Abstract
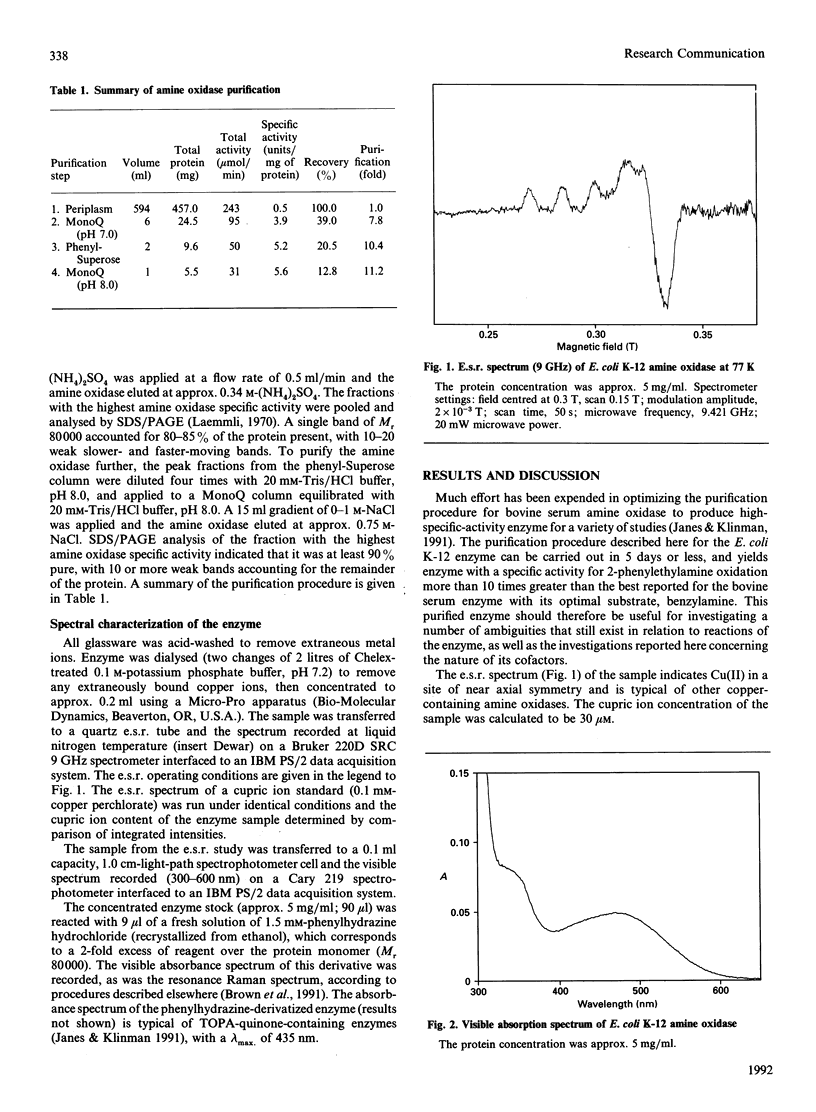
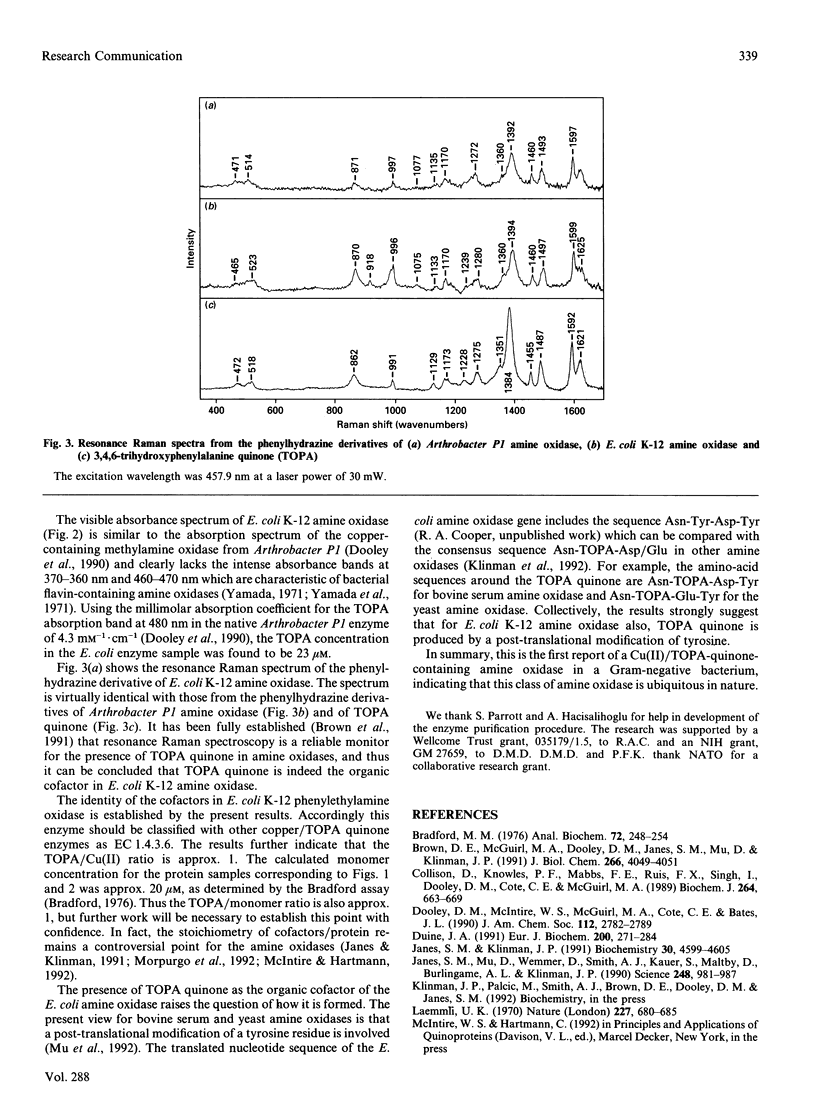
The cofactors present in a amine oxidase induced in Escherichia coli K-12 by growth on 2-phenylethylamine have been studied by spectroscopic methods. E.s.r. spectroscopy establishes the presence of cupric copper while resonance Raman spectroscopy on the phenylhydrazine derivative of the enzyme provides strong evidence for the oxidized form of 3,4,6-trihydroxyphenylalanine (TOPA) quinone. The amine oxidase should accordingly be classified as EC 1.4.3.6. This is the first report of such an amine oxidase in a Gram-negative bacterium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown D. E., McGuirl M. A., Dooley D. M., Janes S. M., Mu D., Klinman J. P. The organic functional group in copper-containing amine oxidases. Resonance Raman spectra are consistent with the presence of topa quinone (6-hydroxydopa quinone) in the active site. J Biol Chem. 1991 Mar 5;266(7):4049–4051. [PubMed] [Google Scholar]
- Collison D., Knowles P. F., Mabbs F. E., Rius F. X., Singh I., Dooley D. M., Cote C. E., McGuirl M. Studies on the active site of pig plasma amine oxidase. Biochem J. 1989 Dec 15;264(3):663–669. doi: 10.1042/bj2640663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duine J. A. Quinoproteins: enzymes containing the quinonoid cofactor pyrroloquinoline quinone, topaquinone or tryptophan-tryptophan quinone. Eur J Biochem. 1991 Sep 1;200(2):271–284. doi: 10.1111/j.1432-1033.1991.tb16183.x. [DOI] [PubMed] [Google Scholar]
