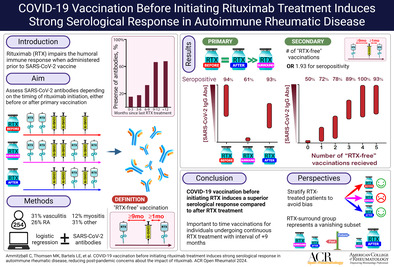
Abstract
Objective
Rituximab (RTX)‐treated patients exhibit suboptimal responses to COVID‐19 vaccines. However, existing research primarily involves patients already receiving RTX when vaccines were introduced, failing to account for the current landscape where patients are vaccinated before initiating RTX. Our objective was to compare the serological response to COVID‐19 vaccines in patients vaccinated before or after RTX initiation.
Methods
We included 254 RTX‐treated patients with autoimmune inflammatory rheumatic diseases (AIIRDs) and 113 blood donors (BDs) in a retrospective, observational cohort study. Patients were categorized based on the timing of RTX treatment relative to primary COVID‐19 vaccination. Serological vaccine responses were assessed using three immunoassays, and logistic regression analysis was used to identify predictors of serological response.
Results
Patients vaccinated before initiating RTX treatment had significantly higher seroconversion rates of SARS‐CoV‐2 immunoglobulin G (87%) and neutralizing antibodies (91%) compared with those receiving RTX before and after vaccination (n = 132) (61% and 65%, respectively). In the logistic regression analysis, a positive serological response was associated with the number of vaccines administered >9 months after the last RTX treatment. Patients receiving the highest number of vaccines with >9 months after RTX showed a response comparable to that of the BDs.
Conclusion
Vaccinating before RTX initiation yields a robust serological response in patients with AIIRDs. Furthermore, we highlight the reversibility of antibody impairment after RTX treatment cessation, provided that adequate vaccinations occur within a minimum of 9 months after RTX. Our findings offer essential insights for clinical decision‐making regarding COVID‐19 vaccination and RTX treatment, alleviating concerns about future RTX use.
INTRODUCTION
Rituximab (RTX), a monoclonal antibody targeting CD20, acts as a potent B cell–depleting agent. It is a highly effective treatment option for various rheumatic diseases by selectively depleting CD20‐positive B cells.
Given its impact on humoral immunity, considerable concern has been emphasized regarding using RTX throughout the COVID‐19 pandemic. Several studies have demonstrated that RTX therapy impairs the humoral immune response to COVID‐19 vaccines 1 , 2 , 3 , 4 without significantly affecting T cell immunity. 5 , 6 , 7 The presence of B cells before vaccination and a sufficient interval between immunization and RTX treatment are known factors promoting a humoral immune response. 1 , 3 , 4 Data have indicated an elevated risk of unfavorable outcomes after COVID‐19, including death, in individuals with rheumatic diseases receiving RTX, particularly before vaccines became available. 8 , 9 , 10 , 11 , 12 Furthermore, a correlation between postacute sequelae of COVID‐19 and RTX treatment has been observed. 13
The impact of RTX on vaccine response and fear of reduced immunogenicity observed during the pandemic has created a significant dilemma for physicians managing these patients. 14 The optimal approach to vaccination in patients with autoimmune inflammatory rheumatic diseases (AIIRDs) receiving RTX therapy has remained a subject of concern. 15 , 16 The American College of Rheumatology does not give specific COVID‐19 vaccination recommendations. 17 The American College of Rheumatology recommends that any vaccination be administered before RTX initiation and RTX be delayed for at least 2 weeks after vaccination to allow for an immune response to develop, assuming disease activity allows this. 13
Understanding the serological response to COVID‐19 vaccines in patients with AIIRDs undergoing RTX therapy is crucial for providing evidence‐based recommendations and optimizing clinical decision‐making. If, for example, vaccination before initiating RTX therapy leads to a robust immune response, it can alleviate the current fear and skepticism among patients and physicians regarding the further use of RTX.
It is essential to recognize that the circumstances experienced at the start of the pandemic, when patients had already initiated RTX treatment at the time of vaccination, are distinct from the current situation in which it is possible to vaccinate patients before initiating RTX therapy. Thus, this study aimed to investigate the serological response to COVID‐19 vaccines in patients with AIIRDs treated with RTX, comparing the outcomes of vaccination administered before or after initiating RTX therapy. The study will contribute to establishing evidence‐based recommendations and guide clinical decision‐making in managing immunization strategies for this population. Moreover, it may help prevent unwarranted RTX abandonment based on unsubstantiated concerns.
PATIENTS AND METHODS
Study design and participants
This retrospective, cross‐sectional, observational cohort study was conducted at a single center at Aarhus University Hospital, Denmark, involving patients with AIIRD who had received treatment with RTX. All participants were included between weeks 4 and 7 of 2023 and had blood withdrawn for antibody analyses at the time of inclusion. Thus, the serology measurements represent cross‐sectional data, whereas information on vaccines and infections were retrospectively collected from patients and patient charts.
The inclusion criteria were adults aged ≥18 years who had received at least one RTX infusion between January 2017 and November 2022. Patients were identified through the hospital's electronic patient registry. The decision to include patients from 2017 and forward was based on previous findings that demonstrated that, even among those who had discontinued RTX for over 12 months, only 60% exhibited a serological response. 3 By including these patients in our study, we could investigate whether they would develop a vaccine response on receiving additional vaccinations, allowing for sufficient time to elapse since their last RTX treatment.”
All eligible patients were observed at the outpatient clinic at the Department of Rheumatology. Before inclusion, all patients had received at least two doses of a COVID‐19 vaccine. Clinical data, treatment characteristics, and vaccination data were obtained from the patients’ medical records.
The patients were categorized based on the timing of their treatment with RTX in relation to receiving their primary COVID‐19 vaccine. Patients who had only received RTX before vaccination were placed in the “RTX before” group. Patients who received RTX before and after the primary COVID‐19 vaccine were placed in the “RTX surround” group. Patients who had only received RTX after their primary COVID‐19 vaccine were placed in the “RTX after” group.
