Abstract
Hereditary colorectal cancer syndromes, such as Lynch syndrome and familial adenomatous polyposis (FAP), present significant clinical challenges due to the heightened cancer risks associated with these genetic conditions. This review explores genetic profiling impact on surgical decisions for hereditary colorectal cancer (HCRC), assessing options, timing, and outcomes. Genotypes of different HCRCs are discussed, revealing a connection between genetic profiles, disease severity, and outcomes. For Lynch syndrome, mutations in the MLH1, MSH2, MSH6, and PMS2 genes guide the choice of surgery. Subtotal colectomy is recommended for patients with mutations in MLH1 and MSH2, while segmental colectomy is preferred for those with MSH6 and PMS2 mutations. In cases of metachronous colon cancer after segmental colectomy, subtotal colectomy with ileorectal anastomosis is advised for all mutations. Surgical strategies for primary rectal cancer include anterior resection or abdominoperineal resection (APR), irrespective of the specific mutation. For rectal cancer occurring after a previous segmental colectomy, proctocolectomy with ileal pouch-anal anastomosis (IPAA) or APR with a permanent ileostomy is recommended. In FAP, surgical decisions are based on genotype-phenotype correlations. The risk of desmoid tumors post-surgery supports a single-stage approach, particularly for certain APC gene variants. Juvenile Polyposis Syndrome (JPS) surgical decisions involve genetic testing, polyp characteristics with attention to vascular lesions in SMAD4 mutation carriers. However, genetic profiling does not directly dictate the specific surgical approach for JPS. In conclusion this review highlights the critical role of personalized surgical plans based on genetic profiles to optimize patient outcomes and reduce cancer risk. Further research is needed to refine these strategies and enhance clinical guidelines.
Keywords: Colorectal cancer, Hereditary non-polyposis colorectal cancer, Lynch syndrome, Familial adenomatous polyposis
Graphical abstract
Highlights
-
•
Hereditary colorectal cancer genetic profiling informs specific surgical approaches.
-
•
Surgical decision making can be determined through genetic, clinical and patient history.
-
•
Genetic profiling can predict risk of metachronous colon cancer.
1. Introduction
Colorectal cancer, one of the most prevalent and lethal malignancies worldwide, presents a multifaceted challenge to both patients and healthcare providers. Its occurrence and progression are influenced by a complex interplay of genetic, environmental, and lifestyle factors. However, for a subset of individuals, the risk of developing colorectal cancer is primarily rooted in their genetic makeup [1]. Hereditary colorectal cancer (HCRC) syndromes, exemplified by Lynch syndrome and familial adenomatous polyposis (FAP), constitute a distinctive and clinically intricate subset of colorectal malignancies [2]. These syndromes are characterized by germline mutations in specific genes, resulting in a significantly heightened risk of colorectal cancer development. In recent years, genetic profiling has emerged as an invaluable tool in the management of HCRC, offering insights into the genetic underpinnings of the disease [3]. The utilization of genetic profiling has notably influenced surgical decisions, enhancing risk assessment, refining treatment strategies, and guiding postoperative management for individuals affected by these syndromes [4]. FAP is an autosomal dominant polyposis syndrome caused by APC gene mutations, leading to nearly 100 % colorectal cancer risk if untreated [5]. Prophylactic surgery, such as total abdominal colectomy or total proctocolectomy, is recommended to prevent cancer [6]. In Lynch syndrome, caused by MLH1, MSH2, MSH6, or PMS2 mutations, total abdominal colectomy is advised in the presence of colon cancer due to the high chance of metachronous colorectal cancer development [6,7]. The effect of genetic profiling extends beyond mere identification to encompass critical implications for risk assessment and early detection, enabling tailored surveillance and screening strategies [8]. This comprehensive review aims to investigate the influence of genetic profiling on surgical decisions in HCRC. It will delve into the significance of genetic testing, including various types of genetic tests, genetic counseling, and their role in screening and diagnosis. The study will also examine the impact of genetic profiling on surgical decision-making, encompassing surgical options, timing of surgery, and surgical outcomes.
2. Definition and significance of hereditary colorectal cancer syndromes
HCRC syndromes are a group of genetic conditions that significantly increase an individual's risk of developing colorectal cancer. These syndromes are characterized by the inheritance of specific gene mutations that predispose affected individuals to the development of colorectal tumors. It's important to note that while these syndromes increase the risk of colorectal cancer, they are relatively rare, and most cases of colorectal cancer are sporadic [9].
Hereditary non-polyposis colorectal cancer (HNPCC), characterized as an autosomal dominant cancer syndrome, is responsible for approximately 1.7–4.2 % of all cases of colorectal cancer on a global scale, equivalent to 3 to 8 cases per one million individuals. It is notably linked to Lynch syndrome (Muir-Torre syndrome), which is the most prevalent form of HCRC [10]. Typically, Lynch syndrome presents with flat polyps located in the right colon, which give rise to colorectal cancer and other malignancies, such as endometrial, stomach, ovarian, pancreatic, ureter and renal pelvis, cerebral glioblastoma, biliary tract, small intestine cancer, sebaceous carcinomas, and keratoacanthomas. Notably, endometrial cancer tends to manifest in relatively young women [11]. Individuals affected by Lynch syndrome typically exhibit fewer than ten adenomatous polyps over the course of their lifetime [12]. Adenomas are commonly observed in patients below the age of 40 and frequently display a villous growth pattern coupled with moderate to high-grade dysplasia. In the case of individuals with Lynch syndrome, adenomas tend to progress into cancers more rapidly than they do in individuals without the syndrome who have adenomas [13,14]. Furthermore, both adenomas and colon tumors linked to Lynch syndrome are predominantly located on the ascending colon. These tumors also display distinct characteristics, including poorly differentiated medullary-type carcinoma, mucinous adenocarcinoma, the presence of signet-ring cells, and a Crohn's disease-like reaction with infiltrating lymphocytes [15]. HPCC, or Hereditary Polyposis Colorectal Cancer, is a relatively rare condition, accounting for approximately 3–5 % of all reported cases of colorectal cancer worldwide. This translates to an incidence rate of 5–9 cases per million individuals across the globe. Within this classification of genetic disorders, HPCC encompasses a diverse range of distinct syndromes, and among them, FAP stands out as a particularly well-recognized and noteworthy condition. FAP is not only prevalent within the HPCC spectrum but also holds a prominent place in the broader landscape of hereditary colorectal disorders [16]. FAP is distinguished by the presence of numerous adenomatous polyps in the colon, ranging from hundreds to thousands, with the potential to progress to colorectal cancer. However, FAP is responsible for only 1 % of the colorectal cancer cases. The impact of FAP extends beyond the colon, encompassing a wide spectrum of extracolonic manifestations, including desmoid tumors, osteomas, epidermoid cysts, papillary thyroid carcinoma, pancreatic carcinoma, gastric cancer, duodenal cancer, hepatobiliary tumors, and central nervous system (CNS) tumors. This comprehensive array of potential health concerns associated with FAP highlights the intricate and severe nature of this hereditary condition, necessitating diligent medical surveillance and management to address both the colorectal and extracolonic aspects of the disease [17]. Juvenile Polyposis Syndrome (JPS) is a rare autosomal dominant hereditary disorder characterized by the formation of multiple juvenile polyps within the gastrointestinal (GI) tract, predominantly in the colon, but also affecting the stomach and small intestine. Onset of symptoms typically occurs during childhood and includes gastrointestinal bleeding, anemia, and abdominal pain. While juvenile polyps themselves are generally benign, individuals with JPS face an elevated risk of gastrointestinal malignancies, notably colorectal cancer [18]. Peutz-Jeghers syndrome (PJS) is an uncommon autosomal dominant genetic disorder characterized by the emergence of hamartomatous polyps within the gastrointestinal tract and distinctive mucocutaneous pigmentation patterns. These benign polyps, distributed throughout the stomach, small intestine, and colon, carry the potential for complications such as intestinal obstruction and hemorrhage. The characteristic mucocutaneous pigmentation, presenting as dark pigmented spots on the lips, buccal mucosa, and extremities, manifests early in childhood [19].
3. Genetic testing in hereditary colorectal cancer syndromes
Genetic profiling of HCRC syndromes has significantly advanced understanding of the molecular basis of these conditions, leading to more personalized approaches for prevention and treatment. The identification of specific genetic markers associated with HCRC syndromes has enabled the development of targeted screening and management strategies [20].
