Abstract
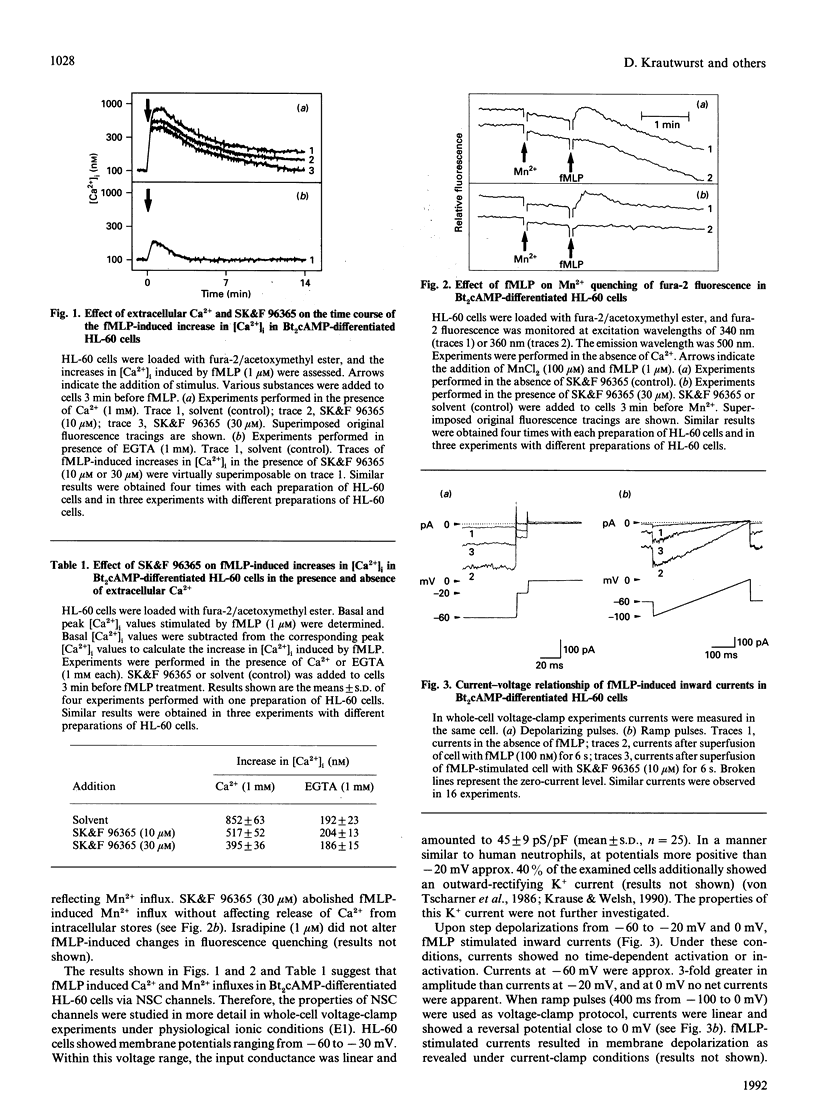
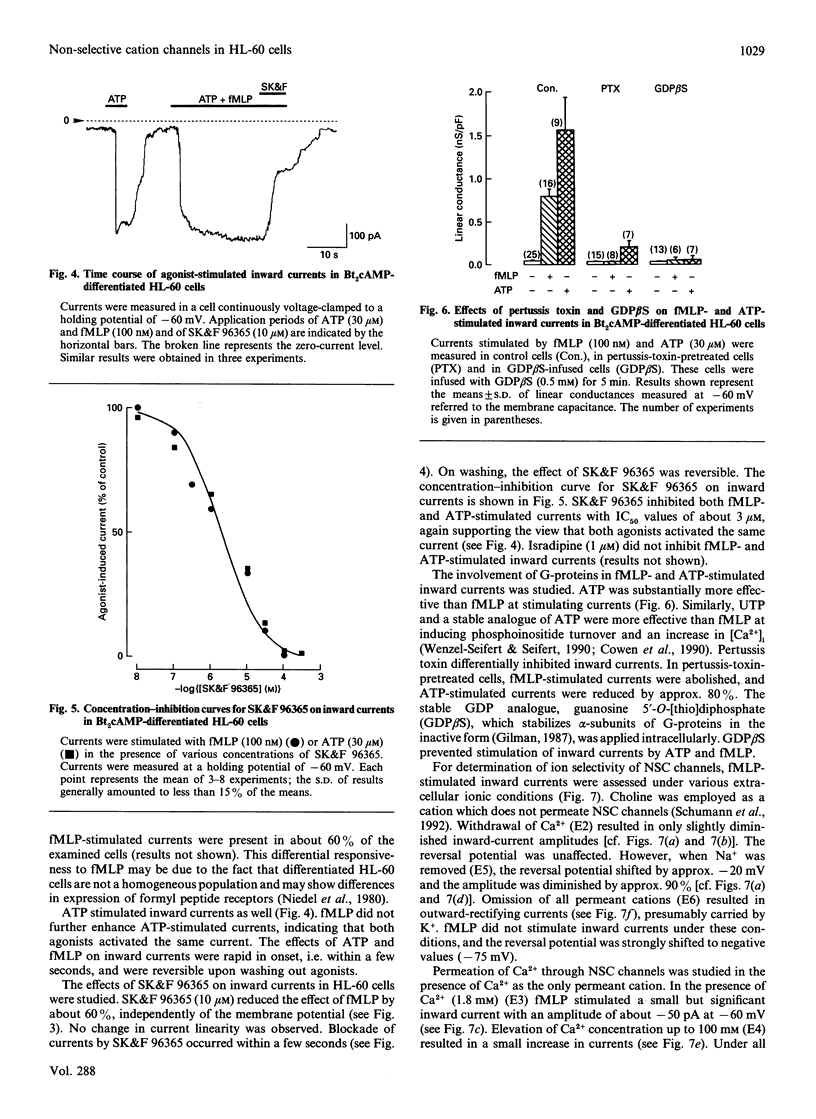
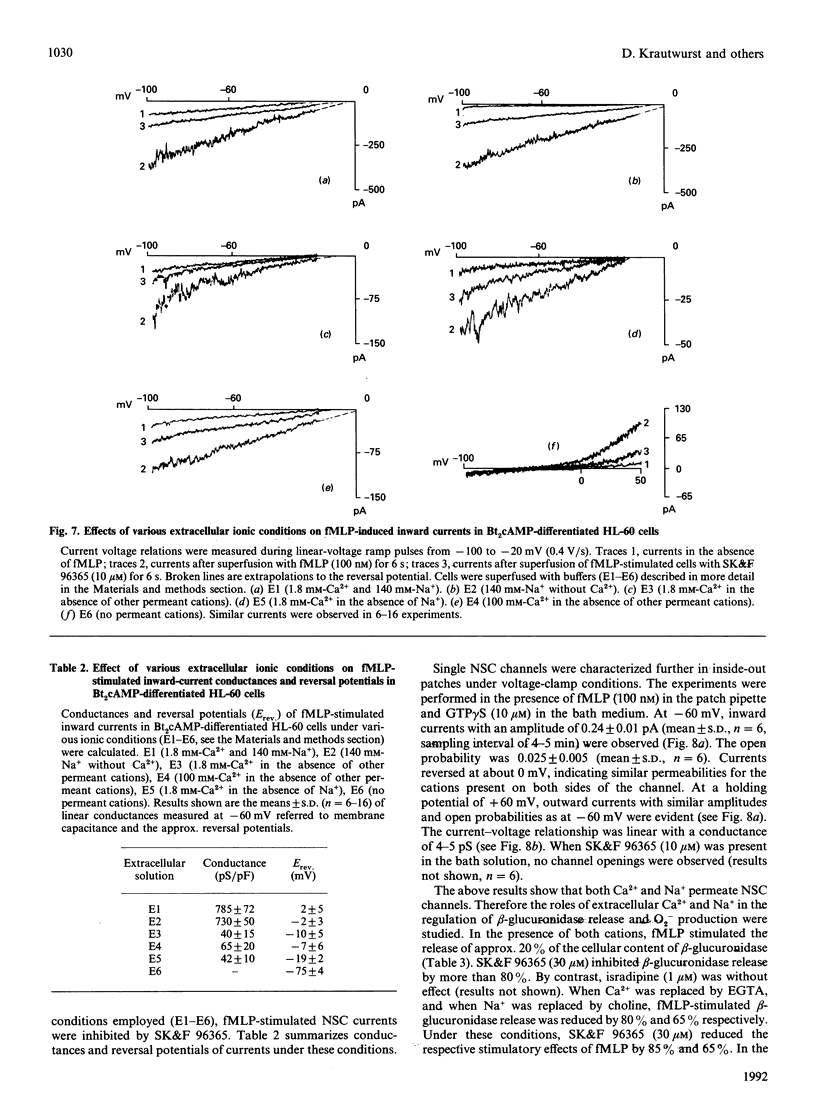
In human neutrophils, the chemotactic peptide N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP) induces increases in the intracellular free Ca2+ concentration ([Ca2+]i) with subsequent activation of beta-glucuronidase release and superoxide (O2-) production. Results from several laboratories suggest that the increase in [Ca2+]i is due to activation of non-selective cation (NSC) channels. We studied the biophysical characteristics, pharmacological modulation and functional role of NSC channels in dibutyryl cyclic AMP (Bt2cAMP)-differentiated HL-60 cells. fMLP increased [Ca2+]i by release of Ca2+ from intracellular stores and influx of Ca2+ from the extracellular space. fMLP also induced Mn2+ influx. Ca2+ and Mn2+ influxes were inhibited by 1-(beta-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl)-1H-imidazole hydrochloride (SK&F 96365). Under whole-cell voltage-clamp conditions, fMLP and ATP (a purinoceptor agonist) activated inward currents characterized by a linear current-voltage relationship and a reversal potential near 0 mV. NSC channels were substantially more permeable to Na+ than to Ca2+. SK&F 96365 inhibited fMLP- and ATP-stimulated currents with a half-maximal effect at about 3 microM. Pertussis toxin prevented stimulation by fMLP of NSC currents and reduced ATP-stimulated currents by about 80%. Intracellular application of the stable GDP analogue, guanosine 5'-O-[2-thio]diphosphate, completely blocked stimulation by agonists of NSC currents. In excised inside-out patches, single channel openings with an amplitude of 0.24 pA were observed in the presence of fMLP and the GTP analogue, guanosine 5'-O-[3-thio]triphosphate. The bath solution contained neither Ca2+ nor ATP. The current/voltage relationship was linear with a conductance of 4-5 pS and reversed at about 0 mV. fMLP-induced beta-glucuronidase release and O2- production were substantially reduced by replacement of extracellular CaCl2 or NaCl by ethylenebis(oxyethylenenitrilo)tetra-acetic acid and choline chloride respectively. In the absence of Ca2+ and Na+, fMLP was ineffective. SK&F 96365 inhibited fMLP-induced beta-glucuronidase release and O2- production in the presence of both Ca2+ and Na+, and in the presence of Ca2+ or Na+ alone. NaCl (25-50 mM) enhanced the basal and absolute extent of fMLP-stimulated GTP hydrolysis of heterotrimeric regulatory G-proteins in HL-60 membranes. The order of effectiveness of salts in enhancing GTP hydrolysis was LiCl > KCl > NaCl > choline chloride.