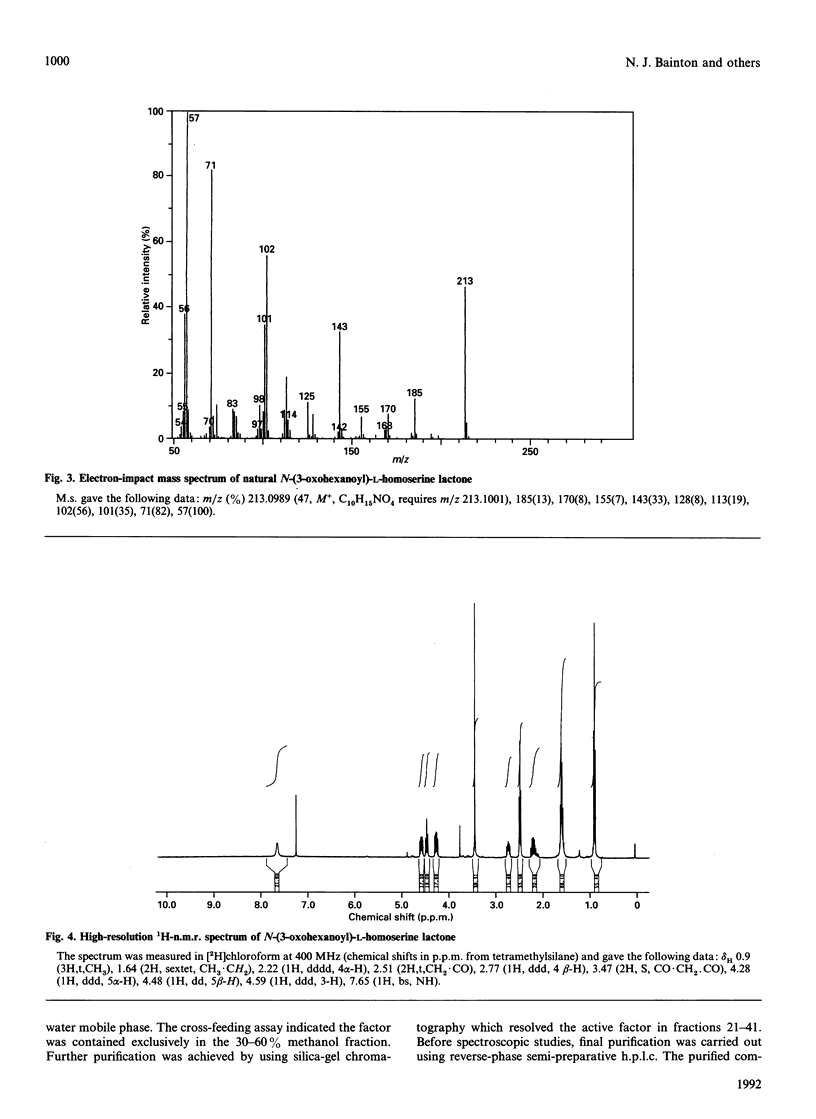
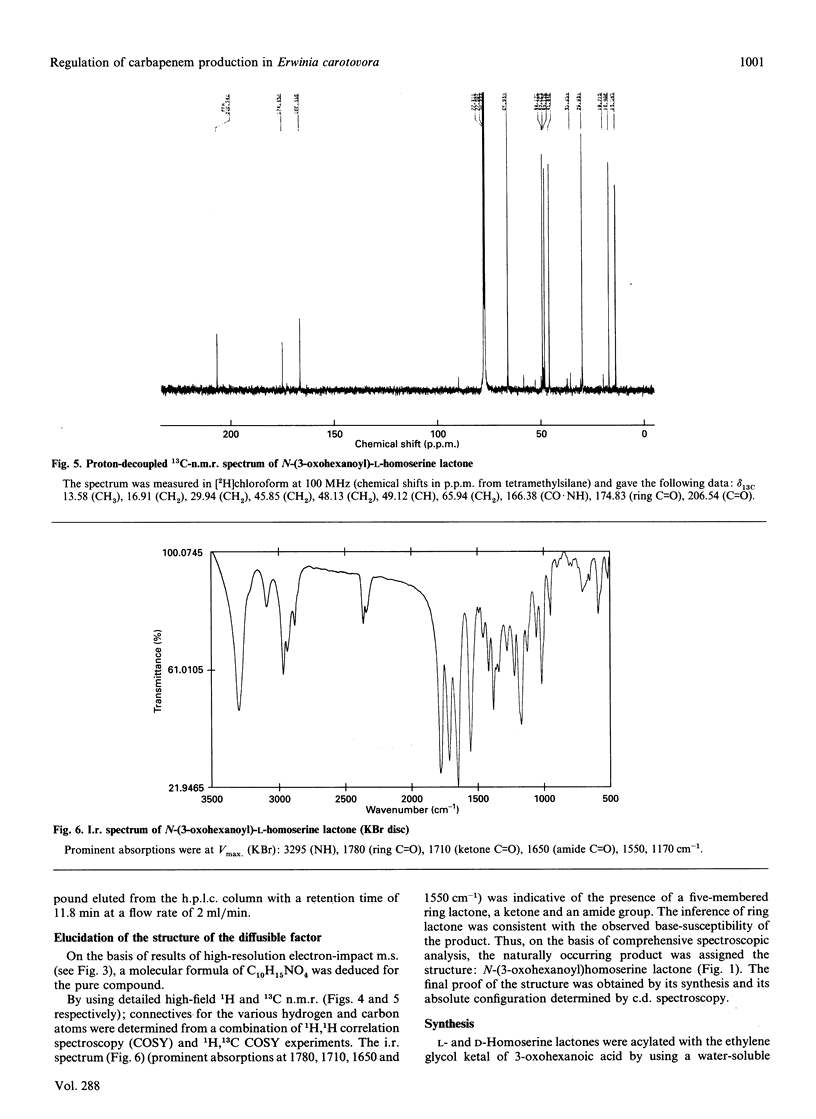
Abstract
Erwinia carotovora A.T.C.C. 39048 produces the antibiotic 1-carbapen-2-em-3-carboxylic acid. A number of mutants with a carbapenem-non-producing phenotype were selected as part of an investigation into the molecular and genetic basis of carbapenem biosynthesis. Cross-feeding studies revealed that the mutants fell into two discrete groups. Group 1 mutants were found to secrete a diffusible low-molecular-mass compound which restored carbapenem production in group 2 mutants. This compound was isolated from the spent culture supernatant of a group 1 mutant using solvent extraction, hydrophobic-interaction chromatography and silica-gel chromatography, and finally purified by reverse-phase semipreparative h.p.l.c. M.s. and n.m.r. spectroscopy revealed that the compound was N-(3-oxohexanoyl)homoserine lactone. Both D- and L-isomers were synthesized, and subsequent analysis by c.d. established that the natural product has the L-configuration. Although carbapenem production was restored by both isomers, dose-response curves indicated that the L-isomer has greater activity, with an induction threshold of about 0.5 micrograms/ml. N-(3-Oxohexanoyl)-L-homoserine lactone is, therefore, an autoregulator of carbapenem biosynthesis rather than a biosynthetic intermediate. This compound is already known for its role in autoinduction of bioluminescence in the marine bacterium Vibrio fischeri. It is also structurally-related to the A- and I-factors which are known to regulate production of antibiotics in some Streptomyces species. Its association in this work with the regulation of carbapenem biosynthesis implies a broader role for autoregulator-controlled gene expression in prokaryotes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton N. J., Bycroft B. W., Chhabra S. R., Stead P., Gledhill L., Hill P. J., Rees C. E., Winson M. K., Salmond G. P., Stewart G. S. A general role for the lux autoinducer in bacterial cell signalling: control of antibiotic biosynthesis in Erwinia. Gene. 1992 Jul 1;116(1):87–91. doi: 10.1016/0378-1119(92)90633-z. [DOI] [PubMed] [Google Scholar]
- Bycroft B. W., Maslen C., Box S. J., Brown A., Tyler J. W. The biosynthetic implications of acetate and glutamate incorporation into (3R,5R)-carbapenam-3-carboxylic acid and (5R)-carbapen-2-em-3-carboxylic acid by Serratia sp. J Antibiot (Tokyo) 1988 Sep;41(9):1231–1242. doi: 10.7164/antibiotics.41.1231. [DOI] [PubMed] [Google Scholar]