3.1. Genetic profiling of lynch syndrome
The suspicion of Lynch syndrome should arise when evaluating an individual known as a proband who exhibits specific criteria. These criteria involve identifying tumors that are part of the Lynch syndrome spectrum, with a notable focus on colorectal cancer but not limited to it. Microsatellite instability (MSI) status stands out as a sanctioned biomarker for clinical application in colorectal cancer by regulatory authorities. The current guidelines advise conducting MSI testing for the majority of patients following a colorectal cancer diagnosis, serving dual purposes of screening for hereditary syndromes and assessing prognostic and treatment implications [21]. Suspicion arises when tumor tissue testing reveals either high MSI, a loss of expression of one or more mismatch repair (MMR) gene products (MLH1, MSH2/EPCAM, MSH6 or PMS2) through immunohistochemistry (IHC), PCR-based method MSI, or identification of a pathogenic variant in an MMR gene [22,23]. The distinct MMR genes linked to HNPCC encompass hMLH1 positioned on chromosome 3p22, hMSH2 and hMSH6 on chromosome 2p16, hPMS1 on chromosome 2q32, hPMS2 on chromosome 7p22, hMSH3 on chromosome 5q14.1, and EXO1 on chromosome 1q43. Notably, mutations in hMLH1, hMSH2, and hMSH6 collectively contribute to around 75–80 % of HNPCC cases. However, the genetic origins of the remaining 20–25 % of HNPCC cases remain undisclosed [10]. The collaboration between the MLH1 and PMS2 proteins results in the formation of the MutLα complex, a pivotal component in DNA mismatch repair. MutLα coordinates the recruitment of various proteins essential for the repair process, including DNA helicase, single-stranded-DNA binding-protein (RPA), and DNA polymerases [24,25]. Simultaneously, the MSH2 protein forms a dimer with MSH6, employing a sliding clamp model to identify mismatches. This model functions as a protein scanning mechanism, detecting errors within the DNA sequence. These molecular interactions underscore the synchronized actions of these protein dimers, ensuring the precision and fidelity of DNA replication and repair mechanisms [26]. In the context of testing for MSI, specific markers such as BAT25/26, D2S123, D5S346, and D17S250 are utilized in PCR-based methodologies to investigate the instability present in these particular loci. This group of markers includes both mononucleotide repeats (BAT25/26) and dinucleotide repeats (D2S123, D5S346, D17S250) and forms a 5-marker panel specifically employed for evaluating MSI [27]. MSI status is divided into three categories: MSI-High (MSI-H) denotes tumors with instability in over 30 % of examined markers, strongly suggesting the presence of Lynch syndrome; MSI-Low (MSI-L) indicates tumors with instability in fewer than 30 % of markers, often associated with sporadic cancers; and Microsatellite Stable (MSS) characterizes tumors demonstrating stability across all tested markers, making Lynch syndrome unlikely in such cases [28].
3.2. Genetic profiling of familial adenomatous polyposis
Comprehensive genetic testing for FAP is centered on examining the APC gene, located on chromosome 5q21.2, where pathogenic alterations drive the development of numerous adenomatous polyps in the colon and rectum. This involves detailed direct sequencing to detect point mutations, small insertions, or deletions [29]. Additionally, techniques such as Multiplex Ligation-dependent Probe Amplification (MLPA) and APC gene deletion/duplication analysis are employed to scrutinize larger structural variations [30]. Methylation analysis of the APC gene promoter region provides insights into potential modifications in gene regulation [31]. The APC gene functions as a tumor suppressor, overseeing the synthesis of APC, a substantial and multifunctional tumor-suppressing protein. This protein serves as a crucial "gatekeeper," preventing the formation of tumors. Specifically, APC regulates β-catenin, a protein integral to cellular communication, signaling, growth, and controlled destruction. Unchecked β-catenin activity can lead to the onset of various cancers. An impairment in the APC gene compromises the effectiveness of APC, increasing the likelihood that some cells, which should have been restrained by APC, will persistently develop and transition into cancerous cells over time. In the context of familial polyposis, these anomalies typically manifest as polyps—minute irregularities on the surface of the intestinal tract [32]. In addition to the APC gene, there are other genes that can be associated with familial adenomatous polyposis, albeit in rarer cases. Mutations in the MUTYH gene, which is involved in DNA repair, can also lead to a condition called MUTYH-associated polyposis (MAP), characterized by the development of polyps in the colon. MUTYH is situated on chromosome 1p34.3–p32. Nevertheless, individuals carrying monoallelic MUTYH mutations within families affected by MAP did not exhibit an elevated risk of colorectal cancer [33].
3.3. Genetic profiling of Juvenile Polyposis Syndrome
Various molecular genetic testing strategies are available for individuals suspected of having JPS, including BMPR1A and SMAD4 (commonly referred to as MADH4/DPC4) concurrent testing, serial single-gene testing, multigene panel use, and more comprehensive genomic testing. For those with clinical features suggestive of JPS, BMPR1A and SMAD4 concurrent testing involves initial sequence analysis, including the examination of promoter regions, and gene-targeted deletion/duplication analysis. If no pathogenic variant is identified, the consideration of a multigene panel, encompassing genes like PTEN, is recommended [34]. Serial single-gene testing is an alternative, beginning with sequence analysis and deletion/duplication analysis of SMAD4, followed by similar analysis of BMPR1A if no SMAD4 pathogenic variant is found. Additional molecular genetic testing of HHT-related genes may be considered if SMAD4 or BMPR1A pathogenic variants are not identified. A multigene panel, including BMPR1A, SMAD4, and other relevant genes, particularly PTEN, may be considered for individuals with JPS. It's important to note that the genes included in multigene panels and their sensitivity may vary, and understanding the limitations of the panel is crucial for interpretation. Chromosomal microarray analysis for detecting deletions in the 10q22-q23 region, involving BMPR1A or both BMPR1A and PTEN, may reveal additional clinical features [35,36].
3.4. Genetic profiling of Peutz-Jeghers syndrome
In the molecular diagnosis of PJS, various genetic testing approaches are considered based on the phenotype observed. Single-gene testing initiates with sequence analysis of STK11, aiming to identify small intragenic deletions/insertions, missense, nonsense, and splice site variants. However, limitations exist, as certain sequencing methods may not detect single-exon, multiexon, or whole-gene deletions/duplications. If no variant is detected through sequencing, subsequent gene-targeted deletion/duplication analysis is performed simultaneously to decrease turnaround time. In cases where no pathogenic STK11 variant is identified, exploring an alternate DNA source for somatic mosaicism is recommended. Additionally, a multigene panel, encompassing STK11 and relevant genes, may be considered, though variations in gene inclusion and panel sensitivity exist among laboratories [37,38] (Table 1).
Table 1.
Approach to testing Strategies, genes involved, and clinical implications of different hereditary colorectal cancer syndromes.
| Hereditary colorectal cancer syndromes | Genetic Markers/Testing Strategies | Inheritance pattern | Genes Involved | Clinical Implications |
|---|---|---|---|---|
| Lynch Syndrome | - Criteria for suspicion: specific tumors within Lynch syndrome spectrum, emphasizing colorectal cancer. - Testing methods: MSI, IHC, PCR, identification of MMR gene variants. | Autosomal dominant | - MMR genes: hMLH1, hMSH2, hMSH6, hPMS1, hPMS2, hMSH3, EXO1. On chromosomes (3p21.3; 2p22-p21; 2p16; 2q31–33; 7p22; 18q21) - MutLα complex formation involving MLH1 and PMS2. - MSH2-MSH6 dimer for mismatch detection. |
- MSI Status: MSI-High (Lynch syndrome likely), MSI-Low, Microsatellite Stable (Lynch syndrome unlikely). - Implications for prognosis and treatment. |
| Familial Adenomatous Polyposis (FAP) and MUTYH associated polyposis | - Comprehensive testing of APC gene (direct sequencing, MLPA, deletion/duplication analysis, methylation analysis). | -Autosomal dominant -Autosomal recessive |
- APC gene on chromosome 5q21.2. - Other genes (rare cases): MUTYH on chromosome 1p34.3–p32. |
- APC as tumor suppressor. - Development of adenomatous polyps. - Risk of colorectal cancer. |
| Juvenile Polyposis Syndrome (JPS) | - Testing strategies: BMPR1A and SMAD4 concurrent testing, serial single-gene testing, multigene panels. | Autosomal dominant | - BMPR1A (on chromosome 10q22-q23), SMAD4 (MADH4/DPC4), PTEN (for multigene panels). | - Clinical features guide testing approach. - Consideration of multigene panels for comprehensive evaluation. |
| Peutz-Jeghers Syndrome (PJS) | - Testing approaches: Single-gene testing (STK11), deletion/duplication analysis, alternative DNA source for mosaicism, multigene panels. | Autosomal dominant | - STK11 (on chromosome 19p13.3). - Other relevant genes (for multigene panels). | - Diagnosis based on phenotype. - Consideration of alternate DNA sources. - Variability in gene inclusion and panel sensitivity. |
4. Genetic counseling for hereditary colorectal cancer syndromes
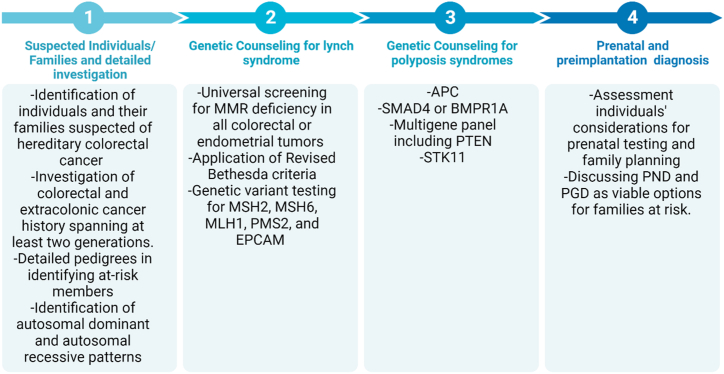
Genetic counseling for HCRC is a comprehensive process that involves assessing an individual's familial and personal medical history, discussing the option of genetic testing, and providing information about the associated risks and implications. Genetic counselors play a key role in supporting informed decision-making, educating individuals about the genetics of colorectal cancer, and addressing psychosocial aspects of the genetic testing process. Through personalized risk management strategies, coordination with healthcare providers, and facilitating family communication, genetic counselors empower individuals to navigate their hereditary cancer risk with knowledge and emotional support. Staying abreast of genetic advancements, these professionals contribute to ongoing updates and improvements in the management of HCRC [39,40]. In the initial step of genetic counseling, suspected individuals and their families, spanning at least two generations, should undergo a detailed investigation of colorectal cancer, endometrial cancer, and other malignancies. Generating a comprehensive family pedigree is crucial for assessing familial cancer risk, understanding inheritance patterns, and identifying the proband [41].