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


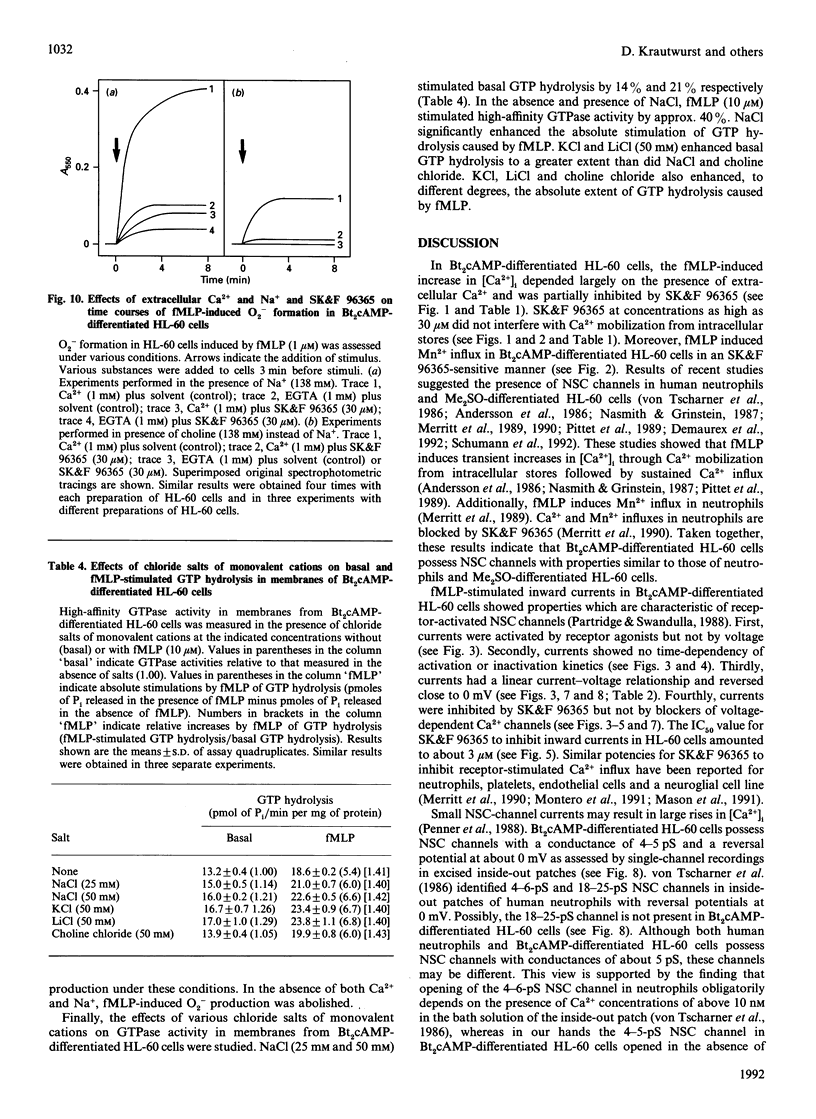



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson T., Dahlgren C., Pozzan T., Stendahl O., Lew P. D. Characterization of fMet-Leu-Phe receptor-mediated Ca2+ influx across the plasma membrane of human neutrophils. Mol Pharmacol. 1986 Nov;30(5):437–443. [PubMed] [Google Scholar]
- Avdonin P. V., Cheglakov I. B., Tkachuk V. A. Stimulation of non-selective cation channels providing Ca2+ influx into platelets by platelet-activating factor and other aggregation inducers. Eur J Biochem. 1991 May 23;198(1):267–273. doi: 10.1111/j.1432-1033.1991.tb16011.x. [DOI] [PubMed] [Google Scholar]
- Aviram A., Rephaeli A., Shaklai M. The role of increased calcium influx rate in receptor mediated function of differentiating HL-60 cells. Cell Calcium. 1990 Apr;11(4):269–274. doi: 10.1016/0143-4160(90)90003-d. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Wymann M. P. Turning on the respiratory burst. Trends Biochem Sci. 1990 Feb;15(2):69–72. doi: 10.1016/0968-0004(90)90179-f. [DOI] [PubMed] [Google Scholar]
- Benham C. D. ATP-gated channels in vascular smooth muscle cells. Ann N Y Acad Sci. 1990;603:275–286. doi: 10.1111/j.1749-6632.1990.tb37679.x. [DOI] [PubMed] [Google Scholar]
- Borin M., Siffert W. Further characterization of the mechanisms mediating the rise in cytosolic free Na+ in thrombin-stimulated platelets. Evidence for inhibition of the Na+,K(+)-ATPase and for Na+ entry via a Ca2+ influx pathway. J Biol Chem. 1991 Jul 15;266(20):13153–13160. [PubMed] [Google Scholar]
- Chaplinski T. J., Niedel J. E. Cyclic nucleotide-induced maturation of human promyelocytic leukemia cells. J Clin Invest. 1982 Nov;70(5):953–964. doi: 10.1172/JCI110707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen D. S., Baker B., Dubyak G. R. Pertussis toxin produces differential inhibitory effects on basal, P2-purinergic, and chemotactic peptide-stimulated inositol phospholipid breakdown in HL-60 cells and HL-60 cell membranes. J Biol Chem. 1990 Sep 25;265(27):16181–16189. [PubMed] [Google Scholar]
