δ41Δ29.
FIG. 1.
Southern blot analysis of recombinant virus genomes. Viral DNAs were digested with EcoRV, electrophoresed, and transferred to nitrocellulose membranes. The blot was probed with a 2-kb fragment of UL29 that was expected to hybridize to 4.6- and 13.3-kb fragments in a mutant virus and a single 14.8-kb fragment in wild-type virus. Lanes are as indicated.
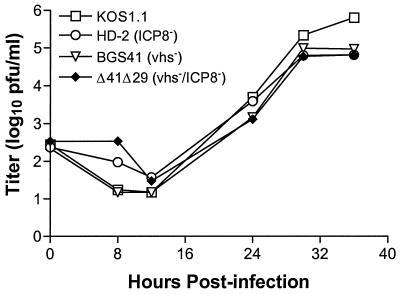
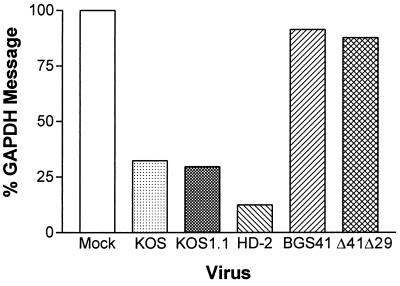
Multistep growth assays were performed in ICP8-complementing S2 cells to compare in vitro replication of Δ41Δ29 with wild-type and single-mutant viruses. Titers of vhs−/ICP8− Δ41Δ29 were not significantly different from those of wild-type KOS1.1, ICP8-defective HD-2, or vhs-defective BGS41 over several rounds of replication in culture (Fig. 2), although slightly higher titers of wild-type virus were consistently observed after 36 h of culture. Growth kinetics of Δ41Δ29 were also similar to those of wild-type KOS and to UL41NHB and d301, the HSV-1 strains used in the original immunization studies with single-mutant viruses (29, 47; data not shown). Single-step growth assays revealed identical kinetics among wild-type, single-mutant, and double-mutant viruses over a 24-h period (data not shown). To verify ablation of vhs activity in Δ41Δ29, Northern blot analysis of GAPDH mRNA levels in cells infected with wild-type, ICP8−, vhs−, or vhs−/ICP8− virus was performed. Vero cells were infected with virus at an MOI of 20 in the presence of actinomycin D, and at 8 h postinfection, total RNA was extracted and electrophoresed. Wild-type KOS- and KOS1.1-infected cells showed decreased levels of GAPDH message compared to mock-infected cells (Fig. 3). Cells infected with replication-incompetent HD-2 virus also showed a decrease in GAPDH message, indicating that ICP8− virus has wild-type vhs activity. Cells infected with BGS41 or Δ41Δ29 exhibited no decrease in GAPDH message compared to the mock-infected control, indicating that vhs function is compromised in these viruses. The vhs deletion, rather than the effect of actinomycin D, is responsible for the observed decrease in vhs activity because a similar decrease in activity was detected in S2 cell cultures infected with Δ41Δ29 in the absence of the drug (data not shown).
FIG. 2.
Multistep growth curve in cultured cells. Replicate monolayers of S2 cells were infected with approximately 100 PFU of HSV-1 strain KOS, KOS1.1, HD-2, BGS41, or Δ41Δ29 and incubated for the indicated periods of time. Monolayers were then collected, and viral titers were determined by standard plaque assay. Two independent experiments gave similar results.
FIG. 3.
RNA degradation assay by Northern blot analysis. Graph shows the percent of GAPDH RNA remaining from 28S-normalized KOS-, KOS1.1-, HD-2-, BGS41-, and Δ41Δ29-infected Vero cells at 8 h postinfection, relative to mock-infected cells in the presence of actinomycin D. Two independent experiments gave similar results.
Immunization studies.
To compare protective efficacy of a vhs-defective, replication-incompetent HSV-1 with that of either vhs-defective or replication-incompetent single mutants, a dose-response experiment was performed. To control for expression of β-Gal by Δ41Δ29, the lacZ+ viruses HD-2 and BGS41 were used in this in vivo comparison. Groups of six BALB/c mice were immunized s.c. with control supernatant or with 4 × 105 PFU, 1 × 105 PFU, or 2.5 × 104 PFU of either HD-2, BGS41, or Δ41Δ29 viruses. Twenty-four days after immunization, mice underwent bilateral corneal challenge with 8 × 105 PFU of virulent HSV-1 mP. Seven parameters were monitored in this experiment: prechallenge IgG titers, acute replication of challenge virus at the site of infection, weight change, blepharitis, keratitis, survival, and reactivation of virus from trigeminal ganglia of mice that survived infection. Altogether, four experiments were performed that yielded remarkably consistent results.