A cohort of 113 randomly selected blood donors (BDs) was included in the study at the Central Denmark Blood Center, Aarhus University Hospital, to establish a comparator for normal serological response. All donors had received at least two COVID‐19 vaccines before inclusion. Patients and BDs received their vaccinations according to the vaccination schedule determined and managed by the Danish National Health Authorities.
The primary outcome was a serological response to COVID‐19 vaccination depending on the timing of RTX treatment assessed by three different immunoassays. Following informed consent, participants were requested to complete an electronic questionnaire that pertained to the incidence of SARS‐CoV‐2 infections, the severity of the disease experienced, and the presence of any post–COVID‐19 symptoms, if applicable.
Quantification of IgG against SARS‐CoV‐2 spike protein
Specific IgG against recombinant trimeric SARS‐CoV‐2 spike protein in serum was evaluated using the LIAISON SARS‐CoV‐2 TrimericS IgG commercial assay (DiaSorin S.p.A) on the LIAISON XL platform. Positive results were defined as samples with a value ≥33.8 binding antibody units (BAU)/mL, whereas negative results were those with <33.8 BAU/mL. The assay has a range of 4.81 to 2,080 BAU/mL. A single test result was used to determine the outcome. The assay's performance characteristics, as reported by Bonelli et al, 18 include a clinical sensitivity of 98.7% (≥15 days after a positive polymerase chain reaction [PCR] result) and a specificity of 99.5% (95% confidence interval [CI] 99.0%–99.7%).
Angiotensin‐converting enzyme 2/receptor‐binding domain antibody inhibition measurement
An in‐house developed, nationally validated pseudoneutralizing enzyme‐linked immunosorbent assay–based assay was used to determine the capacity of the antibodies measured to inhibit the binding of receptor‐binding domain to the angiotensin‐converting enzyme 2 receptor, as described previously. 19 A normal human serum pool from convalescent individuals at a starting dilution of 1:20 in phosphate‐buffered saline with Tween 20 was used as a positive control. A normal human serum pool from uninfected/unvaccinated individuals was used as a negative control. The assay positivity threshold was set at 420 IU/mL. This pseudoneutralizing assay correlates highly (r = 0.9231) with the gold standard plaque reduction neutralization test. 19
Nucleocapsid measurements
To measure SARS‐CoV‐2 antibodies induced because of recent infection, undiluted EDTA plasma was tested for IgG antibodies against the SARS‐CoV‐2 nucleocapsid protein (anti‐N) using a commercial chemiluminescent microparticle immunoassay (CMIA, Abbott Diagnostics). The assay was performed on an automated Architect system. A signal‐to‐cutoff ratio >1.4 was considered positive, as recommended by the manufacturer. Results were based on a single test result. The assay had a sensitivity of 90.0% (95% CI 84.2%–93.8%) and a specificity of 99.5% (95% CI 98.5%–99.8%). 20
Statistical analysis
Unless otherwise specified, all reported values are presented as medians with interquartile ranges (IQRs). The statistical significance of differences was assessed using the Mann‐Whitney nonparametric test for continuous variables and Pearson chi‐square test for categorical variables.
We wanted to investigate the effect of a sufficient interval between the latest RTX treatment before vaccination on the presence of measurable antibodies, as our previous research showed significant effects of a 9‐month interval. 3 Receiver operating characteristic regression analyses on these data, performed as a part of the planning of this study, estimated the optimal interval to 253 days or 8.4 months. Based on these data, we defined a vaccination as having a “sufficient RTX‐free interval” if there were no RTX treatments at least 9 months (270 days) before vaccination and 30 days after.
Logistic regression analyses were conducted with the anti–SARS‐CoV‐2 spike protein IgG as the dependent variable. In the univariate models, explanatory variables included age, sex, diagnosis, body mass index, number of vaccines, number of vaccinations with a sufficient RTX‐free interval, SARS‐CoV‐2 infections, treatment with monoclonal SARS‐CoV‐2 antibodies, symptom duration of COVID‐19, prednisone treatment, accumulated RTX dose, total number of RTX infusions, total RTX treatment duration, and days from the previous vaccination to the blood sample. We opted not to correct for current treatment in our study because previous research from our group has shown that other disease‐modifying antirheumatic drugs did not significantly affect the serological response compared with RTX. 21 A multivariate model compiled all variables demonstrating a statistically significant effect (P < 0.05) in the univariate analyses. Backward selection was performed using the P ≥ 0.05 criterion for removal from the model. It is important to note that correction for multiple hypothesis testing was not performed, because we regard the study as exploratory and because of the relatively small sample size, where correction could increase the risk of Type II errors.
Ethics
The study adhered to the principles outlined in the Helsinki Declaration. Before participation, patients were provided with comprehensive information and gave written consent. The study was granted ethical approval by both the regional Danish Data Protection Agency (1‐16‐02‐467‐22) and the Central Denmark Region Committee on Health Research Ethics (1‐10‐72‐193‐22). Data associated with this paper is available upon request (annetrol@rm.dk).
RESULTS
Participants
Three hundred eighty‐seven patients were eligible: 269 provided informed consent to participate, and 254 had a blood sample collected and completed the study questionnaire (see Supplementary Figure 1). The patients were categorized into three groups based on the timing of their treatment with RTX in relation to receiving the first COVID‐19 vaccine (Table 1). The majority were in the RTX surround group (n = 132), followed by the RTX after group (n = 68) and RTX before (n = 54). Across all three groups, the majority were female (67%), with a median age of 62 (IQR 49–70). The most prevalent patient diagnosis was antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (31%), followed by rheumatoid arthritis (26%) and myositis (12%). All patients had received the COVID‐19 vaccine a median of 4 to 5 times, with the same median duration since their last vaccination (3.9 months) (see Table 1 for additional demographic details). The RTX before group received the highest number of sufficient RTX‐free interval vaccinations with a median of four, followed by the RTX after group with three and, finally, the RTX surround group with zero (Supplementary Figure 2).
Table 1.
