Abstract
Background
Laparoscopic liver surgery is increasingly used for more challenging procedures. The aim of this study was to assess the feasibility and oncological safety of laparoscopic right hepatectomy for colorectal liver metastases after portal vein embolization.
Methods
This was an international retrospective multicentre study of patients with colorectal liver metastases who underwent open or laparoscopic right and extended right hepatectomy after portal vein embolization between 2004 and 2020. The perioperative and oncological outcomes for patients who underwent laparoscopic and open approaches were compared using propensity score matching.
Results
Of 338 patients, 84 patients underwent a laparoscopic procedure and 254 patients underwent an open procedure. Patients in the laparoscopic group less often underwent extended right hepatectomy (18% versus 34.6% (P = 0.004)), procedures in the setting of a two-stage hepatectomy (42% versus 65% (P < 0.001)), and major concurrent procedures (4% versus 16.1% (P = 0.003)). After propensity score matching, 78 patients remained in each group. The laparoscopic approach was associated with longer operating and Pringle times (330 versus 258.5 min (P < 0.001) and 65 versus 30 min (P = 0.001) respectively) and a shorter length of stay (7 versus 8 days (P = 0.011)). The R0 resection rate was not different (71% for the laparoscopic approach versus 60% for the open approach (P = 0.230)). The median disease-free survival was 12 (95% c.i. 10 to 20) months for the laparoscopic approach versus 20 (95% c.i. 13 to 31) months for the open approach (P = 0.145). The median overall survival was 28 (95% c.i. 22 to 48) months for the laparoscopic approach versus 42 (95% c.i. 35 to 52) months for the open approach (P = 0.614).
Conclusion
The advantages of a laparoscopic over an open approach for (extended) right hepatectomy for colorectal liver metastases after portal vein embolization are limited.
In this study, the safety and feasibility of laparoscopic right and extended right hepatectomy for colorectal liver metastases after portal vein embolization were evaluated. After propensity score matching, the only observed benefit in the laparoscopic group was a 1-day shorter hospital stay, with longer operating and Pringle times. The technical complexity of laparoscopy in this particular setting thus seems to limit its advantages over an open approach.
Introduction
Metastases from colorectal cancer most commonly occur in the liver. Up to 20% of patients have liver metastases at the time of diagnosis and more than 30% develop metachronous metastases1–3. Due to the functional, oncological, and technical difficulties related to both disease extent and liver function status, only 10–20% of patients with colorectal liver metastases (CRLM) are eligible for treatment with curative intent4. When major liver resection is needed to clear the liver of disease, the main limiting factor is an insufficient future liver remnant (FLR). Nowadays, there are several methods to increase the volume and function of the FLR. Portal vein embolization (PVE) is often preferred over other FLR modulation techniques. This is likely related to its periprocedural safety and the longer hypertrophy time, which allows for the identification of aggressive disease indicated by tumour progression before the subsequent hepatectomy5–7.
Laparoscopic liver surgery has increasingly been adopted. A plethora of studies have demonstrated the benefits of laparoscopic liver surgery over open liver surgery in selected patients, in terms of reduced length of stay, time to functional recovery, and lower (liver-specific) morbidity rates8–10. Oncological outcomes of laparoscopic liver surgery have been shown to be at least non-inferior to the traditional open approach11,12. With the advancement of surgical techniques and growing experience in laparoscopic liver surgery, the laparoscopic approach is adopted for more challenging procedures11,13–16. Several studies have now shown that, in experienced hands, the laparoscopic approach is also associated with improved short-term outcomes in the setting of major hepatectomies17–20. Preoperative FLR modulation can however further increase the technical difficulty of major hepatectomies, due to the anatomical changes and perivascular inflammation it induces. Evidence for laparoscopic surgery after PVE is scarce21–26. The aim of this study was to compare the feasibility and oncological safety of laparoscopic and open right and extended right hepatectomy for CRLM after PVE.
Methods
This was a retrospective multicentre study of consecutive adult patients with CRLM who underwent open or laparoscopic right and extended right hepatectomy after PVE between 2004 and 2020. An international multicentre database included data from 17 tertiary referral hepatobiliary centres was used to perform this study. The medical ethics committee of Brescia approved the study protocol (protocol number NP5329). Informed consent was not considered necessary due to the its retrospective nature and use of pseudonymized data. This study is reported in accordance with strengthening the reporting of observational studies in epidemiology (STROBE) statement27.
Interventions and surgical technique
PVE was carried out using a transhepatic approach, with patients under sedation or local anaesthesia, and selective embolization was performed after portal venography. After an adequate waiting time, the FLR volume was evaluated using CT. When the FLR was deemed sufficient, the decision was made to proceed to surgery. Although some variability between centres probably existed, laparoscopic resections were generally performed using the following technique. A total of five trocars (one 5 mm to the subxiphoidal, one to the 2 cm above the midpoint between the right midclavicular line and the umbilicus, one 10 mm to the midclavicular line at the level of the umbilicus, one 5 mm to the anterior axillary line at the level of the umbilicus, and one 10 mm to the left midclavicular line at the level of the umbilicus) were placed. Intraoperative ultrasonography was used to identify the number of lesions and the size of the lesions, as well as their relationship with major vascular structures. The liver was fully mobilized by dissecting the falciform and coronary ligament. The right lobe was elevated and venous branches to the retrohepatic vena cava were clipped and dissected. The Makuuchi ligament was dissected and divided using a stapler and the right hepatic vein was slinged. After the complete mobilization of the liver, the hepatoduodenal ligament was dissected and the right hepatic artery and the right portal vein were slinged. At the discretion of the operating surgeon, an intermittent Pringle manoeuvre was used by encircling the hepatoduodenal ligament. After clamping the right liver inflow, the left hemi-liver was controlled using Doppler ultrasonography for venous and arterial flow. Indocyanine green was selectively used to confirm the parenchymal ischaemia demarcation during this phase. Generally, parenchymal transection was performed using an ultrasonic dissector or a bipolar vessel sealer for the superficial part of the liver and an ultrasonic aspirator for the deep parenchyma. Vascular and biliary structures were managed with sealing device or clips based on diameter. When a safe and sufficient transection area was obtained (which could be challenging due to embolic material), the right hepatic duct, the right hepatic vein, and the right portal vein were dissected and usually transected using a stapler.
Definitions and outcomes
The procedure types were defined according to the Brisbane 2000 nomenclature28. A resection of segment five to eight was defined as a right hepatectomy and a resection of segment four to eight was defined as an extended right hepatectomy. Concurrent (non-cholecystectomy) procedures, such as pancreatic, gastric, colorectal, or diaphragmatic resections and biliary or vascular reconstructions, were defined as major concurrent procedures. Two-stage hepatectomy was defined as FLR clearance followed by FLR modulation and surgical removal of the contralateral liver lobe as a second stage29. Intraoperative incidents were defined and graded using the Oslo intraoperative unfavourable events grading system and postoperative morbidity was defined and graded using the Clavien–Dindo classification and is reported as overall and severe (Clavien–Dindo greater than or equal to grade III). Bile leak and liver failure were defined using the definition of the International Study Group of Liver Surgery30,31. Bile leak greater than or equal to grade A and liver failure greater than or equal to grade A were reported. Postoperative morbidity was evaluated within the first 30 days postoperatively and postoperative mortality constituted the 90-day or in-hospital mortality. The resection margin was considered radical (R0) when microscopically greater than 1 mm. Disease-free survival (DFS) and overall survival (OS) were defined as the interval (in months) between the date of surgery and the date when there was clinical evidence of disease recurrence or the date of death, respectively.