Prechallenge serum IgG.
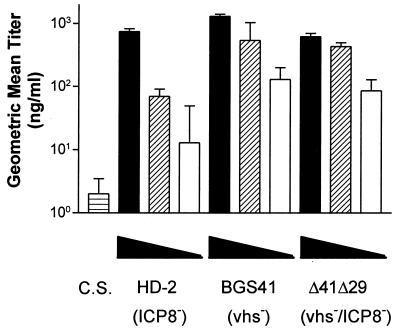
It has been previously shown that immunization with either ICP8− or vhs− mutant viruses can elicit a humoral immune response (29, 47). As one measure of immune induction, we determined whether inactivation of vhs in a replication-incompetent virus alters serum antibody titer. Sera were collected 19 days after immunization, and HSV-specific IgG titers in individual serum samples were analyzed by ELISA. When immunized with the 4 × 105 PFU dose of virus, all mice showed similar high levels of HSV-specific IgG (Fig. 4). Groups immunized with the 105-PFU dose of BGS41 and Δ41Δ29 exhibited almost 1 log10 (six- to eightfold) higher than those immunized IgG titer with HD-2 (P ≤ 0.002). At the 2.5 × 104 PFU dose, titers of mice immunized with BGS41 or Δ41Δ29 again were similar, and they exceeded the titers observed with HD-2, although, due to variability within the HD-2 titers, this difference was not statistically significant. Overall, the titer induced by HD-2 immunization dropped more rapidly with decreasing dose.
FIG. 4.
Prechallenge HSV-1-specific serum IgG titers. Serum was collected from each of the mice immunized with 4 × 105 PFU (filled bars), 1 × 105 PFU (striped bars), or 2.5 × 104 PFU (open bars) of the indicated viruses and analyzed for HSV-specific IgG by ELISA. C.S., control supernatant. Geometric mean titers ± SEM are from groups of six mice and are shown for one representative experiment of three performed. The difference in means was tested for significance by the Student t test (BGS41 or Δ41Δ29 versus HD-2).
Acute replication.
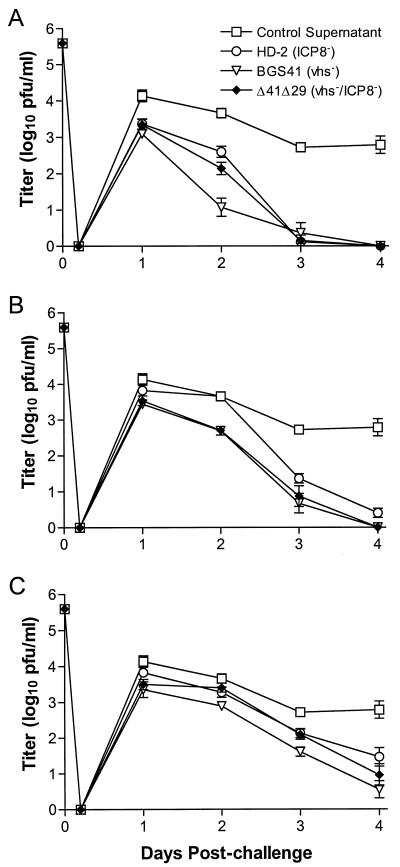
Mice were challenged by application of virulent HSV-1 mP to the scarified corneas, and acute replication of challenge virus was analyzed from days 0 through 4 postchallenge. Immunization with any of the viruses prior to challenge significantly reduced acute replication in the eye compared to control vaccination, and the magnitude of the protection was dose dependent (Fig. 5). In this and subsequent experiments, however, there was no significant difference between the immunizing viruses in their capacities to reduce acute replication.
FIG. 5.
Acute replication of challenge virus in the corneal epithelium. Mouse eyes were swabbed at the indicated times after corneal challenge with 8 × 105 PFU of HSV-1 strain mP. Immunizing doses of (A) 4 × 105 PFU, (B) 1 × 105 PFU, and (C) 2.5 × 104 PFU are shown. Virus content in the tear film was assessed by standard plaque assay. Values represent the geometric mean titer ± SEM from groups of six mice and are from the same experiment as that shown in Fig. 4.
Body weight change.