Patient demographics*
| Demographics | RTX before vaccination | RTX surround vaccination | RTX after vaccination |
|---|---|---|---|
| Patients included, n | 54 | 132 | 68 |
| Female sex, n (%) | 44 (81) | 86 (65) | 40 (59) |
| Age, median (IQR), y | 58 (42–69) | 63 (51–71) | 63 (52–69) |
| BMI, median (IQR), kg/m2 | 25.8 (23.8–28.7) | 25.7 (22.1–29.0) | 25.6 (23.0–29.3) |
| Disease duration, years (IQR) | 9.9 (5.5–18.9) | 9.2 (4.6–15.2) | 3.1 (1.4–10.9) |
| Active/previous/never smoker, % | 13/44/43 | 7/61/32 | 10/44/47 |
| Diagnosis, n (%) | |||
| ANCA vasculitis GPA/EGPA | 7 (13) | 48 (36) | 23 (34) |
| Rheumatoid arthritis | 14 (26) | 37 (28) | 16 (24) |
| Myositis | 7 (13) | 17 (13) | 7 (10) |
| Systemic lupus erythematosus | 5 (9) | 11 (8) | 7 (10) |
| Scleroderma | 7 (13) | 9 (7) | 1 (2) |
| Sjögren syndrome | 3 (6) | 4 (3) | 7 (10) |
| Other diagnosis | 11 (20) | 6 (5) | 7 (10) |
| DMARDs, n (%) | |||
| None | 14 (26) | 53 (40) | 22 (32) |
| Methotrexate bm/sc | 16 (30) | 31 (23) | 14 (21) |
| Hydroxychloroquine | 5 (9) | 11 (8) | 8 (12) |
| Prednisone | 20 (37) | 44 (33) | 32 (47) |
| Leflunomide | 3 (6) | 7 (5) | 4 (6) |
| Mycophenolate mofetil | 6 (11) | 8 (6) | 5 (7) |
| Azathioprine | 7 (7) | 7 (5) | 3 (4) |
| Immunoglobulin | 5 (9) | 8 (6) | 1 (1) |
| Other | 1 (2) | 1 (1) | 3 (4) |
| Biologics and small molecules, n (%) | |||
| None | 31 (57) | 15 (11) | 4 (6) |
| Rituximab a | 0 (0) | 112 (85) | 54 (79) |
| TNF inhibitors | 5 (9) | 0 (0) | 1 (1) |
| JAK inhibitor | 3 (6) | 0 (0) | 1 (1) |
| Anti–IL‐6 | 8 (15) | 3 (2) | 1 (1) |
| Abatacept | 6 (11) | 1 (1) | 1 (1) |
| Other b | 1 (2) | 1 (1) | 6 (9) |
| Previous rituximab treatment, median (IQR) | |||
| Number of infusions | 4 (2–8) | 9 (6–14) | 3 (2–4) |
| Cumulative total dose, g | 4 (2–6.5) | 6 (4.5–9.5) | 2.5 (2–3) |
| Total treatment time, c months | 7 (0.5–28) | 44 (29–78) | 7 (0.5–12) |
| COVID‐19 | |||
| At least 1 SARS‐CoV‐2 infection, n (%) | 40 (74) | 105 (79) | 47 (69) |
| COVID‐19 vaccinations, median (IQR), n | 4 (4–5) | 5 (4–5) | 4 (4–5) |
| Time since last vaccination, median (IQR), months | 3.9 (3.4–10.8) | 3.8 (3.2–6.3) | 3.9 (3.3–7.0) |
“RTX before vaccination” indicates only RTX treatment before vaccination, “RTX surround vaccination” indicates RTX treatment before and after vaccination, and “RTX after vaccination” indicates only RTX treatment after vaccination. “DMARDs” and “biologics and small molecules” indicate active treatment at the time of inclusion. ANCA, antineutrophil cytoplasmic antibody; BMI, body mass index; DMARD, disease‐modifying antirheumatic drug; EGPA, eosinophilic granulomatosis with polyangiitis; GPA, granulomatosis with polyangiitis; IL‐6, interleukin 6; IQR, interquartile range; bm, by mouth; RTX, rituximab; sc, subcutaneous; TNF, tumor necrosis factor.
Indicates RTX treatment within the last 15 months.
Belimumab and intravenous immunoglobulin.
Time between the first and last RTX treatment given.
A total of 1,107 vaccinations were registered in the patients with AIIRD. The Pfizer‐BioNTech messenger RNA (mRNA) vaccine (BNT162b2) was the most frequent (n = 922, 83%), followed by the Moderna mRNA‐1273 vaccine (n = 175, 16%) and the Oxford/AstraZeneca (ChAdOx1‐S) vaccine (n = 10, 1%). The Sankey plot (Supplementary Figure 3) provides an overview of the flow of vaccines received by the included patients.
The BD group had a median age of 37 years (IQR 28–51 years) and mainly consisted of women (58%). The BD group had received fewer COVID‐19 vaccinations than the other patients, with only 25% receiving 4 doses, 63% receiving 3 doses, and 12% receiving 2 doses. The median duration since the last vaccination for the BD group was 13 months (IQR 4.0–13.4 months).
Antibody response
For patients in the RTX surround group, 61% (n = 80) exhibited detectable levels of anti–SARS‐CoV‐2 spike IgG, which was significantly lower compared to both the RTX before (n = 51, 94%) and RTX after (n = 59, 87%) groups (P < 0.001). The difference between the RTX before and RTX after groups was not statistically significant (P = 0.16) (Figure 1A). All BDs (n = 113, 100%) had detectable levels of anti–SARS‐CoV‐2 spike IgG, which was significantly different from all three patient groups (P < 0.001). The BDs exhibited the highest median serum concentration (2,080 AU/mL, IQR 2,050–2,080 AU/mL), which was the upper limit of detection, followed by RTX before (median 1,570 AU/mL, IQR 474–2,080 AU/mL), RTX after (median 1,012 AU/mL, IQR 157–1,730 AU/mL), and RTX surround (median 78 AU/mL, IQR 15–587 AU/mL).
Figure 1.
