Abstract
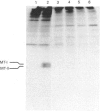
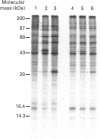
A glutathione S-transferase (GST) was purified from an arsenic-resistant Chinese hamster ovary cell line, SA7. The SA7 GST was shown to catalyse the conjugation of glutathione and ethacrynic acid, a specific substrate for Pi class GST. Its N-terminal amino-acid sequence has 80% identical residues to that of rat GST P and human GST pi. Thus, the GST purified from SA7 cells belongs to the Pi family. Treatment with Cibacron Blue or ethacrynic acid, which are GST inhibitors, significantly decreased the resistance of SA7 cells to sodium arsenite. On the other hand, pretreatment of SA7N cells, a partial revertant of SA7 cells, with sublethal doses of sodium arsenite, cadmium acetate or zinc sulphate resulted in re-elevation of GST activities and the cells regained the arsenic resistance. The regained arsenic resistance was well correlated with the levels of GST pi which were induced dose-dependently by zinc sulphate. Heat-shock treatment (45 degrees C for 10 min) did not increase GST pi expression or arsenic resistance of SA7N cells. The results indicate that GST pi is possibly involved in the mechanism of arsenic detoxification.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang D., Liberek K., Skowyra D., Zylicz M., Georgopoulos C. Biological role and regulation of the universally conserved heat shock proteins. J Biol Chem. 1991 Dec 25;266(36):24233–24236. [PubMed] [Google Scholar]
- Applegate L. A., Luscher P., Tyrrell R. M. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991 Feb 1;51(3):974–978. [PubMed] [Google Scholar]
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Berhane K., Mannervik B. Inactivation of the genotoxic aldehyde acrolein by human glutathione transferases of classes alpha, mu, and pi. Mol Pharmacol. 1990 Feb;37(2):251–254. [PubMed] [Google Scholar]
- Blair P. C., Thompson M. B., Bechtold M., Wilson R. E., Moorman M. P., Fowler B. A. Evidence for oxidative damage to red blood cells in mice induced by arsine gas. Toxicology. 1990 Jul;63(1):25–34. doi: 10.1016/0300-483x(90)90065-o. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buchet J. P., Lauwerys R., Roels H. Comparison of several methods for the determination of arsenic compounds in water and in urine. Their application for the study of arsenic metabolism and for the monitoring of workers exposed to arsenic. Int Arch Occup Environ Health. 1980;46(1):11–29. doi: 10.1007/BF00377456. [DOI] [PubMed] [Google Scholar]
- Buchet J. P., Lauwerys R. Role of thiols in the in-vitro methylation of inorganic arsenic by rat liver cytosol. Biochem Pharmacol. 1988 Aug 15;37(16):3149–3153. doi: 10.1016/0006-2952(88)90313-9. [DOI] [PubMed] [Google Scholar]
- Burdon R. H., Gill V. M., Rice-Evans C. Oxidative stress and heat shock protein induction in human cells. Free Radic Res Commun. 1987;3(1-5):129–139. doi: 10.3109/10715768709069778. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Chuang Y. C., Lin T. M., Wu H. Y. Malignant neoplasms among residents of a blackfoot disease-endemic area in Taiwan: high-arsenic artesian well water and cancers. Cancer Res. 1985 Nov;45(11 Pt 2):5895–5899. [PubMed] [Google Scholar]
- Cherian M. G., Nordberg M. Cellular adaptation in metal toxicology and metallothionein. Toxicology. 1983 Sep;28(1-2):1–15. doi: 10.1016/0300-483x(83)90101-4. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Downes H. F., Mirski S. E., Clements D. J. Alterations in glutathione and glutathione-related enzymes in a multidrug-resistant small cell lung cancer cell line. Mol Pharmacol. 1990 Feb;37(2):192–197. [PubMed] [Google Scholar]
- Georis B., Cardenas A., Buchet J. P., Lauwerys R. Inorganic arsenic methylation by rat tissue slices. Toxicology. 1990 Jul;63(1):73–84. doi: 10.1016/0300-483x(90)90070-w. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gupta V., Singh S. V., Ahmad H., Medh R. D., Awasthi Y. C. Glutathione and glutathione S-transferases in a human plasma cell line resistant to melphalan. Biochem Pharmacol. 1989 Jun 15;38(12):1993–2000. doi: 10.1016/0006-2952(89)90499-1. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Harvey J. W., Beutler E. Binding of heme by glutathione S-transferase: a possible role of the erythrocyte enzyme. Blood. 1982 Nov;60(5):1227–1230. [PubMed] [Google Scholar]
- Ishinishi N., Yamamoto A., Hisanaga A., Inamasu T. Tumorigenicity of arsenic trioxide to the lung in Syrian golden hamsters by intermittent instillations. Cancer Lett. 1983 Dec;21(2):141–147. doi: 10.1016/0304-3835(83)90200-8. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Kamisaka K., Habig W. H., Ketley J. N., Arias M., Jakoby W. B. Multiple forms of human glutathione S-transferase and their affinity for bilirubin. Eur J Biochem. 1975 Dec 1;60(1):153–161. doi: 10.1111/j.1432-1033.1975.tb20987.x. [DOI] [PubMed] [Google Scholar]