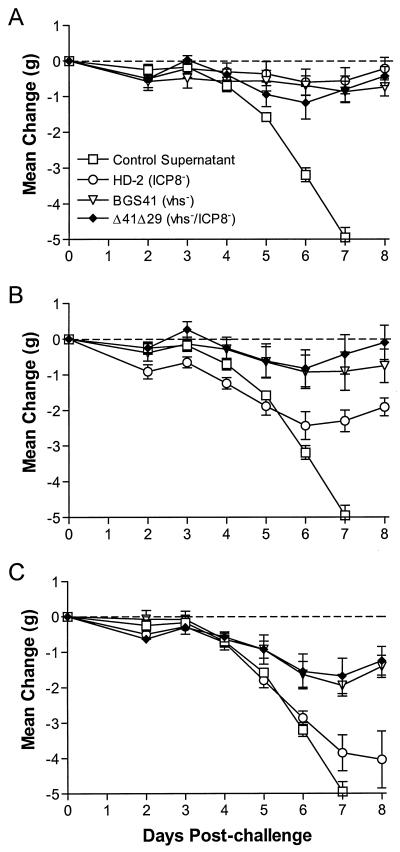
During the progression of HSV-1 infection, mice lose weight in a manner consistent with the severity of disease. Thus, weight change can be used as a sensitive indicator of overall health. Over the first 8 days postchallenge, mice immunized with control supernatant quickly lost weight (Fig. 6). For each immunizing virus, weight loss postchallenge was inversely proportional to the immunizing dose. At the 4 × 105-PFU immunizing dose, all three viruses inhibited weight loss to a similar degree (Fig. 6A). At the 105 PFU dose, HD-2-immunized mice consistently lost an average of 1 g more than BGS41- or Δ41Δ29-immunized mice (Fig. 6B). The difference between HD-2 and Δ41Δ29 and BGS41 was statistically significant from day 3 and beyond (P = 0.01 to 0.05). At later times during infection, HD-2-immunized mice regained weight, but they did not recover to prechallenge weight. Mice immunized with 2.5 × 104 PFU showed an even greater disparity between the BGS41- and Δ41Δ29-immunized groups and the HD-2-immunized group (Fig. 6C). BGS41- and Δ41Δ29-immunized groups consistently exhibited similar weight losses, which were more moderate than that seen with the HD-2 group. By day 8, BGS41- and Δ41Δ29-immunized mice had, in fact, begun to regain weight. The greater weight loss in HD-2-immunized mice was statistically significant beginning at 5 days postchallenge (P ≤ 0.01).
FIG. 6.
Change in body weight postchallenge. Baseline weights of mice (∼20 g) were obtained prior to corneal challenge with 8 × 105 PFU of HSV-1, and mice were weighed each day following challenge through day 8. Immunizing doses of (A) 4 × 105 PFU, (B) 1 × 105 PFU, and (C) 2.5 × 104 PFU are shown. Values represent the mean weight change per group of six mice and are from the same experiment as that shown in Fig. 4.
Blepharitis.
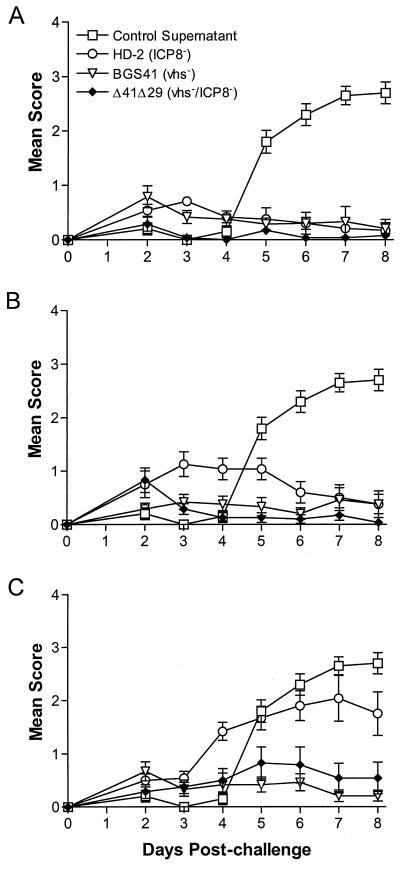
Disease of the eyelid resulting from virulent HSV-1 infection was scored in a masked fashion from days 0 to 8 postinfection. Only occasional mild blepharitis was observed in groups immunized with 4 × 105 PFU of any of the viruses, compared to those given control immunizations (Fig. 7A), and Δ41Δ29-immunized mice differed from HD-2-immunized mice only at 3 days postchallenge (P < 0.013). Greater differences were observed, however, when lower doses of immunizing virus were used. At 105 PFU, mice immunized with HD-2 exhibited significantly more severe blepharitis from days 3 to 6, compared to those given BGS41 and Δ41Δ29 (P < 0.001 to 0.015), which subsided after day 6 (Fig. 7B). Differential protection from blepharitis was most pronounced at the 2.5 × 104 PFU dose, where BGS41- and Δ41Δ29-immunized mice showed minimal disease, but HD-2-immunized mice had significantly elevated scores from days 4 through 8 (P < 0.002 to 0.013) (Fig. 7C).