4.1. Genetic counseling for lynch syndrome
Genetic counseling for Lynch syndrome involves informing individuals about the complexities of genetic testing, emphasizing potential challenges in interpreting results, such as variants of unknown significance (VUS) and the sensitive nature of genetic information shared within families [42]. The shift from traditional single-gene tests to NGS multi-gene panels is highlighted, emphasizing benefits like simultaneous screening of multiple genes, reduced turnaround time, and cost-effectiveness. However, challenges, including interpreting incidental findings and VUS, are acknowledged [43]. Family counseling addresses the importance of detailed pedigrees in identifying at-risk members and recognizes the role of family relationships in disclosure dynamics. The discussion extends to family planning options, incorporating ethical considerations and legal implications surrounding prenatal and preimplantation genetic diagnoses, with consensus-building emphasized for informed decision-making [44]. The family pedigree is instrumental in clinical decision-making, helping determine eligibility for confirmatory genetic testing based on criteria like the Amsterdam II or revised Bethesda guidelines. Computational models like PREMM1,2,6 and MMRpro, assessing Lynch syndrome probability, rely on family pedigree information when direct tumor sequencing is impractical. Additionally, the family pedigree assists in efficiently guiding genetic testing, distinguishing individuals who may benefit from those with no Lynch syndrome risk. It also serves as educational material for patients and their families [45]. Screening universally for MMR deficiency is advocated for all colorectal or endometrial tumors, without regard to the age at diagnosis. This encompasses cases of colorectal cancer diagnosed before the age of 50, instances of synchronous or metachronous colorectal or other Lynch syndrome-associated tumors, irrespective of age, and situations where colorectal cancer is identified in a first-degree relative under the age of 50 [46,47]. The Revised Bethesda criteria further pinpoint individuals with colorectal cancer diagnosed in two or more first- or second-degree relatives, regardless of age. The process involves assessing MSI or conducting immunohistochemical analysis, with negative outcomes suggesting a lower likelihood of Lynch syndrome. Additional examinations, such as MLH1 promoter methylation and/or BRAF p.V600E variant testing, are recommended for specific MSI patterns [48]. Subsequent steps involve scrutinizing methylation status, genetic variant testing for MSH2, MSH6, MLH1, and PMS2, as well as considering EPCAM deletion. The results guide the determination of Lynch syndrome and provide insights for testing at-risk family members based on the identified variants or their absence [49].
4.2. Genetic counseling for familial adenomatous polyposis
Genetic counseling for FAP is an essential component of managing individuals and families affected by this hereditary condition. FAP is an autosomal dominant genetic disorder characterized by the development of numerous adenomatous polyps in the colon, leading to a high risk of colorectal cancer [50]. Testing for hereditary colorectal polyposis is recommended for individuals meeting specific criteria. This includes those with a personal history of 20 or more cumulative adenomas, the presence of multifocal/bilateral congenital hypertrophy of retinal pigment epithelium (CHRPE), and individuals with a personal history of between 10 and 19 cumulative adenomas, a desmoid tumor, hepatoblastoma, cribriform-morular variant of papillary thyroid cancer, unilateral CHRPE, or those meeting the criteria for serrated polyposis syndrome with at least some adenomas [12]. Genetic counseling and consistent monitoring are essential for tailoring the treatment of families affected by cancer due to APC gene expression deficiency. Presently, there are over 2000 documented pathogenic variations in the APC gene, predominantly located in the mutation cluster region (MCR) at the 5′-ends of exon 15 [51]. Notably, codons 1309 and 1061 serve as hotspots for mutations, representing approximately 17 % and 11 % of all germline APC mutations, respectively [52]. Individuals with MCR mutations typically experience a more unfavorable prognosis, with early manifestation of their condition. Identifying the precise APC mutation within a family can facilitate targeted sequencing tests for at-risk family members who have yet to exhibit symptoms [53]. Childhood DNA testing, prenatal diagnosis (PND), and preimplantation genetic diagnosis (PGD) are viable options for individuals from families at elevated risk of FAP [54]. In the majority of cases, FAP follows an autosomal dominant pattern, with an affected parent passing on the condition to their offspring. This familial pattern allows for cascade genetic counseling and testing to identify at-risk family members. However, a significant proportion (about 25 %) of FAP cases result from de novo pathogenic variants, not inherited from either parent. In such cases, where the variant cannot be identified in the parents' DNA, the concept of germline mosaicism is introduced. This concept influences the recommendation for testing, suggesting that while testing siblings of an affected individual is advisable, it is not typically indicated for aunts, uncles, and cousins of the proband unless clear evidence of familial inheritance is present. The nuanced approach to genetic testing considers both familial patterns and de novo variants, ensuring a comprehensive understanding of the genetic basis of FAP within a family context [55].
4.3. Genetic counseling for Juvenile Polyposis Syndrome
JPS follows an autosomal dominant inheritance pattern, wherein up to half of affected individuals inherit the condition from an affected parent. Intriguingly, around 50 % of individuals diagnosed as probands with JPS lack a familial history of polyps, indicating the potential occurrence of a de novo pathogenic variant. In the context of familial inheritance, each child born to an affected individual carries a 50 % likelihood of inheriting the pathogenic variant and subsequently developing JPS [18]. For pregnancies at an increased risk, prenatal testing becomes a viable option, providing insights into the genetic status of the developing fetus. Additionally, advancements in genetic technology have paved the way for PGD, offering prospective parents the opportunity to screen embryos for the pathogenic variant before implantation, thus mitigating the risk of JPS transmission in subsequent generations when the familial variant is known [56]. Genetic testing for the prediction of particular familial mutation has been found in either affected parents or sibling. If there is no identified pathogenic gene mutation in the affected parent or sibling, the child is ineligible for predictive genetic testing and would need to undergo endoscopic screening instead [57]. It's crucial to recognize that a gene mutation may only be identified in around 40 %–60 % of individuals with JPS. In instances where no pathogenic SMAD4 or BMPR1A variant is found, opting for a multigene panel that includes PTEN is recommended [58].
4.4. Genetic counseling for Peutz-Jeghers syndrome
The combined probability of gastrointestinal tumor development in individuals with PJS is approximately 20 % by the age of 40, rising to more than 70 % in patients reaching their eighth decade of life. The uncertainty surrounding this risk, coupled with the substantial clinical implications of PJS, has a notable impact on the overall quality of life experienced by individuals affected by this syndrome [59]. During genetic counseling, individuals with PJS have expressed a consideration for prenatal testing if identified as carriers of the pathogenic STK11 mutation. This proactive approach aims to prevent the birth of children affected by the syndrome. Valuable options for families at an elevated risk of PJS include PND and PGD. There is limited literature available on the utilization of PND and PGD in the context of PJS. In one case described by Wang et al., a positive PND result for PJS was reported, leading to the decision to proceed with the pregnancy, and PGD was subsequently recommended for future pregnancies [60]. An additional study conducted by Woo et al. surveyed 38 individuals with PJS, revealing that 40 % adjusted their reproductive choices due to the syndrome, and 33 % expressed reluctance to have children due to the associated risks, emphasizing the necessity for reproductive counseling [61]. Another survey by Van Lier et al. involving 52 PJS patients indicated that PJS influenced family planning decisions for nearly a third of respondents, with considerations including termination and acceptance of PGD. In light of these findings and experiences with other predisposition syndromes, it is proposed that PJS should be acknowledged as an indication for PND and PGD, underscoring the importance of counseling individuals with PJS about their reproductive options [62]. While some studies have identified specific mutations in the STK11 gene during PND and PGD testing, enhancing the precision and effectiveness of these genetic screening methods [63], findings from specific studies others indicated that in instances of prenatal testing, it was reported that the mutation was not inherited by the fetus, and as a result, the pregnancy proceeded [64] (Fig. 1).