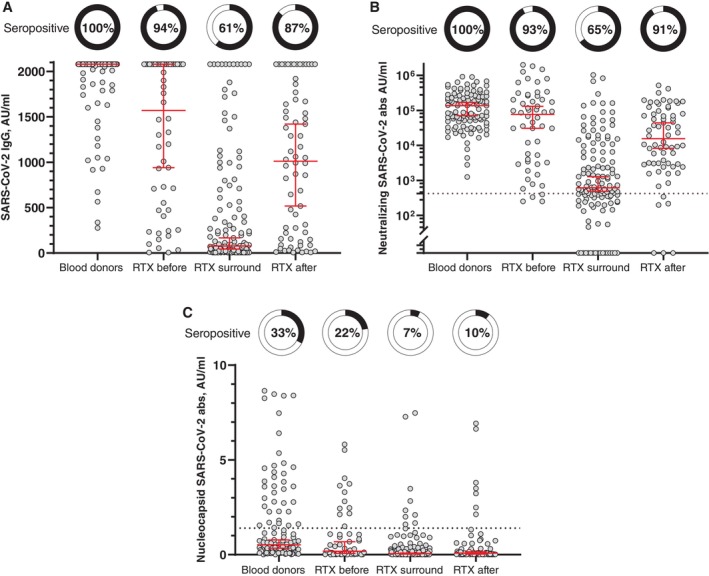
SARS‐CoV‐2 antibody measurements. (A) Concentrations of anti–SARS‐CoV‐2 spike IgG, (B) anti–SARS‐CoV‐2 nucleocapsid IgG, and (C) SARS‐CoV‐2 neutralizing antibodies in RTX‐treated patients and blood donors. RTX‐treated patients are divided according to the sequence of primary COVID‐19 vaccination and RTX treatment: “RTX before” (only RTX treatment before vaccination), “RTX surround” (RTX treatment before and after vaccination), and “RTX after” (only RTX treatment after vaccination). Pie charts indicate the percentage of patients with measurable antibodies/positive neutralizing antibody activity. The horizontal line indicates the mean and the whiskers the 95% confidence interval. The dotted lines indicate the assay positivity threshold. abs, antibodies; AU, arbitrary units; IgG, immunoglobulin G; RTX, rituximab.
When evaluating the neutralizing capability of the anti–SARS‐CoV‐2 antibodies (Figure 1B), it was observed that all BDs and most patients in the RTX before and RTX after groups exhibited a satisfactory antibody response for neutralization (100%, 93%, and 91%, respectively). These percentages were significantly higher than those for the RTX surround group, where a lower proportion of patients had neutralizing antibodies (65%, P < 0.001). The BDs had the highest median serum concentration, followed by RTX before, RTX after, and RTX surround.
Antibodies targeting the nucleocapsid surface antigen of SARS‐CoV‐2 indicate antibodies produced in response to natural infection because this antigen is not included in the vaccines. Among the BDs, one‐third (33%) exhibited detectable antibodies against the nucleocapsid antigen (Figure 1C). The percentages were lower in the RTX before (22%), RTX surround (7%), and RTX after (10%) groups.
Predictors of detectable anti–SARS‐CoV‐2 spike IgG antibodies
After performing univariate and multivariate logistic regression analyses using stepwise backward selection, only the number of vaccinations with a sufficient RTX‐free interval (odds ratio [OR] 1.93, P < 0.001) and the number of RTX infusions (OR 0.94, P = 0.005) were significantly associated with the presence of anti–SARS‐CoV‐2 IgG antibodies (Figure 2). Seroconversion was in the multivariate logistic regression model independent of age, sex, diagnosis, cumulative RTX dose, RTX treatment time, and prednisone treatment. Figure 3A illustrates the dose‐response effect of the number of RTX‐free vaccinations on seroconversion and concentration of anti–SARS‐CoV‐2 IgG. Figure 3B demonstrates the association between increasing RTX infusions and impaired antibody response after vaccination.
Figure 2.
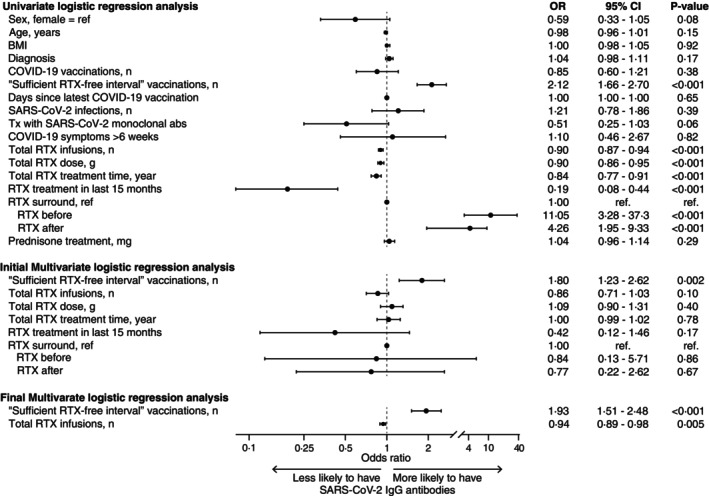
Forest plot of logistic regression analyses. Univariate and multivariate logistic regression analyses with the presence of anti–SARS‐CoV‐2 IgG as the dependent variable in RTX‐treated patients. All significant variables from the univariate analyses were included in the multiple logistic regression model and performed with stepwise backward selection, using the criterion of P ≥ 0.05 for removal from the model. The first and final models of the multiple regression analyses are presented. abs, antibodies; BMI, body mass index; CI, confidence interval; IgG, immunoglobulin G; OR, odds ratio; RTX, rituximab; Tx, treatment.
Figure 3.
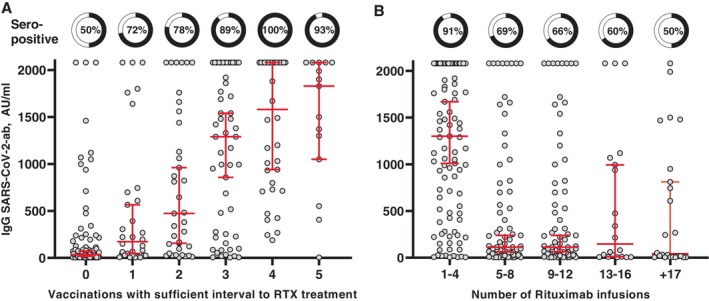
Predictors of seroconversion. SARS‐CoV‐2 antibodies and association with (A) the number of “RTX‐free” vaccinations (a sufficient interval was defined as no RTX treatment 9 months before and 1 month after vaccination) and (B) the number of previous RTX infusions and association with SARS‐CoV‐2 antibodies. Pie charts depict the presence of measurable antibodies. The dot plot shows antibody concentrations. Horizontal lines indicate median and whiskers with 95% confidence intervals. ab, antibody; AU, arbitrary units; IgG, immunoglobulin G; RTX, rituximab.