FIG. 7.
Severity of blepharitis postchallenge. Blepharitis was scored daily postchallenge in masked fashion. Immunizing doses of (A) 4 × 105 PFU, (B) 1 × 105 PFU, and (C) 2.5 × 104 PFU are shown. Values represent the mean score ± SEM for six mice per group and are from the same experiment as that shown in Fig. 4 and 5.
Experimental groups in which mortality was significant could not be used for assessment of keratitis and reactivation frequencies. Thus, several experiments were performed using immunizing doses of 1 × 105 PFU to address keratitis and latent infection and 2 × 104 PFU to assess survival. Data from three such experiments were pooled for these analyses.
Keratitis and latent infection.
Keratitis was scored in masked fashion at 9 days postchallenge. A higher frequency of severe keratitis, defined as scores of 3+ or 4+, was consistently observed in HD-2-immunized mice compared to mice immunized with Δ41Δ29 or BGS41 (Table 1). The incidence of severe keratitis among mice immunized with Δ41Δ29 was not statistically different from that of mice immunized with BGS41.
TABLE 1.
Frequency of severe keratitis and reactivation postchallenge
| Immunization group | Proportion (%) diseased or reactivating
|
|
|---|---|---|
| Eyes with severe keratitisa | Reactivation from TGb | |
| HD-2 | 22/38 (58) | 19/22 (86) |
| Δ41Δ29 | 12/38 (32 [P = 0.0211])c | 12/22 (55 [P = 0.0207])c |
| BGS41 | 5/38 (13 [P < 0.0001])c | 12/22 (55 [P = 0.0207])c |
Severe keratitis defined as scores of 3+ or 4+; keratitis was assessed 9 days postchallenge in experiments 1, 2, and 4. Immunization and challenge doses were 1 × 105 PFU and 8 × 105 PFU, respectively.
TG, trigeminal ganglia. Reactivation was assessed by explant cocultivation at 30 days postchallenge in experiments 1 and 2.
Versus HD-2 (chi-square analysis).
Latent infection of the trigeminal ganglia was assessed by explant cocultivation assay in groups in which all or nearly all mice survived the challenge infection. Results pooled from two experiments showed that mice immunized with BGS41 and Δ41Δ29 had a lower frequency of reactivation than did those immunized with HD-2 (Table 1). In a third experiment using a lower challenge dose, reactivation from ganglia of Δ41Δ29- and HD-2-immunized mice was again significantly different (P < 0.026), but in this case reactivation frequency of BGS41-immunized mice was intermediate (data not shown).
Lethal infection.
Table 2 shows mortality results pooled from three separate experiments in which lethal infection was observed. Significantly fewer mice succumbed to infection in groups immunized with BGS41 or Δ41Δ29 than in those immunized with HD-2. Thus, the trend toward a reduction in lethality and in reactivatable virus in the trigeminal ganglia is consistent with the overall picture of stronger immune protection afforded by immunization with Δ41Δ29.
TABLE 2.
Survival of mice postchallenge
| Immunization group | Proportion (%) survivinga |
|---|---|
| HD-2 | 9/18 (50) |
| Δ41Δ29 | 17/21 (81 [P = 0.0409])b |
| BGS41 | 17/19 (90 [P = 0.0086])b |
Assessed at 30 days postchallenge in experiments 1, 2, and 3; immunization and challenge doses were 2 × 104 and 8 × 105 PFU, respectively.
Versus HD-2 (chi-square analysis).
DISCUSSION
Previous work with vhs-deficient (47) and replication-incompetent (29, 30) viruses and viruses that undergo a single round of replication in the host (11) had suggested the utility of live attenuated viruses as an effective approach to prophylactic vaccination against HSV. Postexposure vaccination of mice with vhs− virus has also been shown to have therapeutic benefit (46). Because vhs is a virion component and is expressed as a γ1 gene product, it would be present upon infection and would also be synthesized de novo in cells infected with ICP8-deficient vaccine viruses. This knowledge led us to hypothesize that a vhs−/ICP8− double-mutant virus might retain the distinct advantages of both the vhs− and ICP8− single-mutant strains: increased immunogenicity and safety, respectively. We have shown that an HSV-1 strain lacking both vhs and ICP8 functions, Δ41Δ29, has immunogenicity and protective capacity similar to that of a replication-competent, vhs− single-mutant virus. In addition, Δ41Δ29 promotes better protection from local and systemic signs of disease and from latent and lethal infection than an equivalent dose of the replication-incompetent ICP8− virus.