- Kano T., Sakai M., Muramatsu M. Structure and expression of a human class pi glutathione S-transferase messenger RNA. Cancer Res. 1987 Nov 1;47(21):5626–5630. [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989 Jan;86(1):99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Oshimura M., Barrett J. C. Comparison of arsenic-induced cell transformation, cytotoxicity, mutation and cytogenetic effects in Syrian hamster embryo cells in culture. Carcinogenesis. 1985 Oct;6(10):1421–1426. doi: 10.1093/carcin/6.10.1421. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Wang-Wuu S., Huang R. Y., Lee K. C., Jan K. Y. Differential effects of pre- and posttreatment of sodium arsenite on the genotoxicity of methyl methanesulfonate in Chinese hamster ovary cells. Cancer Res. 1986 Apr;46(4 Pt 1):1854–1857. [PubMed] [Google Scholar]
- Lee T. C., Wei M. L., Chang W. J., Ho I. C., Lo J. F., Jan K. Y., Huang H. Elevation of glutathione levels and glutathione S-transferase activity in arsenic-resistant Chinese hamster ovary cells. In Vitro Cell Dev Biol. 1989 May;25(5):442–448. doi: 10.1007/BF02624629. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hickson I. D., Robson C. N., Harris A. L., Hayes J. D., Griffiths S. A., Manson M. M., Hall A. E., Moss J. E., Wolf C. R. Amplification and increased expression of alpha class glutathione S-transferase-encoding genes associated with resistance to nitrogen mustards. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8511–8515. doi: 10.1073/pnas.85.22.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. C. Induction of thermotolerance and enhanced heat shock protein synthesis in Chinese hamster fibroblasts by sodium arsenite and by ethanol. J Cell Physiol. 1983 May;115(2):116–122. doi: 10.1002/jcp.1041150203. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Listowsky I., Abramovitz M., Homma H., Niitsu Y. Intracellular binding and transport of hormones and xenobiotics by glutathione-S-transferases. Drug Metab Rev. 1988;19(3-4):305–318. doi: 10.3109/03602538808994138. [DOI] [PubMed] [Google Scholar]
- Léonard A., Lauwerys R. R. Carcinogenicity, teratogenicity and mutagenicity of arsenic. Mutat Res. 1980 Jan;75(1):49–62. doi: 10.1016/0165-1110(80)90027-5. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Moscow J. A., Fairchild C. R., Madden M. J., Ransom D. T., Wieand H. S., O'Brien E. E., Poplack D. G., Cossman J., Myers C. E., Cowan K. H. Expression of anionic glutathione-S-transferase and P-glycoprotein genes in human tissues and tumors. Cancer Res. 1989 Mar 15;49(6):1422–1428. [PubMed] [Google Scholar]
- Nakamuro K., Sayato Y. Comparative studies of chromosomal aberration induced by trivalent and pentavalent arsenic. Mutat Res. 1981 Jan;88(1):73–80. doi: 10.1016/0165-1218(81)90091-4. [DOI] [PubMed] [Google Scholar]
- Ostlund Farrants A. K., Meyer D. J., Coles B., Southan C., Aitken A., Johnson P. J., Ketterer B. The separation of glutathione transferase subunits by using reverse-phase high-pressure liquid chromatography. Biochem J. 1987 Jul 15;245(2):423–428. doi: 10.1042/bj2450423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka F., Koizumi S., Kimura M., Ohsawa M. Silver staining for carboxymethylated metallothioneins in polyacrylamide gels. Anal Biochem. 1988 Jan;168(1):184–192. doi: 10.1016/0003-2697(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Robson C. N., Lewis A. D., Wolf C. R., Hayes J. D., Hall A., Proctor S. J., Harris A. L., Hickson I. D. Reduced levels of drug-induced DNA cross-linking in nitrogen mustard-resistant Chinese hamster ovary cells expressing elevated glutathione S-transferase activity. Cancer Res. 1987 Nov 15;47(22):6022–6027. [PubMed] [Google Scholar]
- Senjo M., Ishibashi T. Possible involvement of glutathione S-transferases in the cell growth of C6 astroglioma cells. J Neurochem. 1988 Jan;50(1):163–166. doi: 10.1111/j.1471-4159.1988.tb13244.x. [DOI] [PubMed] [Google Scholar]
- Suguoka Y., Kano T., Okuda A., Sakai M., Kitagawa T., Muramatsu M. Cloning and the nucleotide sequence of rat glutathione S-transferase P cDNA. Nucleic Acids Res. 1985 Sep 11;13(17):6049–6057. doi: 10.1093/nar/13.17.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M. Biotransformation of trivalent and pentavalent inorganic arsenic in mice and rats. Environ Res. 1981 Aug;25(2):286–293. doi: 10.1016/0013-9351(81)90030-x. [DOI] [PubMed] [Google Scholar]
- Wang Y. Y., Teicher B. A., Shea T. C., Holden S. A., Rosbe K. W., al-Achi A., Henner W. D. Cross-resistance and glutathione-S-transferase-pi levels among four human melanoma cell lines selected for alkylating agent resistance. Cancer Res. 1989 Nov 15;49(22):6185–6192. [PubMed] [Google Scholar]
- Waxman D. J. Glutathione S-transferases: role in alkylating agent resistance and possible target for modulation chemotherapy--a review. Cancer Res. 1990 Oct 15;50(20):6449–6454. [PubMed] [Google Scholar]
- Yamanaka K., Hasegawa A., Sawamura R., Okada S. Dimethylated arsenics induce DNA strand breaks in lung via the production of active oxygen in mice. Biochem Biophys Res Commun. 1989 Nov 30;165(1):43–50. doi: 10.1016/0006-291x(89)91031-0. [DOI] [PubMed] [Google Scholar]