Fig. 1.
Genetic counseling steps for hereditary colorectal cancer syndromes.
5. Surgical options and decision making
Surgery for HNPCC can serve both therapeutic and prophylactic purposes, primarily addressing colon cancer. Surgical choices encompass either segmental resection or total abdominal colectomy (TAC) [65]. The preference for TAC arises from the elevated risk of metachronous neoplasia in the remaining colon. While retrospective studies indicate a reduced risk of metachronous colon cancer following TAC compared to segmental resection, there is a lack of prospective trials supporting a definitive survival advantage for TAC [66]. The likelihood of developing metachronous colon cancer after partial colectomy ranges from 11 to 45 % over 8–13 years. Segmental resection carries a cumulative risk of metachronous colon cancer, reaching 62 % at 30 years. The degree of segmental resection is inversely linked to the risk of metachronous cancer. Survival rates at 5 and 10 years show no substantial disparity between TAC and segmental resection for individuals with HNPCC and colon cancer [67].
5.1. Surgical decision for lynch syndrome
It is recommended that individuals with Lynch syndrome who have completed childbearing or are postmenopausal undergo total abdominal hysterectomy and bilateral salpingo-oophorectomy simultaneously to minimize the risk of endometrial and ovarian cancers [68]. Malik et al. conducted a systematic review and meta-analysis to assess the risk of MCC and mortality in Lynch syndrome following either segmental or extensive colectomy. The findings of this study suggested a fivefold greater risk of MCC after segmental colectomy compared to extensive colectomy in individuals with Lynch syndrome [69]. Approximately 20 %–30 % of individuals diagnosed with Lynch syndrome may develop rectal cancer, and within this group, 15 %–24 % initially present with rectal cancer. Surgical options include either a low anterior resection or abdomino-perineal resection, depending on sphincter involvement [70]. Another option is an extended resection, involving the removal of all colorectal tissue at risk. This extended resection can be accomplished through a total proctocolectomy with an end ileostomy (TPC-EI) or, more commonly, a restorative ileal pouch-anal anastomosis (IPAA) [71]. Preventive surgical interventions for Lynch Syndrome encompass primary, secondary, and tertiary measures. In cases where a patient lacks colonic lesions, primary prophylactic colorectal surgery is generally not recommended, and endoscopic management is favored, associated with a 70 % reduction in the risk of death as per earlier statistical models [72]. However, primary prophylactic colon surgery may be considered for lynch syndrome patients with endometrial cancer (EC), particularly if it represents the initial manifestation of lynch syndrome, owing to the heightened subsequent risk of colorectal cancer [73]. Secondary prophylactic surgery is primarily directed at colorectal cancer, dysplasia, or adenoma, providing choices such as segmental or total colectomy based on lesion location. Surgical decisions take into account patient-specific factors, morbidity, functional consequences, impact on quality of life, and the risk of developing metachronous lesions. Tertiary prophylactic surgery pertains to cases where the lynch syndrome diagnosis is established post-operatively, involving strategies ranging from endoscopic surveillance to extended surgical resection. For women with lynch syndrome, discussions and gynecologic examinations regarding prophylactic surgery typically commence at age 45 or five years before the occurrence of the first family case of endometrial cancer, acknowledging the intricacies of decision-making due to uncertainties in lynch syndrome diagnosis at the time of surgery.
5.2. Surgical decision for familial adenomatous polyposis
Prophylactic colorectal surgery is crucial in managing FAP, given the absolute risk of colorectal cancer by the age of 40 if left untreated [74]. Surgical decision-making, encompassing the timing of prophylactic surgery, extent of bowel resection, and type of reconstruction, is intricately guided by patient-specific factors and disease characteristics [75]. FAP patients have three primary surgical options, including subtotal colectomy with ileorectal anastomosis (IRA), total proctocolectomy with or without mucosectomy and IPAA, and TPC-EI. Key principles of oncologic bowel resection involve the high ligation of the primary blood supply to the bowel and removal of its mesentery [76]. Colectomy is typically recommended when there are multiple polyps exceeding 10 mm in size, polyps displaying high-grade dysplasia, or a notable rapid increase in the polyp count [77]. However, the decision should involve both the healthcare provider and the patient, considering social, educational, and career aspects. Optimal timing aligns with the severity of polyposis and the patient's preferences, ensuring a medically sound decision that respects their overall life circumstances [78]. After deciding on the need for colectomy, the next step involves choosing the type of operation – either subtotal colectomy with IRA/ISA or a more extensive proctocolectomy with IPAA. Performing end ileostomy is rare but may be necessary in specific cases. The decision to preserve the rectum depends on the rectal polyp burden at colectomy, with rectal cancer risk varying [79]. Introduction of Ileal Pouch-Anal Anastomosis (IPAA) in 1978 significantly reduced rectal cancer incidence after colectomy. In low-polyp burden cases like MAP, IRA is a reasonable choice, provided strict endoscopic surveillance [80]. After undergoing colectomy, individuals with MAP encounter the potential risk of developing metachronous colorectal cancer, with reported rates as elevated as 17 %. This heightened risk may be attributed to an accelerated process of carcinogenesis [81].
5.3. Surgical decision for Juvenile Polyposis Syndrome
The decision to opt for surgical intervention in JPS is contingent upon several pivotal factors, underscoring the imperative for timely medical action. These factors encompass an excessive accumulation of polyps, substantial blood loss leading to persistent anemia, and the identification of juvenile polyps displaying characteristics indicative of severe dysplasia or malignancy. Furthermore, a pronounced familial predisposition to colorectal cancer amplifies the necessity for contemplating surgical measures. Within the spectrum of surgical interventions for JPS, colectomy emerges as a prominent option, encompassing variations from segmental or subtotal colectomy to the more encompassing total colectomy. This procedure entails an IRA, offering a surgical remedy to alleviate the impact of the polyp burden and associated complications. Conversely, an alternative viable approach involves a TPC with IPAA [82,83].
5.4. Surgical decision for Juvenile Polyposis Syndrome
For PJS patients with polyps exceeding 0.5 cm detected during upper endoscopy and colonoscopy, it is advisable to consider endoscopic removal [84]. Resection of other small bowel polyps, particularly those surpassing 1 cm or causing symptoms, is recommended to minimize the risk of complications associated with polyps, including anemia, bleeding, obstruction, malignancy, and the potential requirement for urgent surgery [85]. Progress in small bowel endoscopy has elevated the efficacy of non-surgical approaches to small bowel polyps. Successful polyp extraction has been documented with balloon-assisted enteroscopy, encompassing both single and double balloon procedures [84]. The use of double balloon enteroscopy may present challenges in individuals with a history of abdominal surgery, marked by peritoneal adhesions and altered anatomy. Those with PJS confront an increased likelihood of perforation during polyp removal due to serosal invagination within the polyp stalk [86]. Surgical intervention becomes necessary when endoscopic management of polyps is unachievable due to their size or number, or in the presence of neoplasia. Surgery may also be indicated for patients with small bowel obstruction or intussusception. In cases of laparotomy for individuals with PJS, intraoperative enteroscopy is recommended for the identification and excision of small bowel polyps [87].