- Cao J. G., Meighen E. A. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989 Dec 25;264(36):21670–21676. [PubMed] [Google Scholar]
- Distler J., Ebert A., Mansouri K., Pissowotzki K., Stockmann M., Piepersberg W. Gene cluster for streptomycin biosynthesis in Streptomyces griseus: nucleotide sequence of three genes and analysis of transcriptional activity. Nucleic Acids Res. 1987 Oct 12;15(19):8041–8056. doi: 10.1093/nar/15.19.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard A., Burlingame A. L., Eberhard C., Kenyon G. L., Nealson K. H., Oppenheimer N. J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981 Apr 28;20(9):2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- Gambello M. J., Iglewski B. H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991 May;173(9):3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara O., Beppu T. Mutants blocked in streptomycin production in Streptomyces griseus - the role of A-factor. J Antibiot (Tokyo) 1982 Mar;35(3):349–358. doi: 10.7164/antibiotics.35.349. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Wallace J. C., Brown J. P. Finding protein similarities with nucleotide sequence databases. Methods Enzymol. 1990;183:111–132. doi: 10.1016/0076-6879(90)83009-x. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Kumada Y., Beppu T. Unstable genetic determinant of A-factor biosynthesis in streptomycin-producing organisms: cloning and characterization. J Bacteriol. 1984 May;158(2):481–487. doi: 10.1128/jb.158.2.481-487.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Nishiyama M., Suzuki H., Kumada Y., Beppu T. The cloned Streptomyces bikiniensis A-factor determinant. J Antibiot (Tokyo) 1985 May;38(5):636–641. doi: 10.7164/antibiotics.38.636. [DOI] [PubMed] [Google Scholar]
- Kinashi H., Shimaji M., Sakai A. Giant linear plasmids in Streptomyces which code for antibiotic biosynthesis genes. 1987 Jul 30-Aug 5Nature. 328(6129):454–456. doi: 10.1038/328454a0. [DOI] [PubMed] [Google Scholar]
- Kohalmi S. E., Kunz B. A. Role of neighbouring bases and assessment of strand specificity in ethylmethanesulphonate and N-methyl-N'-nitro-N-nitrosoguanidine mutagenesis in the SUP4-o gene of Saccharomyces cerevisiae. J Mol Biol. 1988 Dec 5;204(3):561–568. doi: 10.1016/0022-2836(88)90355-5. [DOI] [PubMed] [Google Scholar]