Statistical analysis
Patients were stratified into two groups, based on surgical approach. The perioperative and oncological outcomes for both groups were compared before and after propensity score matching (PSM). A subgroup analysis, in which the converted laparoscopic procedures were excluded, was performed using PSM. A sensitivity analysis was conducted to assess the outcomes in high-volume minimally invasive liver surgical centres, with centres performing greater than or equal to 50 laparoscopic resections per year being defined as high-volume centres32.
Continuous data, not normally distributed, are reported as the median (interquartile range (i.q.r.)) and comparisons between groups were performed using the Mann–Whitney U test. Normality was assessed by visually inspecting histograms and Q-Q plots. Categorical data are reported as n (%) and comparisons between groups were performed using a chi-squared test or Fisher’s exact test, when appropriate. Single imputation was used to impute missing data, which were present in a ‘missing at random’ pattern (Fig. S1). The outcome data were not imputed. Propensity scores were calculated using a multivariable logistic regression model. Variables that might influence treatment allocation were entered as covariates in this model and included age, sex, ASA grade, history of previous hepatic surgery, number of lesions, size of the largest lesion, procedure type (right hepatectomy or extended right hepatectomy), one- or two-stage procedure, and major concurrent procedures. Patients who underwent laparoscopic liver surgery were matched to their ‘nearest-neighbour’ who underwent open liver surgery in a 1 : 1 ratio without replacement, using a small caliper width. Standardized differences were used to evaluate the balance after PSM. A standardized difference less than 0.1 was considered to indicate optimal balance. After PSM, categorical data were compared using McNemar’s test or marginal homogeneity as appropriate. Continuous data were compared using the Wilcoxon signed rank test. Discrete variables were entered in their original form in the PSM logistic regression model, except for the variables number of lesions (dichotomized to single lesion versus multiple lesions) and size of the largest lesion (dichotomized to less than 51 mm or greater than or equal to 51 mm). DFS and OS were assessed using the Kaplan–Meier method combined with a stratified log rank test (stratification on the propensity score). All analyses were performed using SPSS® (IBM, Armonk, NY, USA; Statistics version 29.0) and R for Mac OS X version 4.2.1. Single imputation was performed using SPSS® and PSM was performed using the MatchIt package in R33. A two-tailed P < 0.050 was considered statistically significant.
Results
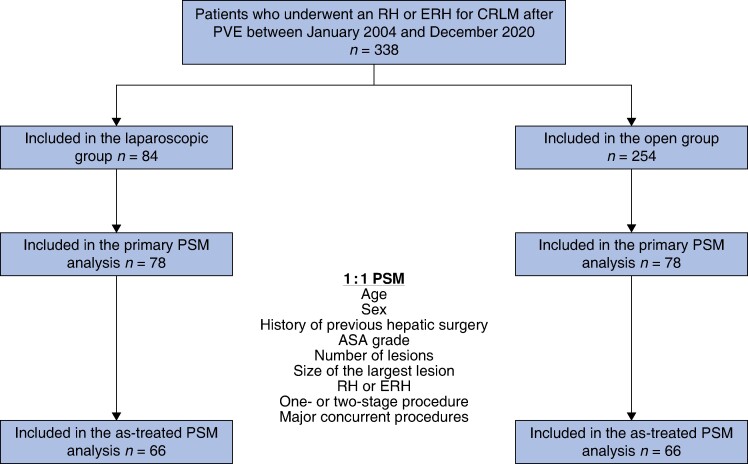
Overall, 338 patients were included, of whom 254 underwent open liver resection and 84 underwent laparoscopic liver resection. See Fig. 1. The use of the laparoscopic approach increased over time (Table S1).
Fig. 1.
Study flow chart
RH, right hepatectomy; ERH, extended right hepatectomy; CRLM, colorectal liver metastases; PVE, portal vein embolization; PSM, propensity score matching.
Characteristics and perioperative outcomes before propensity score matching
Patients in the laparoscopic group less often had a history of previous hepatic surgery and were more often treated with neoadjuvant chemotherapy (Table 1). They less often underwent extended right hepatectomy, procedures in the setting of a two-stage hepatectomy, and major concurrent procedures (Table S2). Patients who underwent laparoscopic surgery generally had less extensive disease, compared with patients who underwent open surgery, indicated by fewer and smaller lesions. The time interval from PVE to the definitive resection did not differ between the groups.
Table 1.
Baseline, procedural, and disease characteristics of patients who underwent right and extended right hepatectomy for colorectal liver metastases after portal vein embolization stratified by surgical approach, before and after propensity score matching
| Before PSM | P | After PSM | Standardized difference | P | |||
|---|---|---|---|---|---|---|---|
| Laparoscopic (n = 84) | Open (n = 254) | Laparoscopic (n = 78) | Open (n = 78) | ||||
| Baseline characteristics | |||||||
| Age (years), median (i.q.r.) | 65.5 (60.2–72.3) | 63 (56–69) | 0.007* | 66 (60.5–72) | 67 (60.3–71) | 0.01 | 0.673 |
| Male | 61 (73) | 171 (67.3) | 0.364 | 55 (71) | 55 (70.5) | 0 | 1.000 |
| BMI (kg/m2), median (i.q.r.) | 25.3 (23.1–28.6) | 25.1 (23–28) | 0.855 | 25.3 (23.2–28.5) | 24.7 (23–28.3) | 0.02 | 0.682 |
| ASA ≥grade III | 41 (49) | 114 (44.9) | 0.531 | 38 (49) | 40 (51) | 0.05 | 0.880 |
| Neoadjuvant chemotherapy | 72 (86) | 165 (65) | <0.001* | 66 (85) | 55 (71) | 0.34 | 0.054 |
| History of previous abdominal surgery | – | – | – | – | – | – | – |
| Extrahepatic | 45 (54) | 128 (50.4) | 0.613 | 42 (54) | 34 (44) | 0.21 | 0.280 |
| Hepatic | 30 (36) | 165 (65) | <0.001* | 30 (39) | 29 (37) | 0.03 | 1.000 |
| Procedural characteristics | |||||||
| Time between PVE and resection (days), median (i.q.r.) | 42.5 (34–59.5) | 42 (31–60) | 0.434 | 42 (34–57) | 40 (30–54.5) | 0.16 | 0.304 |
| Extent of resection | – | – | 0.004* | – | – | 0.03 | 1.000 |
| Right hepatectomy | 69 (82) | 166 (65.4) | – | 63 (81) | 62 (80) | – | – |
| Extended right hepatectomy | 15 (18) | 88 (34.6) | – | 15 (19) | 16 (21) | – | – |
| Part of two-stage hepatectomy | 35 (42) | 165 (65) | <0.001* | 34 (44) | 34 (44) | 0 | 1.000 |
| Major concurrent procedures | 3 (4) | 41 (16.1) | 0.003* | 3 (4) | 4 (5) | 0.06 | 1.000 |
| Disease characteristics | |||||||
| Bilobar distribution† | 27 (32.1) | 102 (40.2) | 0.190 | 27 (35) | 24 (31) | 0.08 | 0.700 |
| Number of lesions | 4 (2–6) | 5 (3–9) | 0.022* | 4 (2–6) | 4 (2.3–7) | 0.08 | 0.778 |
| Size of the largest lesion (mm), median (i.q.r.) | 33.5 (20–50) | 40 (24.3–60) | 0.035* | 35 (21.8–53.8) | 40 (22.5–55) | 0.04 | 0.424 |
Values are n (%) unless otherwise indicated. *Statistically significant. †At the second stage in the case of a two-stage hepatectomy. PSM, propensity score matching; i.q.r., interquartile range; PVE, portal vein embolization.