6. The impact of genetic profiling on surgical decisions and timing
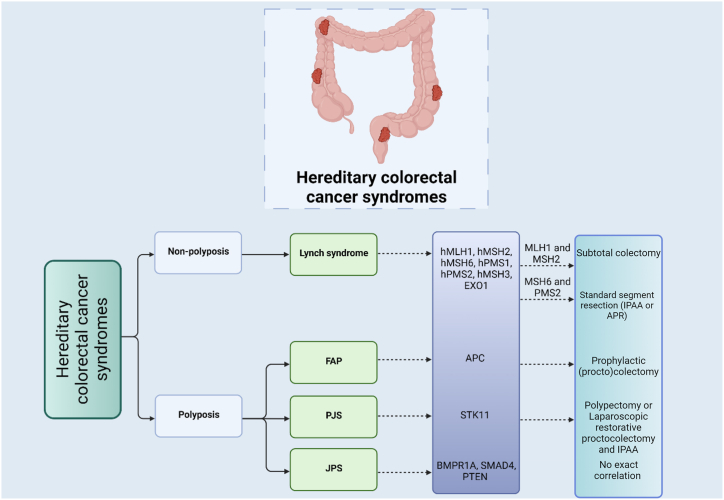
The influence of genetic profiling on surgical decisions in Lynch syndrome is profound. The identification of specific genetic mutations associated with Lynch syndrome has significantly impacted the approach to surgical interventions. With a deeper understanding of the genetic factors involved, healthcare professionals can tailor surgical strategies to the individual's unique genetic profile. Research findings have indicated the occurrence of metachronous colon cancer following surgical procedures for rectal cancer in individuals with path_MMR mutations [88]. Nevertheless, the documented instances of such occurrences are notably lower compared to those of colon cancer [89]. Consequently, there is insufficient empirical evidence to advocate for extended surgery specifically targeting primary rectal cancers in individuals with path_MMR mutations. Consequently, both national and international guidelines suggest anterior resection or abdominoperineal resection (APR) as the preferred primary surgical interventions in these cases [90]. For patients with rectal cancer subsequent to prior segmental colectomy, the European expert group recommends either proctocolectomy with IPAA or APR with a permanent ileostomy. However, it's important to note that there is a lack of available data to substantiate this recommendation [88]. Individuals with variants in MLH1 and MSH2 genes face an elevated risk of developing metachronous colorectal cancer according to current research, emphasizing the potential benefits of extended surgical interventions. Engaging in more extensive surgery in these cases could potentially reduce the necessity for subsequent bowel surgeries [91]. Nevertheless, performing an extended resection does not directly contribute to an improvement in colorectal cancer-related mortality [92]. Reduced cumulative risks of both primary and metachronous colorectal cancer in individuals carrying variants in MSH6 and PMS2 genes justify the adoption of standard segment resection. However, for MSH6 and PMS2 patients with a history of challenging surveillance colonoscopies, there is a rationale for considering extended bowel resection. This is due to the potential complications arising from postoperative bowel adhesions that could further hinder the effectiveness of subsequent endoscopic procedures [88]. The surgical options for colorectal cancer in individuals with path_MMR mutations vary based on the specific gene involved. For primary colon cancer, subtotal colectomy is recommended for those with Path_MLH1 and Path_MSH2 mutations, while segmental colectomy is suggested for those with Path_MSH6 and Path_PMS2 mutations. In cases of metachronous colon cancer after segmental colectomy, the preferred approach is subtotal colectomy with ileorectal anastomosis for all path_MMR mutations. For primary rectal cancer in path_MMR carriers, the recommended surgical procedure is anterior resection or APR, regardless of the specific path_MMR mutation. In instances where rectal cancer occurs after a previous segmental colectomy, the European expert group advises either proctocolectomy with IPAA or abdominoperineal resection with a permanent ileostomy for all path_MMR mutations. It is noteworthy that these recommendations are based on available data and expert opinion, as there may be limited empirical evidence to support certain surgical options [93].
Genotyping the APC gene emerges as a valuable tool for tailoring the management of individuals with FAP [94]. Sarvepalli and collaborators devised a predictive model that demonstrated accuracy levels surpassing 80 % in predicting the probability of surgery within a 2- or 5-year timeframe. Notably, they classified the correlation between genotype and phenotype into four distinct groups: attenuated phenotype (involving mutations in codon <157 or >1595, or alternatively spliced 9th exon), intermediate phenotype (encompassing mutations in codon 157–1249 and 1464–1595, excluding alternatively spliced 9th exon), severe phenotype (linked to mutations in codon 1250–1464), and large APC deletion. The study underscored a substantial connection between the severity of the phenotype and the time elapsed between the initial colonoscopy and the inaugural prophylactic surgery. Specifically, the cohort characterized by large APC deletions demonstrated the briefest duration before undergoing surgery [95]. Certain patients manifest a less severe variant of FAP termed attenuated FAP (aFAP). This distinction from classical FAP relies on factors such as polyp phenotype and family history. Those with aFAP typically present with a lower number of colorectal polyps (ranging from few to one-hundred), experience onset later in life, and often have rectal sparing. The risk of CRC is estimated to be around 70 % by the age of 80, occurring at an average age of approximately 30 years. Given the later onset, the use of colonoscopy with polypectomy is frequently effective, potentially delaying the necessity for surgery until patients reach their 30s or 40s [96]. Recent research conducted by Mori et al., in 2023 [97] proposes that distinct interventional strategies based on genotypes can be considered in the clinical management of FAP patients. Specifically, they observed that individuals with certain germline variants in attenuated FAP-associated regions (Genotype-1) exhibited a lower risk of colorectal cancer stages II–IV compared to those with other variants (Genotype-2). The study highlighted that the risk of developing stage II–IV CRC significantly increased at different ages for Genotype-1 and Genotype-2 patients, suggesting a potential basis for guiding the timing of prophylactic (procto)colectomy. Despite these promising findings, it is imperative to note that the proposed approach awaits validation through further prospective studies, particularly those focusing on long-term endoscopic intervention and determining the optimal age for prophylactic (procto)colectomy. Therefore, while genotyping of the APC gene can provide valuable insights, the decision-making process regarding the type of surgery in FAP should be comprehensive. This involves a thorough assessment of the patient's condition, considering factors such as the number and size of polyps, the presence of symptoms, and the overall health status. The integration of genetic information into the broader clinical context ensures a more nuanced and personalized approach to surgical decisions for individuals with FAP. There is indication that the likelihood of developing desmoid tumors rises after abdominal surgery, with a potentially higher risk associated with procedures necessitating two stages. Those with an elevated risk for desmoids, such as women, individuals with APC pathogenic variants in codons 1395–1493, and those with a family history of desmoids, are advised to contemplate a surgical approach that is likely to be completed in a single stage. This is recommended to minimize the necessity for a subsequent surgery [5].
As mentioned JPS is associated with germline mutations in certain genes, including SMAD4 and BMPR1A. These mutations are associated with an increased risk of juvenile polyps in the gastrointestinal tract, including the colon and rectum. However, the association between specific genes and the degree of dysplasia in the polyps is not always straightforward and might not help choosing the best surgical option. Mostly, finding the best option for the surgery requires a combination of genetic testing, the extent and location of polyps, and individual patient characteristics [98]. Individuals harboring SMAD4 pathogenic mutations should be mindful of vascular lesions associated with hereditary hemorrhagic telangiectasia (HHT) [99].
Surveillance in PJS advises polypectomy, primarily to avert complications rather than significantly altering cancer risk, given the absence of dysplasia in PJS polyps. The primary objective of polypectomy is to prevent complications such as anemia and bleeding, but quantifying this prevention is challenging, and there is a lack of supporting data. In children with PJS, intussusception poses a notable clinical concern, with a cumulative risk ranging from 50 % to 68 % during childhood. Limited therapeutic options are available for the management of PJS concurrent with rectal cancer [100]. Surgical approaches are typically employed to address PJS-related complications, like small bowel intussusception or neoplastic lesions, often necessitating enterectomy. Notably, there is a lack of research specifically investigating laparoscopic restorative proctocolectomy with IPAA in PJS patients [101] (Fig. 2).
Fig. 2.
Illustration of the impact of various genetic profiles on the surgical approach in the management of hereditary colorectal cancer syndromes.
7. Critical view
The application of genetic profiling technologies in surgical decision-making is heavily dependent on the precision and reliability of these tools [102]. Challenges persist in obtaining consistently accurate genetic data, encompassing issues from interpreting variants to identifying new mutations. Additionally, the integration of diverse genetic information into a unified framework for surgical planning necessitates continuous refinement of analytical methods [103]. The American College of Medical Genetics and Genomics (ACMG) has developed a system to classify genetic variants into five categories based on their potential to cause disease: Pathogenic (P), Likely Pathogenic (LP), Variant of Uncertain Significance (VUS), Likely Benign (LB), and Benign (B). This classification system aids clinicians in understanding the clinical significance of genetic variants and tailoring patient care accordingly. Variants identified as pathogenic or likely pathogenic are considered actionable from a clinical standpoint, necessitating specific monitoring and treatment for associated diseases. On the other hand, Variants of Uncertain Significance (VUS) present a challenge due to the uncertainty surrounding their impact on gene function. This uncertainty complicates their role in genetic disorders, emphasizing the need for ongoing monitoring and thorough genetic counseling to evaluate their clinical significance [104]. Another notable challenge emerges in translating genetic findings into actionable insights for surgeons. Clinicians are confronted with the intricate task of interpreting complex genetic data, comprehending the nuanced implications of specific mutations, and assessing their relevance to surgical strategies [105]. Bridging the gap between genetic knowledge and practical clinical application requires collaborative efforts in education and multidisciplinary approaches. With the increasing prevalence of genetic profiling, effective communication between healthcare providers and patients becomes paramount [106]. Navigating the delicate balance of conveying potential risks and benefits, uncertainties, and the enduring impact of surgical decisions presents ethical and communication challenges. The evolving landscape of genetic testing also prompts considerations regarding the appropriate scope and depth of informed consent processes. Ongoing research efforts are essential for advancing the understanding of the genetic foundations of HCRC syndromes. The refinement of predictive models that encompass a broader spectrum of genetic factors, environmental influences, and lifestyle considerations is crucial for enhancing the accuracy and clinical significance of genetic profiling, ultimately enabling more precise surgical recommendations [107]. Looking ahead, the future of genetic profiling involves embracing multi-omics strategies that extend beyond DNA sequencing. Integrating data from genomics, transcriptomics, epigenomics, and proteomics will furnish surgeons with a comprehensive molecular landscape, offering a more holistic perspective on the disease and its potential trajectories [108]. This comprehensive approach holds the potential to uncover novel therapeutic targets and guide personalized surgical interventions. As artificial intelligence progresses, the analysis of data illustrating the connection between the genotype and phenotype in HCRCs can aid in distinguishing various patient classifications and forecasting the results of ongoing surgical procedures [109].