COVID‐19: number of infections and disease severity
No significant differences were observed among the three patient groups and the BD group regarding the number of self‐reported SARS‐CoV‐2 infections (Table 2). Most participants in all groups experienced only one infection (54%–66%), and a minority did not have any infections. However, no significant difference was observed among the groups (19%–31%, P = 0.30). Most participants reported manageable symptoms at home (72%–87%). However, compared with the other patient groups and BDs, the RTX surround group had a higher rate of hospital admissions for both the first (19%) and second (18%) infections (P < 0.001). Nevertheless, only 4% of admitted patients required oxygen during the first admission for COVID‐19, and only 1% needed intensive care. In all cases, oxygen treatment and intensive care were not required during the second infection. The self‐reported disease severity score (measured on a visual analog scale from 0 to 10) was higher in the RTX surround group than in the other three groups (P < 0.001). Additionally, this group had the highest proportion of patients receiving monoclonal antibodies against SARS‐CoV‐2 (39%), and a larger number of patients in this group experienced symptoms of COVID‐19 persisting for >6 weeks after their initial infection. However, this difference was not statistically significant (P = 0.14).
Table 2.
SARS‐CoV‐2 infections and symptoms*
| Number of SARS‐CoV‐2 infections, n (%) | RTX before vaccination | RTX surround vaccination | RTX after vaccination | Blood donors |
|---|---|---|---|---|
| 0 | 14 (26) | 28 (21) | 21 (31) | 22 (19) |
| 1 | 35 (65) | 87 (66) | 37 (54) | 72 (64) |
| 2 | 5 (9) | 11 (8) | 9 (13) | 16 (14) |
| 3 | – | 6 (5) | 1 (2) | 3 (3) |
| Course of first SARS‐CoV‐2 infection | n = 40 | n = 104 | n = 47 | n = 91 |
| No symptoms, n (%) | 3 (8) | 9 (9) | 8 (17) | 11 (12) |
| Symptoms managed at home, n (%) | 35 (87) | 75 (72) | 39 (83) | 78 (87) |
| Admitted to hospital, n (%) | 2 (5) | 15 (14) | 0 | 1 (1) |
| Received oxygen treatment, n (%) | 0 | 4 (4) | 0 | 0 |
| Intensive care unit, n (%) | 0 | 1 (1) | 0 | 0 |
| Self‐reported VAS 0–10 score, median (IQR) | 5.5 (3–7) | 6 (4–8) | 4 (2–5) | 4 (3–6) |
| Course of second SARS‐CoV‐2 infection | n = 5 | n = 17 | n = 10 | n = 19 |
| No symptoms, n (%) | 0 | 1 (6) | 2 (20) | 3 (17) |
| Symptomatic: managed at home, n (%) | 5 (100) | 13 (76) | 8 (80) | 15 (83) |
| Admitted to hospital, n (%) | 0 | 3 (18) | 0 | 0 |
| Self‐reported VAS 0–10 score, median IQR | 4 (4–6) | 5 (4–6) | 4 (1–6) | 3.5 (1–6) |
| Course of third SARS‐CoV‐2 infection | n = 0 | n = 6 | n = 1 | n = 3 |
| No symptoms, n (%) | 0 | 1 (17) | 0 | 0 |
| Symptomatic: managed at home, n (%) | 0 | 5 (83) | 1 (100) | 2 (100) |
| Admitted to hospital, n (%) | 0 | 0 | 0 | 0 |
| Self‐reported VAS 0–10 score, median (IQR) | 0 | 3 (3–4) | 6 (6–6) | 8 (7–9) |
| SARS‐CoV‐2 monoclonal abs treatment, n (%) | 4 (10) | 41 (39) | 9 (19) | 0 |
| PO antiviral treatment for COVID‐19, n (%) | 2 (5) | 4 (4) | 3 (6) | 0 |
| Symptoms of COVID‐19 lasting >6 weeks, n (%) | 7 (18) | 23 (22) | 6 (13) | 9 (10) |
“RTX before vaccination” indicates only RTX treatment before vaccination, “RTX surround vaccination” indicates RTX treatment before and after vaccination, and “RTX after vaccination” indicates only RTX treatment after vaccination. One blood donor patient reported three COVID‐19 infections but did not specify the course of the infections. IQR, interquartile range; BM, by mouth; RTX, rituximab; VAS, visual analog scale.
Supplementary Figure 4 presents a timeline visualization of the pandemic, indicating the timing of vaccinations and COVID‐19 among the included patients. Most of our patients experienced their first infection during the emergence of the Omicron variant in Denmark, which occurred between November 2021 and March 2022. This trend was consistent with the background population, as illustrated by the pink background graph representing the number of positive PCR test results for SARS‐CoV‐2 conducted in Denmark throughout the pandemic. The number of positive test results reached its peak during the Omicron variant period. By this time, all patients had received three doses of vaccination.
DISCUSSION
COVID‐19 vaccination has emerged as a crucial strategy to curb the COVID‐19 pandemic, 22 , 23 but concerns have been raised regarding the vaccine's effectiveness in patients with AIIRD undergoing treatment with RTX. 3 , 4 , 24 , 25 However, previous studies investigating vaccine response in RTX‐treated patients only included individuals who had received RTX before vaccination (in this study called RTX before and RTX surround).
This study aimed to assess the serological response to COVID‐19 vaccination in relation to the initiation of RTX treatment. Our findings revealed that patients who received vaccination before initiating RTX therapy (RTX after) exhibited a robust serological response, including neutralizing antibodies. Additionally, we identified the number of sufficient RTX‐free interval vaccinations and a low number of RTX infusions as predictors of a positive serological response.