- Meighen E. A. Molecular biology of bacterial bioluminescence. Microbiol Rev. 1991 Mar;55(1):123–142. doi: 10.1128/mr.55.1.123-142.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Horinouchi S., Yoshida M., Chiba N., Mori K., Nogawa N., Morikawa N., Beppu T. Detection and properties of A-factor-binding protein from Streptomyces griseus. J Bacteriol. 1989 Aug;171(8):4298–4302. doi: 10.1128/jb.171.8.4298-4302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Kuzuyama T., Horinouchi S., Beppu T. The A-factor-binding protein of Streptomyces griseus negatively controls streptomycin production and sporulation. J Bacteriol. 1990 Jun;172(6):3003–3008. doi: 10.1128/jb.172.6.3003-3008.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuki T., Imanaka T., Aiba S. Self-cloning in Streptomyces griseus of an str gene cluster for streptomycin biosynthesis and streptomycin resistance. J Bacteriol. 1985 Oct;164(1):85–94. doi: 10.1128/jb.164.1.85-94.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker W. L., Rathnum M. L., Wells J. S., Jr, Trejo W. H., Principe P. A., Sykes R. B. SQ 27,860, a simple carbapenem produced by species of Serratia and Erwinia. J Antibiot (Tokyo) 1982 Jun;35(6):653–660. doi: 10.7164/antibiotics.35.653. [DOI] [PubMed] [Google Scholar]
- Richardson K. K., Crosby R. M., Richardson F. C., Skopek T. R. DNA base changes induced following in vivo exposure of unadapted, adapted or ada- Escherichia coli to N-methyl-N'-nitro-N-nitrosoguanidine. Mol Gen Genet. 1987 Oct;209(3):526–532. doi: 10.1007/BF00331159. [DOI] [PubMed] [Google Scholar]
- Tadayyon M., Broome-Smith J. K. TnblaM: a transposon for directly tagging bacterial genes encoding cell envelope and secreted proteins. Gene. 1992 Feb 1;111(1):21–26. doi: 10.1016/0378-1119(92)90598-j. [DOI] [PubMed] [Google Scholar]
- Williamson J. M., Inamine E., Wilson K. E., Douglas A. W., Liesch J. M., Albers-Schönberg G. Biosynthesis of the beta-lactam antibiotic, thienamycin, by Streptomyces cattleya. J Biol Chem. 1985 Apr 25;260(8):4637–4647. [PubMed] [Google Scholar]
- Yamada Y., Sugamura K., Kondo K., Yanagimoto M., Okada H. The structure of inducing factors for virginiamycin production in Streptomyces virginiae. J Antibiot (Tokyo) 1987 Apr;40(4):496–504. doi: 10.7164/antibiotics.40.496. [DOI] [PubMed] [Google Scholar]
- de Vries G. E., Raymond C. K., Ludwig R. A. Extension of bacteriophage lambda host range: selection, cloning, and characterization of a constitutive lambda receptor gene. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6080–6084. doi: 10.1073/pnas.81.19.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]