8. Conclusions
In conclusion, this review underscores the pivotal role of genetic profiling in guiding surgical decisions for HCRC syndromes such as Lynch syndrome and FAP. The understanding of genotypes, particularly mutations in genes like MLH1, MSH2, MSH6, PMS2, and APC, allows for tailored surgical strategies that optimize patient outcomes and mitigate cancer risks. For instance, subtotal colectomy is recommended for Lynch syndrome patients with MLH1 and MSH2 mutations, while segmental colectomy is preferred for those with MSH6 and PMS2 mutations. The genotype-phenotype correlations in FAP further inform surgical timing and approaches, with considerations for desmoid tumor risks influencing the choice of single-stage surgeries. The findings highlight the importance of personalized surgical plans that account not only for the genetic profile but also for the individual's cancer history and risk of metachronous cancer. Such personalized strategies are essential in improving long-term survival and quality of life for patients with HCRC. Despite these advances, further research is necessary to refine these surgical guidelines and ensure they remain aligned with the latest genetic discoveries and clinical practices. This ongoing research will enhance the precision of clinical decision-making and the efficacy of surgical interventions for HCRC.
CRediT authorship contribution statement
Yasaman Goudarzi: Writing – review & editing, Writing – original draft. Khaterehsadat Monirvaghefi: Writing – review & editing, Writing – original draft, Conceptualization. Salar Aghaei: Writing – original draft, Visualization. Seyed Siamak Amiri: Writing – review & editing, Writing – original draft. Mahdi Rezaei: Writing – original draft. Atefeh Dehghanitafti: Visualization, Conceptualization. Ali Azarpey: Writing – original draft. Alireza Azani: Writing – original draft, Visualization. SeyedAbbas Pakmehr: Writing – original draft. Hamid Reza Eftekhari: Writing – original draft, Visualization, Conceptualization. Safa Tahmasebi: Writing – original draft. Shahriar Zohourian Shahzadi: Visualization, Project administration, Conceptualization. Mansour Rajabivahid: Writing – review & editing, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
All figures in this study was designed by biorender.com. This study was not financially supported with any organizations.
Contributor Information
Alireza Azani, Email: Azalireza954@gmail.com.
Shahriar Zohourian Shahzadi, Email: shzohourian@gmail.com.
Mansour Rajabivahid, Email: rajabi.vahid@zums.ac.ir.
References
- 1.Song M., Chan A.T. Environmental factors, gut microbiota, and colorectal cancer prevention. Clin. Gastroenterol. Hepatol. 2019;17(2):275–289. doi: 10.1016/j.cgh.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valle L., et al. Update on genetic predisposition to colorectal cancer and polyposis. Mol. Aspect. Med. 2019;69:10–26. doi: 10.1016/j.mam.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Valle L., et al. Genetic predisposition to colorectal cancer: syndromes, genes, classification of genetic variants and implications for precision medicine. J. Pathol. 2019;247(5):574–588. doi: 10.1002/path.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoun R.J.N., Kalady M.F. The importance of genetics for timing and extent of surgery in inherited colorectal cancer syndromes. Surgical Oncology. 2022;43 doi: 10.1016/j.suronc.2022.101765. [DOI] [PubMed] [Google Scholar]
- 5.Yen T., et al. 2022. APC-Associated Polyposis Conditions. [PubMed] [Google Scholar]
- 6.Vogelsang H.E. Prophylactic surgery and extended oncologic radicality in gastric and colorectal hereditary cancer syndromes. Visc. Med. 2019;35(4):231–239. doi: 10.1159/000501919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llach J., Pellisé M., Monahan K. Lynch syndrome; towards more personalized management? Best Pract. Res. Clin. Gastroenterol. 2022;58 doi: 10.1016/j.bpg.2022.101790. [DOI] [PubMed] [Google Scholar]
- 8.Kastrinos F., Samadder N.J., Burt R.W. Use of family history and genetic testing to determine risk of colorectal cancer. Gastroenterology. 2020;158(2):389–403. doi: 10.1053/j.gastro.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Wells K., Wise P.E. Hereditary colorectal cancer syndromes. Surgical Clinics. 2017;97(3):605–625. doi: 10.1016/j.suc.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Gay J.T., Troxell T., Gross G.P. StatPearls Publishing Copyright © 2023. StatPearls Publishing LLC.; Treasure Island (FL): 2023. Muir-torre syndrome, in StatPearls. [PubMed] [Google Scholar]
- 11.Tiwari A.K., Roy H.K., Lynch H.T. Lynch syndrome in the 21st century: clinical perspectives. QJM: Int. J. Med. 2016;109(3):151–158. doi: 10.1093/qjmed/hcv137. [DOI] [PubMed] [Google Scholar]
- 12.Kalady M.F., Heald B. Diagnostic approach to hereditary colorectal cancer syndromes. Clin. Colon Rectal Surg. 2015;28(4):205–214. doi: 10.1055/s-0035-1564432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vleugels J.L.A., et al. CD31-positive microvessel density within adenomas of Lynch Syndrome patients is similar compared to adenomas of non-Lynch patients. Endosc. Int. Open. 2019;7(5):E701–e707. doi: 10.1055/a-0832-8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kushnir V.M., et al. Advanced colorectal adenomas in patients under 45 years of age are mostly sporadic. Dig. Dis. Sci. 2014;59(11):2757–2764. doi: 10.1007/s10620-014-3245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn G.D., et al. Hereditary nonpolyposis colorectal cancer in association with crohn's disease and lynch syndrome: the importance of a strict endoscopic surveillance. Case Reports in Gastroenterology. 2022;16(1):116–121. doi: 10.1159/000521919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karstensen J.G., et al. Colorectal cancer in individuals with familial adenomatous polyposis, based on analysis of the Danish polyposis registry. Clin. Gastroenterol. Hepatol. 2019;17(11):2294–2300.e1. doi: 10.1016/j.cgh.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Righetti A.E., et al. Familial adenomatous polyposis and desmoid tumors. Clinics. 2011;66(10):1839–1842. doi: 10.1590/S1807-59322011001000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haidle J.L., MacFarland S.P., Howe J.R. GeneReviews®; 2022. Juvenile Polyposis Syndrome. [Internet] [Google Scholar]
- 19.Beggs A.D., et al. Peutz–Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59(7):975–986. doi: 10.1136/gut.2009.198499. [DOI] [PubMed] [Google Scholar]
- 20.Kalady M.F., Church J.M. Prophylactic colectomy: rationale, indications, and approach. J. Surg. Oncol. 2015;111(1):112–117. doi: 10.1002/jso.23820. [DOI] [PubMed] [Google Scholar]
- 21.Sepulveda A.R., et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American society for clinical pathology, College of American pathologists, association for molecular pathology, and American society of clinical oncology. Am. J. Clin. Pathol. 2017;147(3):221–260. doi: 10.1093/ajcp/aqw209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearlman R., et al. Two-stain immunohistochemical screening for Lynch syndrome in colorectal cancer may fail to detect mismatch repair deficiency. Mod. Pathol. 2018;31(12):1891–1900. doi: 10.1038/s41379-018-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latham A., et al. Microsatellite instability is associated with the presence of lynch syndrome pan-cancer. J. Clin. Oncol. 2019;37(4):286–295. doi: 10.1200/JCO.18.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokoyama T., et al. Lynch syndrome-associated endometrial carcinoma with MLH1 germline mutation and MLH1 promoter hypermethylation: a case report and literature review. BMC Cancer. 2018;18(1):576. doi: 10.1186/s12885-018-4489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peltomäki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J. Clin. Oncol. 2003;21(6):1174–1179. doi: 10.1200/JCO.2003.04.060. [DOI] [PubMed] [Google Scholar]
- 26.Tamura K., et al. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int. J. Clin. Oncol. 2019;24(9):999–1011. doi: 10.1007/s10147-019-01494-y. [DOI] [PubMed] [Google Scholar]
- 27.Losso G.M., et al. Microsatellite instability--MSI markers (BAT26, BAT25, D2S123, D5S346, D17S250) in rectal cancer. Arq Bras Cir Dig. 2012;25(4):240–244. doi: 10.1590/s0102-67202012000400006. [DOI] [PubMed] [Google Scholar]
- 28.Battaglin F., et al. Microsatellite instability in colorectal cancer: overview of its clinical significance and novel perspectives. Clin. Adv. Hematol. Oncol. 2018;16(11):735–745. [PMC free article] [PubMed] [Google Scholar]