- Della Bianca V., Bellavite P., De Togni P., Fumarulo R., Rossi F. Studies on stimulus-response coupling in human neutrophils. I. Role of monovalent cations in the respiratory and secretory response to N-formylmethionylleucylphenylalanine. Biochim Biophys Acta. 1983 Feb 22;755(3):497–505. doi: 10.1016/0304-4165(83)90255-6. [DOI] [PubMed] [Google Scholar]
- Demaurex N., Lew D. P., Krause K. H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J Biol Chem. 1992 Feb 5;267(4):2318–2324. [PubMed] [Google Scholar]
- Dubyak G. R., Cowen D. S., Meuller L. M. Activation of inositol phospholipid breakdown in HL60 cells by P2-purinergic receptors for extracellular ATP. Evidence for mediation by both pertussis toxin-sensitive and pertussis toxin-insensitive mechanisms. J Biol Chem. 1988 Dec 5;263(34):18108–18117. [PubMed] [Google Scholar]
- Galietta L. J., Mastrocola T., Nobile M. A class of non-selective cation channels in human fibroblasts. FEBS Lett. 1989 Aug 14;253(1-2):43–46. doi: 10.1016/0014-5793(89)80925-1. [DOI] [PubMed] [Google Scholar]
- Gierschik P., Sidiropoulos D., Steisslinger M., Jakobs K. H. Na+ regulation of formyl peptide receptor-mediated signal transduction in HL 60 cells. Evidence that the cation prevents activation of the G-protein by unoccupied receptors. Eur J Pharmacol. 1989 Dec 5;172(6):481–492. doi: 10.1016/0922-4106(89)90031-x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gusovsky F., Bitran J. A., Yasumoto T., Daly J. W. Mechanism of maitotoxin-stimulated phosphoinositide breakdown in HL-60 cells. J Pharmacol Exp Ther. 1990 Feb;252(2):466–473. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Iizuka T., Kanegasaki S., Makino R., Tanaka T., Ishimura Y. Pyridine and imidazole reversibly inhibit the respiratory burst in porcine and human neutrophils: evidence for the involvement of cytochrome b558 in the reaction. Biochem Biophys Res Commun. 1985 Jul 31;130(2):621–626. doi: 10.1016/0006-291x(85)90462-0. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Krause K. H., Welsh M. J. Voltage-dependent and Ca2(+)-activated ion channels in human neutrophils. J Clin Invest. 