A total of 11 laparoscopic procedures (13%) were converted to open procedures (Table 2); 5 conversions were performed due to bleeding, 4 conversions were performed due to technical difficulties, 1 conversion was performed to achieve oncological safety, and 1 conversion was performed directly after mobilization of the liver. Operating and Pringle times were longer in the laparoscopic group. The laparoscopic approach was associated with a lower bile leak rate and a shorter length of stay.
Table 2.
Perioperative outcomes stratified by surgical approach, before and after propensity score matching
| Before PSM | P | After PSM | P | |||
|---|---|---|---|---|---|---|
| Laparoscopic (n = 84) | Open (n = 254) | Laparoscopic (n = 78) | Open (n = 78) | |||
| Intraoperative outcomes | ||||||
| Operating time (min), median (i.q.r.) | 329.5 (264.8–404.3) | 286 (223.5–358) | 0.002* | 330 (268.3–420) | 258.5 (212.5–313.8) | <0.001* |
| Estimated blood loss (ml), median (i.q.r.) | 500 (236–950) | 555 (292.5–967.3) | 0.651 | 500 (227–940) | 560 (295–1088) | 0.845 |
| Intraoperative PRBC transfusion | 18 (24) | 57 (27.1) | 0.557 | 18 (25) | 12 (19) | 0.404 |
| Number of PRBC transfused†, median (i.q.r.) | 2 (2–2.5) | 2 (2–3) | 0.931 | 2 (2–2.5) | 2 (1, 3.5) | 0.382 |
| Pringle manoeuvre | 58 (69) | 127 (59.3) | 0.120 | 54 (69) | 44 (63) | 0.391 |
| Total Pringle time when used (min), median (i.q.r.) | 62.5 (31.5–93) | 36.5 (24.8–59.3) | 0.001* | 65 (31.5–93.8) | 30 (20–50) | 0.001* |
| Intraoperative unfavourable incidents | – | – | 0.074 | – | – | 0.095 |
| Grade I | 8 (11) | 25 (17.6) | – | 8 (12) | 8 (16) | – |
| Grade II | 4 (5) | 3 (2.1) | – | 4 (6) | 1 (2) | – |
| Grade III | 2 (3) | 0 | – | 2 (3) | 0 | – |
| Conversion | 11 (13) | – | – | 11 (14) | – | – |
| Bleeding | 5 (19) | – | – | 5 (21) | – | – |
| Technical difficulty | 4 (15) | – | – | 4 (17) | – | – |
| Oncological safety | 1 (4) | – | – | 1 (4) | – | – |
| Planned after mobilization | 1 (4) | – | – | 1 (4) | – | – |
| Postoperative outcomes | ||||||
| Overall morbidity | 37 (44) | 124 (53) | 0.160 | 34 (44) | 36 (50) | 0.596 |
| Bile leak ≥grade A | 4 (5) | 34 (15.2) | 0.014* | 4 (5) | 7 (10) | 0.547 |
| Liver failure ≥grade A | 4 (5) | 18 (7.7) | 0.364 | 4 (5) | 4 (6) | 1.000 |
| Severe morbidity | 18 (21) | 44 (19) | 0.638 | 17 (22) | 13 (18) | 0.689 |
| Length of stay (days), median (i.q.r.) | 6 (4–9) | 9 (7–16) | <0.001* | 7 (4–9) | 8 (6–16) | 0.011* |
| Readmission | 7 (10) | 14 (12.1) | 0.576 | 6 (9) | 6 (12) | 1.000 |
| Radical resection margin (R0) | 60 (72) | 189 (77.5) | 0.340 | 54 (70) | 58 (77) | 0.391 |
| Ninety-day or in-hospital mortality | 4 (5) | 12 (4.7) | 0.989 | 4 (5) | 4 (5) | 1.000 |
Values are n (%) unless otherwise indicated. *Statistically significant. †For patients who received a transfusion. PSM, propensity score matching; i.q.r., interquartile range; PRBC, packed red blood cells.
Characteristics and perioperative outcomes after propensity score matching
After PSM, 78 patients remained in each group (Table 1). Operating and Pringle times were longer in the laparoscopic group. Other intraoperative outcomes did not differ between the groups. The length of stay was 1 day shorter in the laparoscopic group. Other postoperative outcomes did not differ between the groups.
Survival
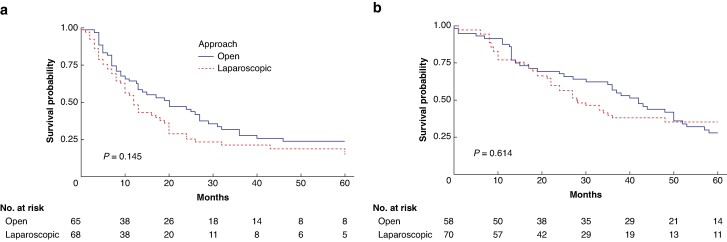
Data regarding DFS and OS were available for 85% and 82% of the matched patients respectively. The median DFS (Fig. 2a) was 12 (95% c.i. 10 to 20) months for the laparoscopic approach versus 20 (95% c.i. 13 to 31) months for the open approach (P = 0.145) and the median OS (Fig. 2b) was 28 (95% c.i. 22 to 48) months for the laparoscopic approach versus 42 (95% c.i. 35 to 52) months for the open approach (P = 0.614).
Fig. 2.
Survival of the propensity score matching cohort
a Disease-free survival. b Overall survival.
Characteristics and perioperative outcomes of the converted procedures and as-treated analysis
Patients who underwent converted procedures more often had a history of previous hepatic surgery (63% versus 32% (P = 0.038)) and more often underwent major concurrent procedures (18% versus 1% (P = 0.005)) compared with patients who underwent non-converted laparoscopic procedures (Table S3). The perioperative outcomes of the procedures that were converted, compared with procedures that were performed fully laparoscopically, are shown in Table S4. Converted procedures were associated with more blood loss, a higher transfusion rate, and higher overall and severe morbidity rates. Of 11 patients in the converted group, 2 patients (18%) died within the first 90 days after surgery, compared with 2 of 73 patients (3%) in the non-converted group (P = 0.025). The median length of stay was longer in the converted group.