It is interesting that less blepharitis occurred after challenge of mice immunized with BGS41 or Δ41Δ29 compared with HD-2. The observations that nude mice develop more severe blepharitis than normal mice (2) and that depletion of both CD4+ and CD8+ T cells before challenge enhances development of periocular skin lesions (16) suggest that T cells are important in clearance of virus from periocular skin and in limiting disease. Such a role for T cells in clearance from dermal lesions has been reported (32–34, 51). It follows then that mice in which a stronger or more competent immune response has been induced would be able to more effectively prevent development of blepharitis (41). It is interesting that a corresponding decrease in virus shed into tear film was not observed in our experiments, although the titers of virus in the periocular skin were not determined. In contrast to blepharitis, keratitis is mediated by an immunopathologic infiltration of the cornea in primary immune responses to virus infection (35) or to a neoantigen revealed by virus infection (53). This raises two possibilities: that some types of immune responses may be protective, while others are pathogenic, and that a more protective type of response is induced by vhs− viruses. Alternatively, HD-2 may not sufficiently prime mice to mount a secondary, protective immune response in the eye and eyelid. The slower kinetics of blepharitis development in HD-2- and control supernatant-immunized mice supports this possibility.
Differences in protection from latent infection, as assessed by frequency of reactivation of ganglia explanted from mice at 4 weeks postchallenge, were observed in experimental groups in which immunizing doses were ≥105 but not among survivors that had been immunized with lower doses of virus. Protection from latent infection may be the most difficult demand placed on a prophylactic vaccine (30) because virus enters the nervous system quickly and latency can be established in mouse trigeminal ganglia in the absence of replication or clinical signs of disease (20, 23). It is possible that the capacity of the double-mutant virus to protect mice against latent infection as compared with that of the ICP8− single mutant would be differentially enhanced if greater immunizing doses or lower challenge doses were given. We currently are examining this possibility.
β-Gal is expressed in different locations and under control of different promoters in the viruses used in this study. β-Gal is known to be immunogenic when encoded by the virus (4), and thus it could have influenced the strength of the immune response to HSV in a bystander fashion if expressed at different levels in cells infected by the three mutant viruses. β-Gal-specific antibody responses in serum from mice immunized with the double- or single-mutant viruses were uniformly low (data not shown), suggesting therefore that virus-expressed β-Gal had little impact on virus-specific immune responses.
With the exception of the vhs protein, Δ41Δ29 has the genetic potential to express the same spectrum of immunogenic proteins as ICP8-deficient HD-2. The equivalent immunogenicity of Δ41Δ29 and BGS41 must be a function of increased viral-protein production due to loss of vhs activity, and/or it is a function of decreased interference with host antigen presentation functions such as major histocompatibility complex (MHC) class I molecule synthesis and cell surface expression. Viruses in which the vhs gene is deleted or inactivated show prolonged viral message stability, resulting in an accumulation of mRNAs of all three kinetic classes (24, 36, 39, 43) and a corresponding increase in the amount of IE, E, and L viral proteins (24, 39). We found clear evidence for a lack of vhs activity in the Δ41Δ29 double mutant, in contrast to ICP8− mutants, by Northern blot analysis of cellular GAPDH message. Thus, an increase in stability of both cellular and viral mRNAs could be expected in cells infected with Δ41Δ29 rather than ICP8− virus. We have not, however, obtained clear-cut evidence of increased viral protein expression, and analyses of specific viral proteins from different kinetic classes are in progress to clarify this issue.
vhs of HSV-1 and HSV-2 has been shown to down-regulate synthesis of MHC class I heavy chain molecules in human fibroblasts (18, 44), resulting in decreased recognition and lysis of the infected cells by MHC class I-restricted cytotoxic T lymphocytes (44). We have extended these findings by demonstrating that HSV-1-infected mouse fibroblasts exhibit lower cell surface expression of class I molecules than cells infected with an HSV-1 strain lacking vhs (L. Thebeau and L. A. Morrison, unpublished observations). Notably, vhs-mediated loss of class I molecules from the cell surface is independent of ICP47, which has been shown to interfere transporter associated with antigen processing (TAP) function in human cells (17) but not mouse cells (45). Thus, maintenance of cell surface MHC class I expression by vhs−/ICP8− virus may contribute to its increased immunogenicity when compared to replication-incompetent virus with wild-type vhs activity. Because the vhs activity of HSV-2 is stronger than that of HSV-1 (10, 12), it will be interesting to assess the immunogenicity of an HSV-2 vhs−/ICP8− mutant compared with an ICP8− HSV-2 strain.