- 29.Chintalacheruvu L.M., et al. Major hereditary gastrointestinal cancer syndromes: a narrative review. J Gastrointestin Liver Dis. 2017;26(2):157–163. doi: 10.15403/jgld.2014.1121.262.maj. [DOI] [PubMed] [Google Scholar]
- 30.Lee J.K., et al. Necessity of multiplex ligation probe amplification in genetic tests: germline variant analysis of the APC gene in familial adenomatous polyposis patients. Cancer Genet. 2022;262–263:95–101. doi: 10.1016/j.cancergen.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Zhu L., et al. APC promoter methylation in gastrointestinal cancer. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.653222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spink K.E., Fridman S.G., Weis W.I. Molecular mechanisms of β-catenin recognition by adenomatous polyposis coli revealed by the structure of an APC–β-catenin complex. EMBO J. 2001;20(22):6203–6212. doi: 10.1093/emboj/20.22.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lubbe S.J., et al. Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J. Clin. Oncol. 2009;27(24):3975–3980. doi: 10.1200/JCO.2008.21.6853. [DOI] [PubMed] [Google Scholar]
- 34.Alimi A., et al. Overlap of Juvenile polyposis syndrome and Cowden syndrome due to de novo chromosome 10 deletion involving BMPR1A and PTEN: implications for treatment and surveillance. Am. J. Med. Genet. 2015;167(6):1305–1308. doi: 10.1002/ajmg.a.36876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dahdaleh F.S., et al. Juvenile polyposis and other intestinal polyposis syndromes with microdeletions of chromosome 10q22-23. Clin. Genet. 2012;81(2):110–116. doi: 10.1111/j.1399-0004.2011.01763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breckpot J., et al. BMPR1A is a candidate gene for congenital heart defects associated with the recurrent 10q22q23 deletion syndrome. Eur. J. Med. Genet. 2012;55(1):12–16. doi: 10.1016/j.ejmg.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Daniell J., et al. vol. 17. Familial cancer; 2018. pp. 421–427. (An Exploration of Genotype-Phenotype Link between Peutz-Jeghers Syndrome and STK11: a Review). [DOI] [PubMed] [Google Scholar]
- 38.Jiang Y.-L., et al. The altered activity of P53 signaling pathway by STK11 gene mutations and its cancer phenotype in Peutz-Jeghers syndrome. BMC Med. Genet. 2018;19(1):1–10. doi: 10.1186/s12881-018-0626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao R., et al. Genetic testing for inherited colorectal cancer and polyposis, 2021 revision: a technical standard of the American College of Medical Genetics and Genomics (ACMG) Genet. Med. 2021;23(10):1807–1817. doi: 10.1038/s41436-021-01207-9. [DOI] [PubMed] [Google Scholar]
- 40.Heald B., et al. Assessment of clinical workload for general and specialty genetic counsellors at an academic medical center: a tool for evaluating genetic counselling practices. NPJ Genom Med. 2016;1 doi: 10.1038/npjgenmed.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhen J.T., et al. Genetic testing for hereditary prostate cancer: current status and limitations. Cancer. 2018;124(15):3105–3117. doi: 10.1002/cncr.31316. [DOI] [PubMed] [Google Scholar]
- 42.Green R.C., et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet. Med. 2013;15(7):565–574. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egalite N., Groisman I.J., Godard B. Genetic counseling practice in next generation sequencing research: implications for the ethical oversight of the informed consent process. J. Genet. Counsel. 2014;23(4):661–670. doi: 10.1007/s10897-014-9703-x. [DOI] [PubMed] [Google Scholar]
- 44.Tafe L.J. Targeted next-generation sequencing for hereditary cancer syndromes: a focus on lynch syndrome and associated endometrial cancer. J. Mol. Diagn. 2015;17(5):472–482. doi: 10.1016/j.jmoldx.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Green R.C., et al. Prediction of Lynch syndrome in consecutive patients with colorectal cancer. J. Natl. Cancer Inst. 2009;101(5):331–340. doi: 10.1093/jnci/djn499. [DOI] [PubMed] [Google Scholar]
- 46.Mills A.M., et al. Lynch syndrome screening should be considered for all patients with newly diagnosed endometrial cancer. Am. J. Surg. Pathol. 2014;38(11):1501–1509. doi: 10.1097/PAS.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Idos G., Valle L. 2021. Lynch Syndrome. [Google Scholar]
- 48.Wang W., et al. A modified screening strategy for Lynch syndrome among MLH1-deficient CRCs: analysis from consecutive Chinese patients in a single center. Transl Oncol. 2021;14(5) doi: 10.1016/j.tranon.2021.101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sorscher S. Primary care implications of the expanded national guidelines for germline testing of patients previously diagnosed with colorectal cancer. J. Am. Board Fam. Med. 2023;36(2):360–365. doi: 10.3122/jabfm.2022.220288R1. [DOI] [PubMed] [Google Scholar]
- 50.Dinarvand P., et al. Familial adenomatous polyposis syndrome: an update and review of extraintestinal manifestations. Arch. Pathol. Lab Med. 2019;143(11):1382–1398. doi: 10.5858/arpa.2018-0570-RA. [DOI] [PubMed] [Google Scholar]
- 51.Jung S.M., et al. Clinicopathological features of familial adenomatous polyposis in Korean patients. World J. Gastroenterol. 2016;22(17):4380–4388. doi: 10.3748/wjg.v22.i17.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leoz M.L., et al. The genetic basis of familial adenomatous polyposis and its implications for clinical practice and risk management. Appl. Clin. Genet. 2015;8:95–107. doi: 10.2147/TACG.S51484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghadamyari F., et al. Mutational screening through comprehensive bioinformatics analysis to detect novel germline mutations in the APC gene in patients with familial adenomatous polyposis (FAP) J. Clin. Lab. Anal. 2021;35(5) doi: 10.1002/jcla.23768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lemke T., Rüppel J. Social dimensions of preimplantation genetic diagnosis: a literature review. New Genet. Soc. 2019;38(1):80–112. [Google Scholar]
- 55.Bisgaard M.L., et al. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum. Mutat. 1994;3(2):121–125. doi: 10.1002/humu.1380030206. [DOI] [PubMed] [Google Scholar]
- 56.Jelsig A.M., et al. Familial Cancer; 2023. Whole Genome Sequencing and Disease Pattern in Patients with Juvenile Polyposis Syndrome: a Nationwide Study; pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burt R., Neklason D.W. Genetic testing for inherited colon cancer. Gastroenterology. 2005;128(6):1696–1716. doi: 10.1053/j.gastro.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 58.Cohen S., et al. Management of juvenile polyposis syndrome in children and adolescents: a position paper from the espghan polyposis working group. J. Pediatr. Gastroenterol. Nutr. 2019;68(3):453–462. doi: 10.1097/MPG.0000000000002246. [DOI] [PubMed] [Google Scholar]
- 59.van Lier M.G.F., et al. BMJ Publishing Group; 2011. High Cancer Risk and Increased Mortality in Patients with Peutz–Jeghers Syndrome; pp. 141–147. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z., et al. Prenatal diagnosis in a hereditary Peutz-Jeghers syndrome family with high cancer risk. BMC Med. Genet. 2018;19:1–7. doi: 10.1186/s12881-018-0594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woo A., et al. Psychosocial impact of peutz-jeghers syndrome. Fam. Cancer. 2009;8(1):59–65. doi: 10.1007/s10689-008-9202-z. [DOI] [PubMed] [Google Scholar]
- 62.van Lier M.G., et al. Peutz-Jeghers syndrome and family planning: the attitude towards prenatal diagnosis and pre-implantation genetic diagnosis. Eur. J. Hum. Genet. 2012;20(2):236–239. doi: 10.1038/ejhg.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Z., et al. Prenatal diagnosis in a hereditary Peutz-Jeghers syndrome family with high cancer risk. BMC Med. Genet. 2018;19(1):66. doi: 10.1186/s12881-018-0594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thakur N., et al. A novel mutation in STK11gene is associated with Peutz-Jeghers Syndrome in Indian patients. BMC Med. Genet. 2006;7(1):1–6. doi: 10.1186/1471-2350-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stupart D.A., et al. Surgery for colonic cancer in HNPCC: total vs segmental colectomy. Colorectal Dis. 2011;13(12):1395–1399. doi: 10.1111/j.1463-1318.2010.02467.x. [DOI] [PubMed] [Google Scholar]
- 66.Kalady M.F., et al. Risk of colorectal adenoma and carcinoma after colectomy for colorectal cancer in patients meeting Amsterdam criteria. Ann. Surg. 2010;252(3):507–511. doi: 10.1097/SLA.0b013e3181f20bd2. ; discussion 511-3. [DOI] [PubMed] [Google Scholar]