After excluding the converted procedures, 66 of 67 remaining patients who underwent laparoscopic surgery were matched with 66 patients who underwent open surgery. The covariates were well balanced, but some imbalance remained for the covariates age (standardized difference 0.15), major concurrent procedures (standardized difference 0.18), and number of lesions (standardized difference 0.26). See Table S5. The length of stay was shorter for patients who underwent laparoscopic liver surgery without conversion. No other differences were observed. See Table 3.
Table 3.
Perioperative outcomes after propensity score matching, excluding converted procedures
| Laparoscopic (n = 66) | Open (n = 66) | P | |
|---|---|---|---|
| Intraoperative outcomes | |||
| Operating time (min), median (i.q.r.) | 300 (255–390) | 252 (212.5–313.3) | 0.015* |
| Estimated blood loss (ml), median (i.q.r.) | 400 (200–700) | 525 (265–805) | 0.436 |
| Intraoperative PRBC transfusion | 9 (15) | 7 (14) | 0.773 |
| Number of PRBC transfused†, median (i.q.r.) | 2 (2–2) | 2 (1–2) | 0.832 |
| Pringle manoeuvre | 46 (70) | 36 (59) | 0.176 |
| Total Pringle time when used (min), median (i.q.r.) | 65 (32–93.5) | 30 (23–45) | <0.001* |
| Intraoperative unfavourable incidents | – | – | 0.257 |
| Grade I | 4 (7) | 5 (12) | – |
| Grade II | 0 | 0 | – |
| Grade III | 0 | 0 | – |
| Postoperative outcomes | |||
| Overall morbidity | 23 (35) | 26 (42) | 0.596 |
| Bile leak ≥grade A | 3 (5) | 4 (7) | 1.000 |
| Liver failure ≥grade A | 3 (5) | 3 (5) | 1.000 |
| Severe morbidity | 9 (14) | 8 (13) | 1.000 |
| Length of stay (days), median (i.q.r.) | 6 (4–8) | 7.8 (6–11.8) | 0.004* |
| Readmission | 6 (11) | 5 (12) | 1.000 |
| Radical resection margin (R0) | 49 (75) | 56 (85) | 0.286 |
| Ninety-day or in-hospital mortality | 1 (2) | 5 (8) | 0.221 |
Values are n (%) unless otherwise indicated. *Statistically significant. †For patients who received a transfusion. i.q.r., interquartile range; PRBC, packed red blood cells.
Sensitivity analysis
The sensitivity analysis in high-volume centres generally yielded comparable results to the primary analysis. See Tables S6 and S7. The laparoscopic approach had a more pronounced benefit in terms of length of stay in these centres, with a median length of stay of 6 (i.q.r. 4–9) versus 8 (i.q.r. 7–14) days (P < 0.001).
Discussion
Laparoscopic right and extended right hepatectomies for CRLM after PVE can be performed safely and effectively by experienced surgeons working in specialized centres. However, the advantages over the open approach in this setting are very limited. In this technically complex setting, most patients underwent open surgery. Patients were probably carefully selected for the laparoscopic approach, indicated by the fact that they had less extensive disease. Differences between the groups included longer operating and Pringle times, but only a 1-day shorter length of stay, for patients who underwent laparoscopic hepatectomy. Other outcomes, including R0 rates, DFS, and OS, did not differ between the groups.
A system for scoring the complexity of laparoscopic liver resections has been formulated. A positive correlation was observed between the difficulty index and prolonged operating time34. In this study, the operating and Pringle times were longer in the laparoscopic group, highlighting the technical complexity of these procedures. Laparoscopic liver resections have generally been associated with less blood loss35,36. However, in the context of major hepatectomy, the laparoscopic approach has been associated with comparable amounts of blood loss to open surgery, thereby losing this advantage12,17–19. This study had concordant results. Even when the converted cases were excluded, intraoperative blood loss did not differ between the two groups. Overall morbidity, severe morbidity, and liver-specific complications did not differ between the groups either. Observed liver failure rates were slightly lower than generally reported in the literature, possibly due to the selection of patients diagnosed with CRLM only37.
These findings support the importance of expanding indications for laparoscopic liver surgery only after completing the learning curve and employing proper patient selection. A history of previous hepatic surgery and the need for major concurrent procedures demand specific consideration during patient selection. In this study, the conversion rate was 13%, concordant with rates reported for laparoscopic major liver resections in general of between 0% and 11%38. It is well known that emergency conversions are associated with poor outcomes39.
Several studies have provided insights into the impact of centre volume on the outcomes of minimally invasive liver surgery32,40. The proficiency of the surgeon constitutes another pivotal determinant impacting postoperative results41–43. Notably, the surgeons participating in this study routinely perform more than 20 minimally invasive hepatectomies on an annual basis.
The most frequently mentioned benefit of minimally invasive surgery is its ability to accelerate functional recovery, indicated by a shorter hospital stay8,44. The clinical relevance of a 1-day reduction in hospital stay in the laparoscopic group, and its associated cost-effectiveness, is a matter of debate44,45.
This study has several limitations. These include the relatively small sample size and retrospective design, without standardization of perioperative care and surgical techniques. Data on FLR changes after PVE, which could be a crucial factor in the occurrence of liver failure, were not available. It is also important to note that the laparoscopic approach was generally used for more straightforward cases. PSM might not have been completely able to adjust for confounding by indication. Concludingly, the findings of this study indicate that laparoscopic right hepatectomy for CRLM after PVE can be performed safely and effectively by experienced surgeons working in specialized centres. However, it should be acknowledged that the advantages over the open approach in this setting are very limited when compared to those seen in other less complex resections.
Supplementary Material
Acknowledgements
Emre Bozkurt and Jasper P. Sijberden contributed equally. The following surgeons are acknowledged: Marco Vivarelli (Hepatobiliary and Abdominal Transplantation Surgery, Department of Experimental and Clinical Medicine, Riuniti Hospital, Polytechnic University of Marche, Ancona, Italy), Adnan Alseidi (Department of Surgery, Virginia Mason Medical Center, Seattle, WA, USA and Department of Surgery, University of California San Francisco, San Francisco, CA, USA), and Elio Jovine (Department of Surgery, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy).
Contributor Information
Emre Bozkurt, Department of Surgery, Fondazione Poliambulanza Istituto Ospedaliero, Brescia, Italy; Department of Surgery, Koç University School of Medicine, Istanbul, Turkey.
Jasper P Sijberden, Department of Surgery, Fondazione Poliambulanza Istituto Ospedaliero, Brescia, Italy; Department of Surgery, Amsterdam UMC, location University of Amsterdam, Amsterdam, The Netherlands; Cancer Center Amsterdam, Amsterdam, The Netherlands.
Serena Langella, Department of General and Oncological Surgery, Umberto I Mauriziano Hospital, Turin, Italy.
Federica Cipriani, Hepatobiliary Surgery Division, IRCCS San Raffaele Hospital, Milan, Italy.