Whether maintenance of MHC class I expression results in enhanced HSV-specific cytotoxic T-lymphocyte activity in mice immunized with Δ41Δ29 is not yet known. We have, however, demonstrated that antibody responses are increased in mice immunized with Δ41Δ29 as compared to HD-2. By inference, this suggests that helper T-cell responses may be enhanced in mice immunized with vhs-deficient viruses. The higher antibody titer correlates with better protective efficacy, but it may be responsible for only certain aspects of the enhanced protection. We have previously shown that serum antibody affects development of encephalitis but does not alter acute replication in the cornea when passively transferred at physiologic levels prior to challenge (31). Regardless of the mechanism by which the immunogenicity of replication-incompetent Δ41Δ29 is enhanced to levels near that of replication-competent, vhs− virus, our data argue that the inactivation of vhs is an important feature for the future engineering of a safe and efficacious vaccine strain.
ACKNOWLEDGMENTS
We thank Li Zhu and John Patton for technical assistance and Mae Gordon and Julia Beiser for expert statistical analyses. Helpful discussions with Sam Speck, Skip Virgin, Peggy MacDonald, and members of their laboratories are gratefully acknowledged.
This work was supported by GA97011 from the Fight for Sight Foundation and Public Health Service awards CA75052 to L.A.M., EY10707 to D.A.L., and P30-EY02689 to the Department of Ophthalmology and Visual Sciences, Washington University School of Medicine. D.A.L. is also supported by a Robert E. McCormick scholarship from Research to Prevent Blindness.
REFERENCES
- 1.Anonymous. Guide for the care and use of laboratory animals. DHHS publication 85-23 (NIH). Bethesda, Md: Committee on Care and Use of Laboratory Animals, National Institutes of Health; 1985. [Google Scholar]
- 2.Brandt C R. Susceptibility of +/+ +/nu and nu/nu BALB/c mice to ocular herpes simplex virus infection. Ophthalmic Res. 1992;24:332–337. doi: 10.1159/000267189. [DOI] [PubMed] [Google Scholar]
- 3.Brehm M A, Bonneau R H, Knipe D M, Tevethia S S. Immunization with a replication-deficient mutant of herpes simplex virus type 1 (HSV-1) induces a CD8+ cytotoxic T-lymphocyte response and confers a level of protection comparable to that of wild-type HSV-1. J Virol. 1997;71:3534–3544. doi: 10.1128/jvi.71.5.3534-3544.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brubaker J O, Thompson C M, Morrison L A, Knipe D M, Siber G R, Finberg R W. Induction of Th1-associated immune responses to beta-galactosidase expressed by a replication-defective mutant of herpes simplex virus 1. J Immunol. 1996;157:1598–1604. [PubMed] [Google Scholar]
- 5.Coen D M, Irmiere A F, Jacobson J G, Kerns K M. Low levels of herpes simplex virus thymidine-thymidylate kinase are not limiting for sensitivity to certain antiviral drugs or for latency in a mouse model. Virology. 1989;168:221–231. doi: 10.1016/0042-6822(89)90261-4. [DOI] [PubMed] [Google Scholar]
- 6.Conley A J, Knipe D M, Jones P C, Roizman B. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of polypeptides. J Virol. 1981;37:413–428. doi: 10.1128/jvi.37.1.191-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Da Costa X J, Bourne N, Stanberry L R, Knipe D M. Construction and characterization of a replication-defective HSV-2. Virology. 1997;232:1–12. doi: 10.1006/viro.1997.8564. [DOI] [PubMed] [Google Scholar]
- 8.Da Costa X J, Jones C A, Knipe D M. Immunization against genital herpes with a vaccine virus that has defects in productive and latent infection. Proc Natl Acad Sci USA. 1999;96:6994–6998. doi: 10.1073/pnas.96.12.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elgardi M M, Hayes C E, Smiley J R. The herpes simplex virus vhs protein induces endoribonucleolytic cleavage of target RNAs in cell extracts. J Virol. 1999;73:7153–7164. doi: 10.1128/jvi.73.9.7153-7164.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everly D N, Read G S. Mutational analysis of the virion host shutoff gene (UL41) of herpes simplex virus (HSV) characterization of HSV type 1 (HSV-1)/HSV-2 chimeras. J Virol. 1997;71:7157–7166. doi: 10.1128/jvi.71.10.7157-7166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrell H E, McLean C S, Harley C, Efstathiou S, Inglis S, McLean A C. Vaccine potential of a herpes simplex virus type 1 mutant with an essential glycoprotein deleted. J Virol. 1994;68:927–932. doi: 10.1128/jvi.68.2.927-932.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenwick M L, Everett R D. Transfer of UL41, the gene controlling virion-associated host cell shutoff, between different strains of herpes simplex virus. J Gen Virol. 1990;71:411–418. doi: 10.1099/0022-1317-71-2-411. [DOI] [PubMed] [Google Scholar]