It is not new that infection risk increases with RTX treatment and low levels of immunoglobulins are a risk predictor of infections. 26 , 27 However, administering the COVID‐19 vaccine before initiating RTX treatment allows patients to develop an immune response unimpeded by the B cell depletion caused by RTX. This strategy aligns with the general principle of vaccinating individuals before exposure to the pathogen and has been the recommendation for patients receiving RTX even before the pandemic. 17
A serological vaccine response is essential for protection against COVID‐19. 28 T cell responses remain intact despite RTX treatment. 5 , 7 , 15 , 29 A study on immunocompromised patients demonstrated that the combined deficient B and T cell response against SARS‐CoV‐2 was associated with insufficient viral clearance and persistent infection. 30 We did not investigate T cell responses in the present study because we had already conducted such analysis in previous research. 27 A study revealed heightened reactivity and proliferative capacity of effector and memory CD4+ and CD8+ T cell responses to SARS‐CoV‐2 following both infection and vaccination in B cell–deficient individuals. 29 This effect was notably prominent within the CD8+ T cell compartment. The findings suggest a potential explanation for reduced hospitalizations in these individuals, even in the absence of an antibody response. It has, however, been demonstrated that breakthrough COVID‐19 in patients with AIIRD is associated with postvaccination seronegativity. 24 Although T cell responses may mitigate risk in patients with low antibody responses, seronegativity continues to pose a significant risk factor despite the presence of T cell responses. 31 In the current study, we found that both the serological response and the neutralizing capacity of patients vaccinated before initiating RTX were intact. Thus, fear and hesitancy surrounding the current use of RTX in the context of COVID‐19 vaccination are based on outdated data that do not reflect the current situation. 14 , 32
Some studies have demonstrated that breakthrough COVID‐19 has been associated with a significant increase in mortality and post‐COVID morbidity in patients treated with RTX. 9 , 10 A new study demonstrated that breakthrough infections were more frequent among RTX‐treated patients, 33 and the same has been shown for repeat infections. 34 In some countries (excluding Denmark), the option of prophylactic treatment with tixagevimab/cilgavimab was available; however, it did not appear to significantly reduce breakthrough infections in RTX‐treated patients compared with those receiving other disease‐modifying antirheumatic drugs. 35 A study from the United Kingdom also reported frequent breakthrough infections (30%) in RTX‐treated patients. 12 Similar to the current study, infections were generally mild, and severity decreased with the number of vaccinations and with the number of infections.
The incidence of hospital admissions during COVID‐19 was low among the patients included in our study. In hindsight, a large percentage of these admissions were perhaps not necessary. At least we can see that very few admitted patients needed oxygen treatment or intensive care. Fear surrounding COVID‐19 led to a more cautious approach, resulting in increased hospital admissions for RTX‐treated patients, particularly in the first years of the pandemic.
Our previous findings showed that the serological response to COVID‐19 vaccination in patients receiving RTX was strongly influenced by two key factors: the “time since last RTX treatment” and the presence of measurable B cells. 3 , 4 The presence of quantifiable B cells indicates a degree of recovery in the immune system, which is time‐dependent and is associated with a higher likelihood of a serological vaccine response. B cell recovery in patients receiving RTX occurs within 6 to 9 months after the last RTX treatment, with normal levels typically reached after 9 to 12 months. 36
Building upon our previous investigations, 3 we calculated the optimal timing since RTX treatment initiation to achieve a detectable serological response. Our results indicated that a period just below 9 months after RTX treatment was associated with an optimal serological response. The current study established a clear correlation between the number of sufficient RTX‐free interval vaccines and the likelihood of a serological response. Notably, our analysis identified the number of sufficient RTX‐free interval vaccines as the strongest predictor of a serological response. These findings offer hope for patients who have already undergone RTX treatment but have not received a COVID‐19 vaccine or did not produce an antibody response. A window of opportunity exists approximately 9 months after the latest RTX treatment in which a detectable serological response can be achieved through vaccination. This is supported by the noteworthy discovery in this study demonstrating a robust immunologic response in the RTX before group. Previous investigations, including our research, consistently indicated that patients who underwent RTX therapy before receiving their COVID‐19 vaccination (designated as the RTX before group) exhibited a compromised antibody response. 1 , 2 , 3 , 4 Among patients who had received RTX treatment within the last 18 to 60 months before vaccination, only two‐thirds displayed detectable antibodies following their primary COVID‐19 vaccination. 3 However, in the current study, the RTX before group received the highest number of vaccinations with a sufficient RTX‐free interval, resulting in a significantly enhanced antibody response. This finding carries substantial implications for individuals previously treated with RTX because it underscores the potential reversibility of the antibody impairment experienced after RTX treatment cessation, provided patients receive an adequate number of RTX‐free vaccinations.
The landscape of medical practice has changed since the onset of the pandemic. Moving forward, patients commencing RTX treatment will be categorized as RTX after (those who have received vaccination or been infected before RTX therapy). In contrast, the RTX surround group will represent a vanishing subset, comprising only those individuals who initiated RTX treatment before the pandemic and continue to receive it. It is crucial to acknowledge that previous studies highlighting an elevated risk of morbidity and mortality were conducted on patients falling within the RTX surround category. 9 , 37 , 38 This cohort no longer aligns with the current clinical scenario. Consequently, it becomes imperative to stratify patients into three distinct groups (RTX before, RTX surround, and RTX after) when assessing studies reporting vaccine responses in individuals with AIIRD to eliminate potential bias. Guidelines formulated for the future should be separate from the pandemic time warp, as they fail to represent the contemporary landscape of RTX‐treated patients.
The key strength of this study lies in its considerable cohort of patients with AIIRD treated with RTX, addressing a relevant question regarding the future initiation of RTX treatment. The limitations of this study include the following. First, given our study's retrospective design, there is a risk of inherent biases and confounding factors. Additionally, its cross‐sectional nature limits our ability to establish causal relationships; longitudinal data would have allowed for more definitive conclusions. Second, information regarding COVID‐19 was obtained from patient recollections, leaving room for recall bias. Third, the study population comprises patients with diverse rheumatic diseases, which may contribute to variability in immune response and clinical outcomes. However, our previous investigations of vaccine responses in rheumatic diseases did not show differences between diagnoses. 3 , 4 , 39 Fourth, we lack data on disease activity in patients during vaccination, and it is plausible that disease activity may influence the vaccine response. Lastly, the study does not include information on patients who died during the pandemic. Although our clinic has experienced minimal COVID‐19–related deaths, this aspect has not been systematically evaluated and could potentially skew the results of our disease severity analysis.