1990 Feb;85(2):491–498. doi: 10.1172/JCI114464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malech H. L., Gallin J. I. Current concepts: immunology. Neutrophils in human diseases. N Engl J Med. 1987 Sep 10;317(11):687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- Mason M. J., Garcia-Rodriguez C., Grinstein S. Coupling between intracellular Ca2+ stores and the Ca2+ permeability of the plasma membrane. Comparison of the effects of thapsigargin, 2,5-di-(tert-butyl)-1,4-hydroquinone, and cyclopiazonic acid in rat thymic lymphocytes. J Biol Chem. 1991 Nov 5;266(31):20856–20862. [PubMed] [Google Scholar]
- McCarthy S. A., Hallam T. J., Merritt J. E. Activation of protein kinase C in human neutrophils attenuates agonist-stimulated rises in cytosolic free Ca2+ concentration by inhibiting bivalent-cation influx and intracellular Ca2+ release in addition to stimulating Ca2+ efflux. Biochem J. 1989 Dec 1;264(2):357–364. doi: 10.1042/bj2640357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J., Clementi E., Fasolato C., Zacchetti D., Pozzan T. Ca2+ influx following receptor activation. Trends Pharmacol Sci. 1991 Aug;12(8):289–292. doi: 10.1016/0165-6147(91)90577-f. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Armstrong W. P., Benham C. D., Hallam T. J., Jacob R., Jaxa-Chamiec A., Leigh B. K., McCarthy S. A., Moores K. E., Rink T. J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990 Oct 15;271(2):515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Jacob R., Hallam T. J. Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem. 1989 Jan 25;264(3):1522–1527. [PubMed] [Google Scholar]
- Mizuno T., Kaibuchi K., Ando S., Musha T., Hiraoka K., Takaishi K., Asada M., Nunoi H., Matsuda I., Takai Y. Regulation of the superoxide-generating NADPH oxidase by a small GTP-binding protein and its stimulatory and inhibitory GDP/GTP exchange proteins. J Biol Chem. 1992 May 25;267(15):10215–10218. [PubMed] [Google Scholar]
- Montero M., Alvarez J., Garcia-Sancho J. Agonist-induced Ca2+ influx in human neutrophils is secondary to the emptying of intracellular calcium stores. Biochem J. 1991 Jul 1;277(Pt 1):73–79. doi: 10.1042/bj2770073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P. M., Eide B., Goldsmith P., Brann M., Gierschik P., Spiegel A., Malech H. L. Detection of multiple forms of Gi alpha in HL60 cells. FEBS Lett. 1987 Aug 31;221(1):81–86. doi: 10.1016/0014-5793(87)80356-3. [DOI] [PubMed] [Google Scholar]