- 13.Fenwick M L, Clark J. Early and delayed shut-off of host protein synthesis in cells infected with herpes simplex virus. J Gen Virol. 1982;61:121–125. doi: 10.1099/0022-1317-61-1-121. [DOI] [PubMed] [Google Scholar]
- 14.Fort P, Marty L, Piechaczyk M, el Sabrouty S, Dani C, Jeanteur P, Blanchard J M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985;13:1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao M, Knipe D M. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J Virol. 1989;63:5258–5267. doi: 10.1128/jvi.63.12.5258-5267.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendricks R L, Tumpey T M. Concurrent regeneration of T lymphocytes and susceptibility to HSV-1 corneal stromal disease. Curr Eye Res. 1991;10:47–53. doi: 10.3109/02713689109020357. [DOI] [PubMed] [Google Scholar]
- 17.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 18.Hill A B, Barnett B C, McMichael A J, McGeoch D J. HLA class I molecules are not transported to the cell surface in cells infected with herpes simplex virus types 1 and 2. J Immunol. 1994;152:2736–2741. [PubMed] [Google Scholar]
- 19.Hoggan M D, Roizman B. The isolation and properties of a variant of herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- 20.Katz J P, Bodin E T, Coen D M. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J Virol. 1990;64:4288–4295. doi: 10.1128/jvi.64.9.4288-4295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knipe D M, Quinlan M P, Spang A E. Characterization of two conformational forms of the major DNA-binding protein encoded by herpes simplex virus 1. J Virol. 1982;44:736–741. doi: 10.1128/jvi.44.2.736-741.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knipe D M, Spang A E. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J Virol. 1982;43:314–324. doi: 10.1128/jvi.43.1.314-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer M F, Chen S H, Knipe D M, Coen D M. Accumulation of viral transcripts and DNA during establishment of latency by herpes simplex virus. J Virol. 1998;72:1177–1185. doi: 10.1128/jvi.72.2.1177-1185.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwong A D, Frenkel N. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc Natl Acad Sci USA. 1987;84:1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwong A D, Frenkel N. The herpes simplex virus virion host shutoff function. J Virol. 1989;63:4834–4839. doi: 10.1128/jvi.63.11.4834-4839.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwong A D, Frenkel N, Kruper J A. Herpes simplex virus virion host shutoff function. Proc Natl Acad Sci USA. 1988;62:912–921. doi: 10.1128/jvi.62.3.912-921.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Littler E, Purifoy D, Minson A, Powell K L. Herpes simplex virus nonstructural proteins. III. Function of the major DNA-binding protein. J Virol. 1983;64:983–995. doi: 10.1099/0022-1317-64-5-983. [DOI] [PubMed] [Google Scholar]
- 28.Morrison L A, Da Costa X J, Knipe D M. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology. 1998;243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- 29.Morrison L A, Knipe D M. Immunization with replication-defective mutants of herpes simplex virus type 1: sites of immune intervention in pathogenesis of challenge virus infection. J Virol. 1994;68:689–696. doi: 10.1128/jvi.68.2.689-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison L A, Knipe D M. Mechanisms of immunization with a replication-defective mutant of herpes simplex virus 1. Virology. 1996;320:402–413. doi: 10.1006/viro.1996.0328. [DOI] [PubMed] [Google Scholar]
- 31.Morrison L A, Knipe D M. Contributions of antibody and T cell subsets to protection elicited by immunization with a replication-defective mutant of herpes simplex virus type 1. Virology. 1997;239:315–326. doi: 10.1006/viro.1997.8884. [DOI] [PubMed] [Google Scholar]
- 32.Nagafuchi S, Hayashida I, Higa K, Wada T, Mori R. Role of Lyt-1 positive immune T cells in recovery from herpes simplex virus infection in mice. Microbiol Immunol. 1982;26:359–362. doi: 10.1111/j.1348-0421.1982.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 33.Nash A A, Gell P G H, Wildy P. Tolerance and immunity in mice infected with herpes simplex virus: simultaneous induction of protective immunity and tolerance to delayed-type hypersensitivity. Immunology. 1981;43:153–159. [PMC free article] [PubMed] [Google Scholar]