In conclusion, our study provides important insights into the serological response to COVID‐19 vaccination in patients with AIIRD receiving RTX therapy. The results suggest that the timing of RTX treatment in relation to COVID‐19 vaccination significantly impacts the serological response, with adequate responses when vaccination is administered before initiating RTX therapy or a sufficient number of RTX‐free vaccines have been administered.
Although previous vaccine studies have primarily involved patients already receiving RTX during vaccination, the future landscape will involve vaccinating patients before initiating RTX treatment. This approach yields more favorable serological responses and mitigates the risks associated with SARS‐CoV‐2 infection during immunosuppression. Our findings could lead to a paradigm shift in our clinical decision‐making regarding COVID‐19 vaccination and RTX treatment and should alleviate apprehension regarding the future use of RTX.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Ammitzbøll, Thomsen, Erikstrup, Hauge, Troldborg.
Acquisition of data
Thomsen, Bartels, Hansen, Hermansen, Hänel, Klose‐Jensen, Larsen, Lauritsen, Mistegaard, Mikkelsen, Olesen, Næser, Nielsen.
Analysis and interpretation of data
Ammitzbøll, Thomsen, Hansen, Hermansen, Hänel, Klose‐Jensen, Larsen, Lauritsen, Mistegaard, Mikkelsen, Olesen, Næser, Nielsen, Erikstrup, Troldborg.
Supporting information
Disclosure form
Appendix S1: Supplementary Figures.
ACKNOWLEDGMENTS
We extend our gratitude to Sif Kaas Nielsen and Victoria Linderod Larsen for their exceptional technical support. Furthermore, we express our acknowledgment to all the patients who participated in this study for their invaluable contribution.
Supported by the Carlsberg Foundation (CF20‐0045), the Novo Nordisk Foundation (NFF205A0063505 and NNF20SA0064201), the Svend Andersen Research Foundation (SARF2021), and the Danish Rheumatism Association (R203‐A7217). The study's funders played no part in the study's design; collection, analysis, or interpretation of the data; or the writing of the report.
Additional supplementary information cited in this article can be found online in the Supporting Information section (https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/acr2.11681).
Author disclosures and graphical abstract are available at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11681.
REFERENCES
- 1. Furer V, Eviatar T, Zisman D, et al. Predictors of immunogenic response to the BNT162b2 mRNA COVID‐19 vaccination in patients with autoimmune inflammatory rheumatic diseases treated with rituximab. Vaccines (Basel) 2022;10(6):901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ammitzbøll C, Bartels LE, Bøgh Andersen J, et al. Impaired antibody response to the BNT162b2 messenger RNA coronavirus disease 2019 vaccine in patients with systemic lupus erythematosus and rheumatoid arthritis. ACR Open Rheumatol 2021;3(9):622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Troldborg A, Thomsen MK, Bartels LE, et al. Time since rituximab treatment is essential for developing a humoral response to COVID‐19 mRNA vaccines in patients with rheumatic diseases. J Rheumatol 2022;49(6):644–649. [DOI] [PubMed] [Google Scholar]
- 4. Ammitzbøll C, Kragh Thomsen M, Bøgh Andersen J, et al. Rituximab‐treated rheumatic patients: B cells predict seroconversion after COVID‐19 boost or revaccination in initial vaccine non‐responders. Rheumatology (Oxford) 2023;62(7):2544–2549. [DOI] [PubMed] [Google Scholar]
- 5. Prendecki M, Clarke C, Edwards H, et al. Humoral and T‐cell responses to SARS‐CoV‐2 vaccination in patients receiving immunosuppression. Ann Rheum Dis 2021;80(10):1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bitoun S, Henry J, Desjardins D, et al. Rituximab impairs B‐cell response but not T‐cell response to COVID‐19 vaccine in auto‐immune diseases. Arthritis Rheumatol 2022;74(6):927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ammitzbøll C, Thomsen MK, Andersen JB, et al. Revaccination of patients with systemic lupus erythematosus or rheumatoid arthritis without an initial COVID‐19 vaccine response elicits seroconversion in half of the patients. Clin Exp Rheumatol 2024;42(1):157–165. [DOI] [PubMed] [Google Scholar]
- 8. Patel NJ, D'Silva KM, Hsu TYT, et al. Coronavirus disease 2019 outcomes among recipients of anti‐CD20 monoclonal antibodies for immune‐mediated diseases: a comparative cohort study. ACR Open Rheumatol 2022;4(3):238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lawson‐Tovey S, Hyrich KL, Gossec L, et al. SARS‐CoV‐2 infection after vaccination in patients with inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis 2022;81(1):145–150. [DOI] [PubMed] [Google Scholar]
- 10. Cook C, Patel NJ, D'Silva KM, et al. Clinical characteristics and outcomes of COVID‐19 breakthrough infections among vaccinated patients with systemic autoimmune rheumatic diseases. Ann Rheum Dis 2022;81(2):289–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh N, Madhira V, Hu C, et al. Rituximab is associated with worse COVID‐19 outcomes in patients with rheumatoid arthritis: a retrospective, nationally sampled cohort study from the U.S. National COVID Cohort Collaborative (N3C). Semin Arthritis Rheum 2023;58:152149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Md Yusof MY, Arnold J, Saleem B, et al. Breakthrough SARS‐CoV‐2 infections and prediction of moderate‐to‐severe outcomes during rituximab therapy in patients with rheumatic and musculoskeletal diseases in the UK: a single‐centre cohort study. Lancet Rheumatol 2023;5(2):e88–e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Venkat RK, Wang X, Patel NJ, et al. Associations of DMARDs with post‐acute sequelae of COVID‐19 in patients with systemic autoimmune rheumatic diseases: a prospective study. Rheumatology (Oxford) Published online December 9, 2023. 10.1093/rheumatology/kead662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elahee M, Sattui SE. Navigating use of rituximab during the COVID‐19 pandemic. Lancet Rheumatol 2023;5(2):e63–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonelli MM, Mrak D, Perkmann T, et al. SARS‐CoV‐2 vaccination in rituximab‐treated patients: evidence for impaired humoral but inducible cellular immune response. Ann Rheum Dis 2021;80(10):1355–1356. [DOI] [PubMed] [Google Scholar]