- Murphy P. M., McDermott D. Functional expression of the human formyl peptide receptor in Xenopus oocytes requires a complementary human factor. J Biol Chem. 1991 Jul 5;266(19):12560–12567. [PubMed] [Google Scholar]
- Nasmith P. E., Grinstein S. Are Ca2+ channels in neutrophils activated by a rise in cytosolic free Ca2+? FEBS Lett. 1987 Aug 31;221(1):95–100. doi: 10.1016/0014-5793(87)80359-9. [DOI] [PubMed] [Google Scholar]
- Neher E. Cell physiology. Controls on calcium influx. Nature. 1992 Jan 23;355(6358):298–299. doi: 10.1038/355298a0. [DOI] [PubMed] [Google Scholar]
- Niedel J., Kahane I., Lachman L., Cuatrecasas P. A subpopulation of cultured human promyelocytic leukemia cells (HL-60) displays the formyl peptide chemotactic receptor. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1000–1004. doi: 10.1073/pnas.77.2.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann J. J., Stuenkel E. L. Ca(2+)-independent regulation of neurosecretion by intracellular Na+. FEBS Lett. 1991 Nov 4;292(1-2):37–41. doi: 10.1016/0014-5793(91)80828-q. [DOI] [PubMed] [Google Scholar]
- Partridge L. D., Swandulla D. Calcium-activated non-specific cation channels. Trends Neurosci. 1988 Feb;11(2):69–72. doi: 10.1016/0166-2236(88)90167-1. [DOI] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Pittet D., Lew D. P., Mayr G. W., Monod A., Schlegel W. Chemoattractant receptor promotion of Ca2+ influx across the plasma membrane of HL-60 cells. A role for cytosolic free calcium elevations and inositol 1,3,4,5-tetrakisphosphate production. J Biol Chem. 1989 May 5;264(13):7251–7261. [PubMed] [Google Scholar]
- Rahamimoff R., Meiri H., Erulkar S. D., Barenholz Y. Changes in transmitter release induced by ion-containing liposomes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5214–5216. doi: 10.1073/pnas.75.10.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F. The O2- -forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and function. Biochim Biophys Acta. 1986 Nov 4;853(1):65–89. doi: 10.1016/0304-4173(86)90005-4. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Rink T. J., Mahaut-Smith M. P. Resting and ADP-evoked changes in cytosolic free sodium concentration in human platelets loaded with the indicator SBFI. J Physiol. 1991 Sep;441:559–573. doi: 10.1113/jphysiol.1991.sp018767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborg R. R., Smolen J. E. Early biochemical events in leukocyte activation. Lab Invest. 1988 Sep;59(3):300–320. [PubMed] [Google Scholar]