- Janes S. M., Klinman J. P. An investigation of bovine serum amine oxidase active site stoichiometry: evidence for an aminotransferase mechanism involving two carbonyl cofactors per enzyme dimer. Biochemistry. 1991 May 7;30(18):4599–4605. doi: 10.1021/bi00232a034. [DOI] [PubMed] [Google Scholar]
- Janes S. M., Mu D., Wemmer D., Smith A. J., Kaur S., Maltby D., Burlingame A. L., Klinman J. P. A new redox cofactor in eukaryotic enzymes: 6-hydroxydopa at the active site of bovine serum amine oxidase. Science. 1990 May 25;248(4958):981–987. doi: 10.1126/science.2111581. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morpurgo L., Agostinelli E., Mondovi B., Avigliano L., Silvestri R., Stefancich G., Artico M. Bovine serum amine oxidase: half-site reactivity with phenylhydrazine, semicarbazide, and aromatic hydrazides. Biochemistry. 1992 Mar 10;31(9):2615–2621. doi: 10.1021/bi00124a023. [DOI] [PubMed] [Google Scholar]
- Mu D., Janes S. M., Smith A. J., Brown D. E., Dooley D. M., Klinman J. P. Tyrosine codon corresponds to topa quinone at the active site of copper amine oxidases. J Biol Chem. 1992 Apr 25;267(12):7979–7982. [PubMed] [Google Scholar]
- Parrott S., Jones S., Cooper R. A. 2-Phenylethylamine catabolism by Escherichia coli K12. J Gen Microbiol. 1987 Feb;133(2):347–351. doi: 10.1099/00221287-133-2-347. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Binetruy B., Cuzin F. High frequency of gene transfer after fusion between bacteria and eukaryotic cells. Nature. 1982 Jan 21;295(5846):257–259. doi: 10.1038/295257a0. [DOI] [PubMed] [Google Scholar]