Francesc Collado-Roura, Servei de Cirurgia General i Digestiva, Hospital Universitari Doctor Josep Trueta de Girona, Girona, Spain.
Victoria Morrison-Jones, Department of Surgery, University Hospital Southampton NHS Foundation Trust, Southampton, UK.
Burak Görgec, Department of Surgery, Amsterdam UMC, location University of Amsterdam, Amsterdam, The Netherlands; Cancer Center Amsterdam, Amsterdam, The Netherlands.
Gabriel Zozaya, Department of Surgery, HPB and Liver Transplantation Unit, University Clinic, Universidad de Navarra, Institute of Health Research of Navarra (IdisNA), Pamplona, Spain.
Jacopo Lanari, Department of Surgical, Oncological and Gastroenterological Sciences, General Surgery 2, Hepato-pancreato-biliary Surgery and Liver Transplantation, Padua University Hospital, Padua, Italy.
Davit Aghayan, The Intervention Centre and Department of HPB Surgery, Oslo University Hospital and Institute of Medicine, University of Oslo, Oslo, Norway.
Celine De Meyere, Department of Digestive and Hepatobiliary/Pancreatic Surgery, Groeninge Hospital, Kortrijk, Belgium.
David Fuks, Department of Digestive, Oncologic and Metabolic Surgery, Institut Mutualiste Montsouris, Université Paris Descartes, Paris, France.
Giuseppe Zimmiti, Department of Surgery, Fondazione Poliambulanza Istituto Ospedaliero, Brescia, Italy.
Benedetto Ielpo, Hepatobiliary and Pancreatic Surgery Unit, Hospital del Mar, Hospital del Mar Medical Research Institute (IMIM), Universitat Pompeu Fabra, Barcelona, Spain.
Mikhail Efanov, Department of Hepato-Pancreato-Biliary Surgery, Moscow Clinical Research Centre, Moscow, Russia.
Robert P Sutcliffe, Liver Unit, Queen Elizabeth Hospital, Birmingham, UK.
Nadia Russolillo, Department of General and Oncological Surgery, Umberto I Mauriziano Hospital, Turin, Italy.
Miquel Gomez-Artacho, Servei de Cirurgia General i Digestiva, Hospital Universitari Doctor Josep Trueta de Girona, Girona, Spain.
Francesca Ratti, Hepatobiliary Surgery Division, IRCCS San Raffaele Hospital, Milan, Italy.
Mathieu D’Hondt, Department of Digestive and Hepatobiliary/Pancreatic Surgery, Groeninge Hospital, Kortrijk, Belgium.
Bjørn Edwin, The Intervention Centre and Department of HPB Surgery, Oslo University Hospital and Institute of Medicine, University of Oslo, Oslo, Norway.
Umberto Cillo, Department of Surgical, Oncological and Gastroenterological Sciences, General Surgery 2, Hepato-pancreato-biliary Surgery and Liver Transplantation, Padua University Hospital, Padua, Italy.
Fernando Rotellar, Department of Surgery, HPB and Liver Transplantation Unit, University Clinic, Universidad de Navarra, Institute of Health Research of Navarra (IdisNA), Pamplona, Spain.
Marc G Besselink, Department of Surgery, Amsterdam UMC, location University of Amsterdam, Amsterdam, The Netherlands; Cancer Center Amsterdam, Amsterdam, The Netherlands.
John N Primrose, Department of Surgery, University Hospital Southampton NHS Foundation Trust, Southampton, UK.
Santi Lopez-Ben, Servei de Cirurgia General i Digestiva, Hospital Universitari Doctor Josep Trueta de Girona, Girona, Spain.
Luca A Aldrighetti, Hepatobiliary Surgery Division, IRCCS San Raffaele Hospital, Milan, Italy.
Alessandro Ferrero, Department of General and Oncological Surgery, Umberto I Mauriziano Hospital, Turin, Italy.
Mohammad Abu Hilal, Department of Surgery, Fondazione Poliambulanza Istituto Ospedaliero, Brescia, Italy; Department of Surgery, University Hospital Southampton NHS Foundation Trust, Southampton, UK.
Funding
The authors have no funding to declare.
Author contributions
Emre Bozkurt (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), Jasper P. Sijberden (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), Serena Langella (Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Validation, Writing—original draft), Federica Cipriani (Conceptualization, Data curation, Formal analysis, Methodology, Resources, Supervision, Validation, Visualization, Writing—original draft), Francesc Collado-Roura (Conceptualization, Data curation, Formal analysis, Methodology, Resources, Supervision, Validation, Writing—original draft), Victoria Morrison-Jones (Conceptualization, Data curation, Formal analysis, Methodology, Resources, Supervision, Validation, Writing—original draft), Burak Görgec (Conceptualization, Data curation, Formal analysis, Methodology, Resources, Supervision, Validation, Writing—original draft), Gabriel Zozaya (Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Visualization, Writing—original draft), Jacopo Lanari (Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Writing—original draft), Davit Aghayan (Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Writing—original draft), Celine De Meyere (Conceptualization, Data curation, Methodology, Validation, Writing—original draft), David Fuks (Conceptualization, Data curation, Formal analysis, Methodology, Resources, Supervision, Validation, Writing—original draft, Writing—review & editing), Giuseppe Zimmitti (Conceptualization, Data curation, Methodology, Supervision, Validation, Writing—original draft, Writing—review & editing), Benedetto Ielpo (Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Writing—original draft, Writing—review & editing), Mikhail Efanov (Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Validation, Writing—review & editing), Robert P. Sutcliffe (Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Visualization, Writing—review & editing), Nadia Russolillo (Conceptualization, Data curation, Methodology, Resources, Validation, Writing—review & editing), Miquel Gomez-Artacho (Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Visualization, Writing—review & editing), Francesca Ratti (Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Visualization, Writing—review & editing), Mathieu D’Hondt (Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Visualization, Writing—review & editing), Bjørn Edwin (Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), Umberto Cillo (Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Visualization, Writing—review & editing), Fernando Rotellar (Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), Marc G. Besselink (Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), John N. Primrose (Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), Santi Lopez-Ben (Conceptualization, Formal analysis, Methodology, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), Luca A. Aldrighetti (Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Visualization, Writing—review & editing), Alessandro Ferrero (Conceptualization, Data curation, Methodology, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), and Mohammed Abu Hilal (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing)
Disclosure
Fernando Rotellar has received fees from Olympus, Sitex, Integra, Medtronic, and Johnson & Johnson outside of the submitted work. Santi Lopez-Ben has received fees from Baxter, Olympus, and Johnson & Johnson outside of the submitted work. The authors declare no other conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
The data that support the findings of this study are available from the corresponding author, M.A.H., upon reasonable request. The data are not publicly available as this could compromise the privacy of research participants.