- 16. Seree‐Aphinan C, Ratanapokasatit Y, Suchonwanit P, et al. Optimal time for COVID‐19 vaccination in rituximab‐treated dermatologic patients. Front Immunol 2023;14:1138765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bass AR, Chakravarty E, Akl EA, et al. 2022 American College of Rheumatology guideline for vaccinations in patients with rheumatic and musculoskeletal diseases. Arthritis Care Res (Hoboken) 2023;75(3):449–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bonelli F, Blocki FA, Bunnell T, et al. Evaluation of the automated LIAISON® SARS‐CoV‐2 TrimericS IgG assay for the detection of circulating antibodies. Clin Chem Lab Med 2021;59(8):1463–1467. [DOI] [PubMed] [Google Scholar]
- 19. Bayarri‐Olmos R, Idorn M, Rosbjerg A, et al. SARS‐CoV‐2 neutralizing antibody responses towards full‐length spike protein and the receptor‐binding domain. J Immunol 2021;207(3):878–887. [DOI] [PubMed] [Google Scholar]
- 20. Harritshøj LH, Gybel‐Brask M, Afzal S, et al. Comparison of sixteen serological SARS‐CoV‐2 immunoassays in sixteen clinical laboratories. J Clin Microbiol 2021;59:e02596‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ammitzbøll C, Thomsen MK, Andersen JB, et al. COVID‐19 vaccination in patients with rheumatic diseases leads to a high seroconversion rate and reduced self‐imposed isolation and shielding behaviour. Mod Rheumatol 2023;33(4):777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS‐CoV‐2 infections and COVID‐19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397(10287):1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rennert L, Ma Z, McMahan CS, et al. Effectiveness and protection duration of Covid‐19 vaccines and previous infection against any SARS‐CoV‐2 infection in young adults. Nat Commun 2022;13(1):3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jyssum I, Kared H, Tran TT, et al. Humoral and cellular immune responses to two and three doses of SARS‐CoV‐2 vaccines in rituximab‐treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol 2022;4(3):e177–e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Furer V, Eviatar T, Freund T, et al. Immunogenicity induced by two and three doses of the BNT162b2 mRNA vaccine in patients with autoimmune inflammatory rheumatic diseases and immunocompetent controls: a longitudinal multicentre study. Ann Rheum Dis 2022;81(11):1594–1602. [DOI] [PubMed] [Google Scholar]
- 26. Barmettler S, Ong MS, Farmer JR, et al. Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Netw Open 2018;1(7):e184169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Md Yusof MY, Vital EM, McElvenny DM, et al. Predicting severe infection and effects of hypogammaglobulinemia during therapy with rituximab in rheumatic and musculoskeletal diseases. Arthritis Rheumatol 2019;71(11):1812–1823. [DOI] [PubMed] [Google Scholar]
- 28. Goldblatt D, Fiore‐Gartland A, Johnson M, et al. Towards a population‐based threshold of protection for COVID‐19 vaccines. Vaccine 2022;40(2):306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zonozi R, Walters LC, Shulkin A, et al. T cell responses to SARS‐CoV‐2 infection and vaccination are elevated in B cell deficiency and reduce risk of severe COVID‐19. Sci Transl Med 2023;15(724):eadh4529. [DOI] [PubMed] [Google Scholar]
- 30. Li Y, Choudhary MC, Regan J, et al. SARS‐CoV‐2 viral clearance and evolution varies by type and severity of immunodeficiency. Sci Transl Med 2024;16(731):eadk1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahmed S, Mehta P, Paul A, et al. Postvaccination antibody titres predict protection against COVID‐19 in patients with autoimmune diseases: survival analysis in a prospective cohort. Ann Rheum Dis 2022;81(6):868–874. [DOI] [PubMed] [Google Scholar]
- 32. Boekel L, Wolbink GJ. Rituximab during the COVID‐19 pandemic: time to discuss treatment options with patients. Lancet Rheumatol 2022;4(3):e154–e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schiff AE, Wang X, Patel NJ, et al. Immunomodulators and risk for breakthrough COVID‐19 after third SARS‐CoV‐2 mRNA vaccine among patients with rheumatoid arthritis: a cohort study. Ann Rheum Dis 2024;83(5):680–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kowalski EN, Wang X, Patel NJ, et al. Risk factors and outcomes for repeat COVID‐19 infection among patients with systemic autoimmune rheumatic diseases: a case‐control study. Semin Arthritis Rheum 2023;63:152286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kawano Y, Wang X, Patel NJ, et al. Breakthrough COVID‐19 after tixagevimab/cilgavimab among patients with systemic autoimmune rheumatic diseases. J Rheumatol. 2024;51(3):305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thiel J, Rizzi M, Engesser M, et al. B cell repopulation kinetics after rituximab treatment in ANCA‐associated vasculitides compared to rheumatoid arthritis, and connective tissue diseases: a longitudinal observational study on 120 patients. Arthritis Res Ther 2017;19(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strangfeld A, Schäfer M, Gianfrancesco MA, et al; COVID‐19 Global Rheumatology Alliance . Factors associated with COVID‐19‐related death in people with rheumatic diseases: results from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis 2021;80(7):930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gianfrancesco M, Hyrich KL, Al‐Adely S, et al; COVID‐19 Global Rheumatology Alliance . Characteristics associated with hospitalisation for COVID‐19 in people with rheumatic disease: data from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis 2020;79(7):859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ammitzbøll C, Andersen JB, Vils SR, et al. Isolation, behavioral changes and low seroprevalence of SARS‐CoV‐2 antibodies in patients with systemic lupus erythematosus or rheumatoid arthritis. Arthritis Care Res (Hoboken) 2022;74(11):1780–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure form
Appendix S1: Supplementary Figures.