- Schumann M. A., Tanigaki T., Heller D. N., Raffin T. A. Ca(2+)-dependent and Ca(2+)-independent mechanisms modulate whole-cell cationic currents in human neutrophils. Biochem Biophys Res Commun. 1992 Jun 15;185(2):531–538. doi: 10.1016/0006-291x(92)91657-c. [DOI] [PubMed] [Google Scholar]
- Schwaner I., Seifert R., Schultz G. Receptor-mediated increases in cytosolic Ca2+ in the human erythroleukaemia cell line involve pertussis toxin-sensitive and -insensitive pathways. Biochem J. 1992 Jan 15;281(Pt 2):301–307. doi: 10.1042/bj2810301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R., Burde R., Schultz G. Activation of NADPH oxidase by purine and pyrimidine nucleotides involves G proteins and is potentiated by chemotactic peptides. Biochem J. 1989 May 1;259(3):813–819. doi: 10.1042/bj2590813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R., Höer A., Offermanns S., Buschauer A., Schunack W. Histamine increases cytosolic Ca2+ in dibutyryl-cAMP-differentiated HL-60 cells via H1 receptors and is an incomplete secretagogue. Mol Pharmacol. 1992 Aug;42(2):227–234. [PubMed] [Google Scholar]
- Seifert R., Jungblut P., Schultz G. Differential expression of cytosolic activation factors for NADPH oxidase in HL-60 leukemic cells. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1109–1117. doi: 10.1016/0006-291x(89)91357-0. [DOI] [PubMed] [Google Scholar]
- Seifert R., Schultz G. Reversible activation of NADPH oxidase in membranes of HL-60 human leukemic cells. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1296–1302. doi: 10.1016/0006-291x(87)90790-x. [DOI] [PubMed] [Google Scholar]
- Seifert R., Schultz G. The superoxide-forming NADPH oxidase of phagocytes. An enzyme system regulated by multiple mechanisms. Rev Physiol Biochem Pharmacol. 1991;117:1–338. [PubMed] [Google Scholar]
- Seifert R., Serke S., Huhn D., Bessler W. G., Hauschildt S., Metzger J., Wismüller K. H., Jung G. Incomplete functional differentiation of HL-60 leukemic cells by synthetic lipopeptides. Partial inhibition by pertussis toxin of enhanced superoxide formation. Eur J Biochem. 1992 Jan 15;203(1-2):143–151. doi: 10.1111/j.1432-1033.1992.tb19839.x. [DOI] [PubMed] [Google Scholar]