References
- 1. Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases—a population-based study on incidence, management and survival. BMC Cancer 2018;18:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer 2014;14:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kawaguchi Y, Lillemoe HA, Vauthey JN. Dealing with an insufficient future liver remnant: portal vein embolization and two-stage hepatectomy. J Surg Oncol 2019;119:594–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charalel RA, Sung J, Askin G, Jo J, Mitry M, Chung C et al. Systematic reviews and meta-analyses of portal vein embolization, associated liver partition and portal vein ligation, and radiation lobectomy outcomes in hepatocellular carcinoma patients. Curr Oncol Rep 2021;23:135. [DOI] [PubMed] [Google Scholar]
- 6. Baumgart J, Jungmann F, Bartsch F, Kloth M, Mittler J, Heinrich S et al. Two-stage hepatectomy and ALPPS for advanced bilateral liver metastases: a tailored approach balancing risk and outcome. J Gastrointest Surg 2019;23:2391–2400 [DOI] [PubMed] [Google Scholar]
- 7. Shindoh J, Vauthey JN, Zimmitti G, Curley SA, Huang SY, Mahvash A et al. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg 2013;217:126–133; discussion 133–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heinrich S, Seehofer D, Corvinus F, Tripke V, Huber T, Hüttl F et al. [Advantages and future perspectives of laparoscopic liver surgery]. Chirurg 2021;92:542–549 [DOI] [PubMed] [Google Scholar]
- 9. van der Heijde N, Ratti F, Aldrighetti L, Benedetti Cacciaguerra A, Can MF, D'Hondt M et al. Laparoscopic versus open right posterior sectionectomy: an international, multicenter, propensity score-matched evaluation. Surg Endosc 2021;35:6139–6149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gumbs AA, Lorenz E, Tsai TJ, Starker L, Flanagan J, Benedetti Cacciaguerra A et al. Study: international multicentric minimally invasive liver resection for colorectal liver metastases (SIMMILR-CRLM). Cancers (Basel) 2022;14:1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Görgec B, Suhool A, Al-Jarrah R, Fontana M, Tehami NA, Modi S et al. Surgical technique and clinical results of one- or two-stage laparoscopic right hemihepatectomy after portal vein embolization in patients with initially unresectable colorectal liver metastases: a case series. Int J Surg 2020;77:69–75 [DOI] [PubMed] [Google Scholar]
- 12. Robles-Campos R, Lopez-Lopez V, Brusadin R, Lopez-Conesa A, Gil-Vazquez PJ, Navarro-Barrios Á et al. Open versus minimally invasive liver surgery for colorectal liver metastases (LapOpHuva): a prospective randomized controlled trial. Surg Endosc 2019;33:3926–3936 [DOI] [PubMed] [Google Scholar]
- 13. Malerba V, Benedetti Cacciaguerra A, Zimmitti G, Manzoni A, Sallemi C, Monfardini L et al. From PVE to HVE to fully laparoscopic rescue ALPPS: a case report of multidisciplinary management of giant HCC. Hepatoma Res 2021;7:19 [Google Scholar]
- 14. Zimmitti G, Sijberden JP, Osei-Bordom D, Russolillo N, Aghayan D, Lanari J et al. Indications, trends, and perioperative outcomes of minimally invasive and open liver surgery in non-obese and obese patients: an international multicentre propensity score matched retrospective cohort study of 9963 patients. Int J Surg 2022;107:106957. [DOI] [PubMed] [Google Scholar]
- 15. Di Fabio F, Whistance R, Rahman S, Primrose JN, Pearce NW, Abu Hilal M. Exploring the role of laparoscopic surgery in two-stage hepatectomy for bilobar colorectal liver metastases. J Laparoendosc Adv Surg Tech A 2012;22:647–650 [DOI] [PubMed] [Google Scholar]
- 16. Jain G, Parmar J, Mohammed MM, Bryant T, Kitteringham L, Pearce N et al. “Stretching the limits of laparoscopic surgery”: two-stage laparoscopic liver resection. J Laparoendosc Adv Surg Tech A 2010;20:51–54 [DOI] [PubMed] [Google Scholar]
- 17. Kasai M, Cipriani F, Gayet B, Aldrighetti L, Ratti F, Sarmiento JM et al. Laparoscopic versus open major hepatectomy: a systematic review and meta-analysis of individual patient data. Surgery 2018;163:985–995 [DOI] [PubMed] [Google Scholar]
- 18. Cipriani F, Alzoubi M, Fuks D, Ratti F, Kawai T, Berardi G et al. Pure laparoscopic versus open hemihepatectomy: a critical assessment and realistic expectations—a propensity score-based analysis of right and left hemihepatectomies from nine European tertiary referral centers. J Hepatobiliary Pancreat Sci 2020;27:3–15 [DOI] [PubMed] [Google Scholar]
- 19. Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW et al. Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg 2018;267:199–207 [DOI] [PubMed] [Google Scholar]
- 20. Hoogteijling TJ, Sijberden JP, Primrose JN, Morrison-Jones V, Modi S, Zimmitti G et al. Laparoscopic right hemihepatectomy after future liver remnant modulation: a single surgeon’s experience. Cancers (Basel) 2023;15:2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bozkurt E, Sijberden JP, Abu Hilal M. Safety and feasibility of laparoscopic right or extended right hemi hepatectomy following modulation of the future liver remnant in patients with colorectal liver metastases: a systematic review. J Laparoendosc Adv Surg Tech A 2023;33:654–664 [DOI] [PubMed] [Google Scholar]
- 22. Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R et al. The Southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg 2018;268:11–18 [DOI] [PubMed] [Google Scholar]
- 23. Wang ZY, Chen QL, Sun LL, He SP, Luo XF, Huang LS et al. Laparoscopic versus open major liver resection for hepatocellular carcinoma: systematic review and meta-analysis of comparative cohort studies. BMC Cancer 2019;19:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G. Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg 2016;263:761–777 [DOI] [PubMed] [Google Scholar]
- 25. Delvecchio A, Conticchio M, Ratti F, Gelli M, Anelli FM, Laurent A et al. Laparoscopic major hepatectomy for hepatocellular carcinoma in elderly patients: a multicentric propensity score-based analysis. Surg Endosc 2021;5:3642–3652 [DOI] [PubMed] [Google Scholar]
- 26. Heid F, Toti J, Balzarotti Canger R, Cristaudi A, Breitenstein S, Majno-Hurst P et al. Is laparoscopic major hepatectomy feasible and safe in Swiss cantonal hospitals? Swiss Med Wkly 2021;151:w30044. [DOI] [PubMed] [Google Scholar]
- 27. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007;4:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 2005;12:351–355 [DOI] [PubMed] [Google Scholar]
- 29. Imai K, Allard MA, Baba H, Adam R. Optimal patient selection for successful two-stage hepatectomy of bilateral colorectal liver metastases. Ann Gastroenterol Surg 2021;5:634–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680–688 [DOI] [PubMed] [Google Scholar]
- 31. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713–724 [DOI] [PubMed] [Google Scholar]