- 34.Nash A A, Jayasuriya A, Phelan J, Cobbold S P, Waldmann H, Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987;68:825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- 35.Newell C K, Martin S, Sendele D, Mercadal C M, Rouse B T. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J Virol. 1989;63:769–775. doi: 10.1128/jvi.63.2.769-775.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oroskar A A, Read G S. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J Virol. 1989;63:1897–1906. doi: 10.1128/jvi.63.5.1897-1906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pachl C, Burke R L, Stuve L L, Sanchez-Pescador L, VanNest G, Masiarz F, Dina D. Expression of cell-associated and secreted forms of herpes simplex virus type 1 glycoproteins in mammalian cells. J Virol. 1987;61:315–325. doi: 10.1128/jvi.61.2.315-325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rader K A, Ackland-Berglund C E, Miller J K, Pepose J S, Leib D A. In vivo characterization of site-directed mutations in the promoter of the herpes simplex virus type 1 latency-associated transcripts. J Gen Virol. 1993;74:1859–1869. doi: 10.1099/0022-1317-74-9-1859. [DOI] [PubMed] [Google Scholar]
- 39.Read G S, Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of α (immediate early) viral polypeptides. J Virol. 1983;46:498–512. doi: 10.1128/jvi.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 41.Smith T J, Ackland-Berglund C E, Leib D A. Herpes simplex virus virion host shutoff (vhs) activity alters periocular disease in mice. J Virol. 2000;74:3598–3604. doi: 10.1128/jvi.74.8.3598-3604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strelow L I, Leib D A. Role of the viron host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J Virol. 1995;69:6779–6786. doi: 10.1128/jvi.69.11.6779-6786.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strom T, Frenkel N. Effects of herpes simplex virus on mRNA stability. J Virol. 1987;61:2198–2207. doi: 10.1128/jvi.61.7.2198-2207.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tigges M, Leng S, Johnson D C, Burke R L. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2 infected fibroblasts after treatment with IFN-γ or when virion host shutoff functions are disabled. J Immunol. 1996;156:3901–3910. [PubMed] [Google Scholar]
- 45.Tomazin R, vanShoot N, Goldsmith K, Jugovic P, Sempe P, Fruh K, Johnson D C. Herpes simplex virus type 2 ICP47 inhibits human TAP but not mouse TAP. J Virol. 1998;72:2560–2563. doi: 10.1128/jvi.72.3.2560-2563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker J, Laycock K A, Pepose J S, Leib D A. Postexposure vaccination with a virion host shutoff defective mutant reduces UV-B radiation-induced ocular herpes simplex virus shedding in mice. Vaccine. 1998;16:6–8. doi: 10.1016/s0264-410x(97)00177-1. [DOI] [PubMed] [Google Scholar]
- 47.Walker J, Leib D A. Protection from primary infection and establishment of latency by vaccination with herpes simplex virus type 1 recombinants deficient in ICP4 and the virion host shutoff (vhs) function. Vaccine. 1998;16:1–5. doi: 10.1016/s0264-410x(97)00164-3. [DOI] [PubMed] [Google Scholar]
- 48.Weller S K, Lee J, Sabourin J, Schaffer P A. Genetic analysis of temperature-sensitive mutants which define the gene for the major herpes simplex virus type 1 DNA-binding protein. J Virol. 1983;45:354–366. doi: 10.1128/jvi.45.1.354-366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitley R J. Herpes simplex viruses. In: Fields B, Knipe D, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2297–2342. [Google Scholar]
- 50.Wu C A, Nelson N J, McGeoch D J, Challberg M D. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J Virol. 1988;62:435–443. doi: 10.1128/jvi.62.2.435-443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yasumoto S, Okabe N, Mori R. Role of epidermal Langerhans cells in resistance to herpes simplex virus. Arch Virol. 1986;90:261–267. doi: 10.1007/BF01317375. [DOI] [PubMed] [Google Scholar]
- 52.Zelus B D, Stewart R S, Ross J. The virion host shutoff protein of herpes simplex virus type 1: messenger ribonucleolytic activity in vitro. J Virol. 1996;70:2411–2419. doi: 10.1128/jvi.70.4.2411-2419.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Z H, Granucci F, Yeh L, Schaffer P A, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1998;279:1344–1347. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]