- Seifert R., Wenzel K., Eckstein F., Schultz G. Purine and pyrimidine nucleotides potentiate activation of NADPH oxidase and degranulation by chemotactic peptides and induce aggregation of human neutrophils via G proteins. Eur J Biochem. 1989 Apr 15;181(1):277–285. doi: 10.1111/j.1432-1033.1989.tb14722.x. [DOI] [PubMed] [Google Scholar]
- Simchowitz L. Chemotactic factor-induced activation of Na+/H+ exchange in human neutrophils. I. Sodium fluxes. J Biol Chem. 1985 Oct 25;260(24):13237–13247. [PubMed] [Google Scholar]
- Simchowitz L., Cragoe E. J., Jr Na+-Ca2+ exchange in human neutrophils. Am J Physiol. 1988 Jan;254(1 Pt 1):C150–C164. doi: 10.1152/ajpcell.1988.254.1.C150. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: evidence for the role of sodium. J Immunol. 1979 Nov;123(5):2428–2435. [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. The roles of extracellular and intracellular calcium in lysosomal enzyme release and superoxide anion generation by human neutrophils. Biochim Biophys Acta. 1981 Nov 5;677(3-4):512–520. doi: 10.1016/0304-4165(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Tohkin M., Morishima N., Iiri T., Takahashi K., Ui M., Katada T. Interaction of guanine-nucleotide-binding regulatory proteins with chemotactic peptide receptors in differentiated human leukemic HL-60 cells. Eur J Biochem. 1991 Jan 30;195(2):527–533. doi: 10.1111/j.1432-1033.1991.tb15733.x. [DOI] [PubMed] [Google Scholar]
- Uhing R. J., Polakis P. G., Snyderman R. Isolation of GTP-binding proteins from myeloid HL-60 cells. Identification of two pertussis toxin substrates. J Biol Chem. 1987 Nov 15;262(32):15575–15579. [PubMed] [Google Scholar]
- Wenzel-Seifert K., Seifert R. Nucleotide-, chemotactic peptide- and phorbol ester-induced exocytosis in HL-60 leukemic cells. Immunobiology. 1990 Nov;181(4-5):298–316. doi: 10.1016/S0171-2985(11)80499-7. [DOI] [PubMed] [Google Scholar]
- von Tscharner V., Prod'hom B., Baggiolini M., Reuter H. Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. 1986 Nov 27-Dec 3Nature. 324(6095):369–372. doi: 10.1038/324369a0. [DOI] [PubMed] [Google Scholar]