- 32. Görgec B, Fichtinger RS, Ratti F, Aghayan D, Van der Poel MJ, Al-Jarrah R et al. Comparing practice and outcome of laparoscopic liver resection between high-volume expert centres and nationwide low-to-medium volume centres. Br J Surg 2021;108:983–990 [DOI] [PubMed] [Google Scholar]
- 33. Ho D, Imai K, King G, Stuart EA. Matchit: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011;42:1–28 [Google Scholar]
- 34. Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 2014;21:745–753 [DOI] [PubMed] [Google Scholar]
- 35. Topal B, Fieuws S, Aerts R, Vandeweyer H, Penninckx F. Laparoscopic versus open liver resection of hepatic neoplasms: comparative analysis of short-term results. Surg Endosc 2008;22:2208–2213 [DOI] [PubMed] [Google Scholar]
- 36. Nguyen KT, Marsh JW, Tsung A, Steel JJ, Gamblin TC, Geller DA. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg 2011;146:348–356 [DOI] [PubMed] [Google Scholar]
- 37. Hammond JS, Guha IN, Beckingham IJ, Lobo DN. Prediction, prevention and management of postresection liver failure. Br J Surg 2011;98:1188–1200 [DOI] [PubMed] [Google Scholar]
- 38. Perri G, Marchegiani G, Reich F, Casetti L, Fontana M, Esposito A et al. Intraoperative blood loss estimation in hepato-pancreato-biliary surgery—relevant, not reported, not standardized: results from a systematic review and a worldwide snapshot survey. Ann Surg 2023;277:e849–e855 [DOI] [PubMed] [Google Scholar]
- 39. Halls MC, Cipriani F, Berardi G, Barkhatov L, Lainas P, Alzoubi M et al. Conversion for unfavorable intraoperative events results in significantly worse outcomes during laparoscopic liver resection: lessons learned from a multicenter review of 2861 cases. Ann Surg 2018;268:1051–1057 [DOI] [PubMed] [Google Scholar]
- 40. Viganò L, Cimino M, Aldrighetti L, Ferrero A, Cillo U, Guglielmi A et al. Multicentre evaluation of case volume in minimally invasive hepatectomy. Br J Surg 2020;107:443–451 [DOI] [PubMed] [Google Scholar]
- 41. Chen PD, Wu CY, Hu RH, Chen CN, Yuan RH, Liang JT et al. Robotic major hepatectomy: is there a learning curve? Surgery 2017;161:642–649 [DOI] [PubMed] [Google Scholar]
- 42. Halls MC, Alseidi A, Berardi G, Cipriani F, Van der Poel M, Davila D et al. A comparison of the learning curves of laparoscopic liver surgeons in differing stages of the IDEAL paradigm of surgical innovation: standing on the shoulders of pioneers. Ann Surg 2019;269:221–228 [DOI] [PubMed] [Google Scholar]
- 43. Gumbs AA, Hilal MA, Croner R, Gayet B, Chouillard E, Gagner M. The initiation, standardization and proficiency (ISP) phases of the learning curve for minimally invasive liver resection: comparison of a fellowship-trained surgeon with the pioneers and early adopters. Surg Endosc 2021;35:5268–5278 [DOI] [PubMed] [Google Scholar]
- 44. Fichtinger RS, Aldrighetti LA, Abu Hilal M, Troisi RI, Sutcliffe RP, Besselink MG et al. ; ORANGE II PLUS Collaborative . Laparoscopic versus open hemihepatectomy: the ORANGE II PLUS multicenter randomized controlled trial. J Clin Oncol 2024;42:1799–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jackson NR, Hauch A, Hu T, Buell JF, Slakey DP, Kandil E. The safety and efficacy of approaches to liver resection: a meta-analysis. JSLS 2015;19:e2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, M.A.H., upon reasonable request. The data are not publicly available as this could compromise the privacy of research participants.
References
- 1. Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases—a population-based study on incidence, management and survival. BMC Cancer 2018;18:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer 2014;14:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kawaguchi Y, Lillemoe HA, Vauthey JN. Dealing with an insufficient future liver remnant: portal vein embolization and two-stage hepatectomy. J Surg Oncol 2019;119:594–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charalel RA, Sung J, Askin G, Jo J, Mitry M, Chung C et al. Systematic reviews and meta-analyses of portal vein embolization, associated liver partition and portal vein ligation, and radiation lobectomy outcomes in hepatocellular carcinoma patients. Curr Oncol Rep 2021;23:135. [DOI] [PubMed] [Google Scholar]
- 6. Baumgart J, Jungmann F, Bartsch F, Kloth M, Mittler J, Heinrich S et al. Two-stage hepatectomy and ALPPS for advanced bilateral liver metastases: a tailored approach balancing risk and outcome. J Gastrointest Surg 2019;23:2391–2400 [DOI] [PubMed] [Google Scholar]
- 7. Shindoh J, Vauthey JN, Zimmitti G, Curley SA, Huang SY, Mahvash A et al. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg 2013;217:126–133; discussion 133–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heinrich S, Seehofer D, Corvinus F, Tripke V, Huber T, Hüttl F et al. [Advantages and future perspectives of laparoscopic liver surgery]. Chirurg 2021;92:542–549 [DOI] [PubMed] [Google Scholar]
- 9. van der Heijde N, Ratti F, Aldrighetti L, Benedetti Cacciaguerra A, Can MF, D'Hondt M et al. Laparoscopic versus open right posterior sectionectomy: an international, multicenter, propensity score-matched evaluation. Surg Endosc 2021;35:6139–6149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gumbs AA, Lorenz E, Tsai TJ, Starker L, Flanagan J, Benedetti Cacciaguerra A et al. Study: international multicentric minimally invasive liver resection for colorectal liver metastases (SIMMILR-CRLM). Cancers (Basel) 2022;14:1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Görgec B, Suhool A, Al-Jarrah R, Fontana M, Tehami NA, Modi S et al. Surgical technique and clinical results of one- or two-stage laparoscopic right hemihepatectomy after portal vein embolization in patients with initially unresectable colorectal liver metastases: a case series. Int J Surg 2020;77:69–75 [DOI] [PubMed] [Google Scholar]
- 12. Robles-Campos R, Lopez-Lopez V, Brusadin R, Lopez-Conesa A, Gil-Vazquez PJ, Navarro-Barrios Á et al. Open versus minimally invasive liver surgery for colorectal liver metastases (LapOpHuva): a prospective randomized controlled trial. Surg Endosc 2019;33:3926–3936 [DOI] [PubMed] [Google Scholar]
- 13. Malerba V, Benedetti Cacciaguerra A, Zimmitti G, Manzoni A, Sallemi C, Monfardini L et al. From PVE to HVE to fully laparoscopic rescue ALPPS: a case report of multidisciplinary management of giant HCC. Hepatoma Res 2021;7:19 [Google Scholar]
- 14. Zimmitti G, Sijberden JP, Osei-Bordom D, Russolillo N, Aghayan D, Lanari J et al. Indications, trends, and perioperative outcomes of minimally invasive and open liver surgery in non-obese and obese patients: an international multicentre propensity score matched retrospective cohort study of 9963 patients. Int J Surg 2022;107:106957. [DOI] [PubMed] [Google Scholar]
- 15. Di Fabio F, Whistance R, Rahman S, Primrose JN, Pearce NW, Abu Hilal M. Exploring the role of laparoscopic surgery in two-stage hepatectomy for bilobar colorectal liver metastases. J Laparoendosc Adv Surg Tech A 2012;22:647–650 [DOI] [PubMed] [Google Scholar]
- 16. Jain G, Parmar J, Mohammed MM, Bryant T, Kitteringham L, Pearce N et al. “Stretching the limits of laparoscopic surgery”: two-stage laparoscopic liver resection. J Laparoendosc Adv Surg Tech A 2010;20:51–54 [DOI] [PubMed] [Google Scholar]
- 17. Kasai M, Cipriani F, Gayet B, Aldrighetti L, Ratti F, Sarmiento JM et al. Laparoscopic versus open major hepatectomy: a systematic review and meta-analysis of individual patient data. Surgery 2018;163:985–995 [DOI] [PubMed] [Google Scholar]
- 18. Cipriani F, Alzoubi M, Fuks D, Ratti F, Kawai T, Berardi G et al. Pure laparoscopic versus open hemihepatectomy: a critical assessment and realistic expectations—a propensity score-based analysis of right and left hemihepatectomies from nine European tertiary referral centers. J Hepatobiliary Pancreat Sci 2020;27:3–15 [DOI] [PubMed] [Google Scholar]
- 19. Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW et al. Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg 2018;267:199–207 [DOI] [PubMed] [Google Scholar]
- 20. Hoogteijling TJ, Sijberden JP, Primrose JN, Morrison-Jones V, Modi S, Zimmitti G et al. Laparoscopic right hemihepatectomy after future liver remnant modulation: a single surgeon’s experience. Cancers (Basel) 2023;15:2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bozkurt E, Sijberden JP, Abu Hilal M. Safety and feasibility of laparoscopic right or extended right hemi hepatectomy following modulation of the future liver remnant in patients with colorectal liver metastases: a systematic review. J Laparoendosc Adv Surg Tech A 2023;33:654–664 [DOI] [PubMed] [Google Scholar]
- 22. Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R et al. The Southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg 2018;268:11–18 [DOI] [PubMed] [Google Scholar]
- 23. Wang ZY, Chen QL, Sun LL, He SP, Luo XF, Huang LS et al. Laparoscopic versus open major liver resection for hepatocellular carcinoma: systematic review and meta-analysis of comparative cohort studies. BMC Cancer 2019;19:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G. Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg 2016;263:761–777 [DOI] [PubMed] [Google Scholar]
- 25. Delvecchio A, Conticchio M, Ratti F, Gelli M, Anelli FM, Laurent A et al. Laparoscopic major hepatectomy for hepatocellular carcinoma in elderly patients: a multicentric propensity score-based analysis. Surg Endosc 2021;5:3642–3652 [DOI] [PubMed] [Google Scholar]
- 26. Heid F, Toti J, Balzarotti Canger R, Cristaudi A, Breitenstein S, Majno-Hurst P et al. Is laparoscopic major hepatectomy feasible and safe in Swiss cantonal hospitals? Swiss Med Wkly 2021;151:w30044. [DOI] [PubMed] [Google Scholar]
- 27. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007;4:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 2005;12:351–355 [DOI] [PubMed] [Google Scholar]
- 29. Imai K, Allard MA, Baba H, Adam R. Optimal patient selection for successful two-stage hepatectomy of bilateral colorectal liver metastases. Ann Gastroenterol Surg 2021;5:634–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680–688 [DOI] [PubMed] [Google Scholar]
- 31. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713–724 [DOI] [PubMed] [Google Scholar]
- 32. Görgec B, Fichtinger RS, Ratti F, Aghayan D, Van der Poel MJ, Al-Jarrah R et al. Comparing practice and outcome of laparoscopic liver resection between high-volume expert centres and nationwide low-to-medium volume centres. Br J Surg 2021;108:983–990 [DOI] [PubMed] [Google Scholar]
- 33. Ho D, Imai K, King G, Stuart EA. Matchit: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011;42:1–28 [Google Scholar]
- 34. Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 2014;21:745–753 [DOI] [PubMed] [Google Scholar]
- 35. Topal B, Fieuws S, Aerts R, Vandeweyer H, Penninckx F. Laparoscopic versus open liver resection of hepatic neoplasms: comparative analysis of short-term results. Surg Endosc 2008;22:2208–2213 [DOI] [PubMed] [Google Scholar]
- 36. Nguyen KT, Marsh JW, Tsung A, Steel JJ, Gamblin TC, Geller DA. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg 2011;146:348–356 [DOI] [PubMed] [Google Scholar]
- 37. Hammond JS, Guha IN, Beckingham IJ, Lobo DN. Prediction, prevention and management of postresection liver failure. Br J Surg 2011;98:1188–1200 [DOI] [PubMed] [Google Scholar]
- 38. Perri G, Marchegiani G, Reich F, Casetti L, Fontana M, Esposito A et al. Intraoperative blood loss estimation in hepato-pancreato-biliary surgery—relevant, not reported, not standardized: results from a systematic review and a worldwide snapshot survey. Ann Surg 2023;277:e849–e855 [DOI] [PubMed] [Google Scholar]
- 39. Halls MC, Cipriani F, Berardi G, Barkhatov L, Lainas P, Alzoubi M et al. Conversion for unfavorable intraoperative events results in significantly worse outcomes during laparoscopic liver resection: lessons learned from a multicenter review of 2861 cases. Ann Surg 2018;268:1051–1057 [DOI] [PubMed] [Google Scholar]
- 40. Viganò L, Cimino M, Aldrighetti L, Ferrero A, Cillo U, Guglielmi A et al. Multicentre evaluation of case volume in minimally invasive hepatectomy. Br J Surg 2020;107:443–451 [DOI] [PubMed] [Google Scholar]
- 41. Chen PD, Wu CY, Hu RH, Chen CN, Yuan RH, Liang JT et al. Robotic major hepatectomy: is there a learning curve? Surgery 2017;161:642–649 [DOI] [PubMed] [Google Scholar]
- 42. Halls MC, Alseidi A, Berardi G, Cipriani F, Van der Poel M, Davila D et al. A comparison of the learning curves of laparoscopic liver surgeons in differing stages of the IDEAL paradigm of surgical innovation: standing on the shoulders of pioneers. Ann Surg 2019;269:221–228 [DOI] [PubMed] [Google Scholar]
- 43. Gumbs AA, Hilal MA, Croner R, Gayet B, Chouillard E, Gagner M. The initiation, standardization and proficiency (ISP) phases of the learning curve for minimally invasive liver resection: comparison of a fellowship-trained surgeon with the pioneers and early adopters. Surg Endosc 2021;35:5268–5278 [DOI] [PubMed] [Google Scholar]
- 44. Fichtinger RS, Aldrighetti LA, Abu Hilal M, Troisi RI, Sutcliffe RP, Besselink MG et al. ; ORANGE II PLUS Collaborative . Laparoscopic versus open hemihepatectomy: the ORANGE II PLUS multicenter randomized controlled trial. J Clin Oncol 2024;42:1799–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jackson NR, Hauch A, Hu T, Buell JF, Slakey DP, Kandil E. The safety and efficacy of approaches to liver resection: a meta-analysis. JSLS 2015;19:e2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]