- 67.Parry S., et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut. 2011;60(7):950–957. doi: 10.1136/gut.2010.228056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tomita N., et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2020 for the clinical practice of hereditary colorectal cancer. Int. J. Clin. Oncol. 2021;26(8):1353–1419. doi: 10.1007/s10147-021-01881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malik S.S., et al. Metachronous colorectal cancer following segmental or extended colectomy in Lynch syndrome: a systematic review and meta-analysis. Fam. Cancer. 2018;17(4):557–564. doi: 10.1007/s10689-017-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quan J., et al. Surgical outcomes of left hemicolon sparing resection versus extensive resection in treating synchronous colorectal cancer involving the right-sided colon and sigmoid colon or rectum. World J. Surg. Oncol. 2023;21(1):131. doi: 10.1186/s12957-023-03012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kudchadkar S., et al. Current guidelines in the surgical management of hereditary colorectal cancers. World J. Gastrointest. Oncol. 2022;14(4):833–841. doi: 10.4251/wjgo.v14.i4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Menahem B., et al. Lynch syndrome: current management in 2019. Journal of Visceral Surgery. 2019;156(6):507–514. doi: 10.1016/j.jviscsurg.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 73.Win A.K., et al. Risks of colorectal and other cancers after endometrial cancer for women with Lynch syndrome. J. Natl. Cancer Inst.: Journal of the National Cancer Institute. 2013;105(4):274–279. doi: 10.1093/jnci/djs525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Warrier S.K., Kalady M.F. Familial adenomatous polyposis: challenges and pitfalls of surgical treatment. Clin. Colon Rectal Surg. 2012;25(2):83–89. doi: 10.1055/s-0032-1313778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinberger A.E., Westfal M.L., Wise P.E. Surgical decision-making in familial adenomatous polyposis. Clin. Colon Rectal Surg. 2023 doi: 10.1055/s-0043-1770732. (EFirst) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horisberger K., Mann C., Lang H. Current surgical concepts in lynch syndrome and familial adenomatous polyposis. Visc. Med. 2023;39(1):1–9. doi: 10.1159/000530030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Monahan K.J., et al. Guidelines for the management of hereditary colorectal cancer from the British society of gastroenterology (BSG)/Association of coloproctology of great britain and Ireland (ACPGBI)/United Kingdom cancer genetics group (UKCGG) Gut. 2020;69(3):411–444. doi: 10.1136/gutjnl-2019-319915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aelvoet A.S., et al. Management of familial adenomatous polyposis and MUTYH-associated polyposis; new insights. Best Pract. Res. Clin. Gastroenterol. 2022;58–59 doi: 10.1016/j.bpg.2022.101793. [DOI] [PubMed] [Google Scholar]
- 79.Pasquer A., et al. Prophylactic colectomy and rectal preservation in FAP: systematic endoscopic follow-up and adenoma destruction changes natural history of polyposis. Endosc. Int. Open. 2021;9(7):E1014–E1022. doi: 10.1055/a-1467-6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bülow S., et al. Colectomy and ileorectal anastomosis is still an option for selected patients with familial adenomatous polyposis. Dis. Colon Rectum. 2008;51:1318–1323. doi: 10.1007/s10350-008-9307-3. [DOI] [PubMed] [Google Scholar]
- 81.van Leerdam M.E., et al. Endoscopic management of Lynch syndrome and of familial risk of colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2019;51(11):1082–1093. doi: 10.1055/a-1016-4977. [DOI] [PubMed] [Google Scholar]
- 82.Mogere E., et al. Juvenile polyposis syndrome: a case report. Clinical Case Reports. 2023;11(1):e6798. doi: 10.1002/ccr3.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dal Buono A., et al. Juvenile polyposis syndrome: an overview. Best Pract. Res. Clin. Gastroenterol. 2022;58–59 doi: 10.1016/j.bpg.2022.101799. [DOI] [PubMed] [Google Scholar]
- 84.Li B.-R., et al. Primary experience of small bowel polypectomy with balloon-assisted enteroscopy in young pediatric Peutz–Jeghers syndrome patients. Eur. J. Pediatr. 2020;179:611–617. doi: 10.1007/s00431-019-03534-1. [DOI] [PubMed] [Google Scholar]
- 85.Wagner A., et al. The management of peutz-jeghers syndrome: European hereditary tumour group (EHTG) guideline. J. Clin. Med. 2021;10(3) doi: 10.3390/jcm10030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y.X., et al. The role of double‐balloon enteroscopy in reducing the maximum size of polyps in patients with Peutz‐Jeghers syndrome: 12‐year experience. Journal of Digestive Diseases. 2019;20(8):415–420. doi: 10.1111/1751-2980.12784. [DOI] [PubMed] [Google Scholar]
- 87.Nakayama Y., et al. Urgent double-balloon enteroscopy for reduction of jejuno-jejunal intussusception and polypectomy in Peutz-Jeghers syndrome. Journal of Pediatric Surgery Case Reports. 2020;59 [Google Scholar]
- 88.Seppälä T.T., et al. European guidelines from the EHTG and ESCP for Lynch syndrome: an updated third edition of the Mallorca guidelines based on gene and gender. Br. J. Surg. 2021;108(5):484–498. doi: 10.1002/bjs.11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Win A.K., et al. Risk of metachronous colon cancer following surgery for rectal cancer in mismatch repair gene mutation carriers. Ann. Surg Oncol. 2013;20:1829–1836. doi: 10.1245/s10434-012-2858-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Møller P., et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut. 2018;67(7):1306–1316. doi: 10.1136/gutjnl-2017-314057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Møller P., et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. 2017;66(3):464–472. doi: 10.1136/gutjnl-2015-309675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Renkonen-Sinisalo L., et al. Subtotal colectomy for colon cancer reduces the need for subsequent surgery in Lynch syndrome. Dis. Colon Rectum. 2017;60(8):792–799. doi: 10.1097/DCR.0000000000000802. [DOI] [PubMed] [Google Scholar]
- 93.Doerner J. Risk of metachronous colorectal cancer in lynch syndrome: who needs an extended resection? Surgeries. 2022;3:185–191. doi: 10.3390/surgeries3030020. [DOI] [Google Scholar]
- 94.Fodde R. In: Encyclopedia of Cancer. Schwab M., editor. Springer Berlin Heidelberg; Berlin, Heidelberg: 2011. APC gene in familial adenomatous polyposis; pp. 234–235. [Google Scholar]
- 95.Sarvepalli S., et al. Web‐based model for predicting time to surgery in young patients with familial adenomatous polyposis: an internally validated study. Am. J. Gastroenterol. 2018;113(12):1881. doi: 10.1038/s41395-018-0278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Knudsen A.L., et al. Attenuated familial adenomatous polyposis: results from an international collaborative study. Colorectal Dis. 2010;12(10Online):e243–e249. doi: 10.1111/j.1463-1318.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 97.Mori Y., et al. Usefulness of genotyping APC gene for individualizing management of patients with familial adenomatous polyposis. Int. J. Clin. Oncol. 2023;28(12):1641–1650. doi: 10.1007/s10147-023-02419-6. [DOI] [PubMed] [Google Scholar]
- 98.MacFarland S.P., et al. Phenotypic differences in juvenile polyposis syndrome with or without a disease-causing SMAD4/bmpr1a variant. Cancer Prev. Res. 2021;14(2):215–222. doi: 10.1158/1940-6207.CAPR-20-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matsumoto T., et al. Clinical guidelines for diagnosis and management of juvenile polyposis syndrome in children and adults-secondary publication. J Anus Rectum Colon. 2023;7(2):115–125. doi: 10.23922/jarc.2023-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van Lier M.G.F., et al. High cumulative risk of intussusception in patients with Peutz–Jeghers syndrome: time to update surveillance guidelines? Official journal of the American College of Gastroenterology| ACG. 2011;106(5):940–945. doi: 10.1038/ajg.2010.473. [DOI] [PubMed] [Google Scholar]
- 101.Zhong M.E., et al. Laparoscopic restorative proctocolectomy with ileal pouch-anal anastomosis for Peutz-Jeghers syndrome with synchronous rectal cancer. World J. Gastroenterol. 2016;22(22):5293–5296. doi: 10.3748/wjg.v22.i22.5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y., et al. Progress of gastric cancer surgery in the era of precision medicine. Int. J. Biol. Sci. 2021;17(4):1041. doi: 10.7150/ijbs.56735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hoffman T.L., et al. Next-generation universal hereditary cancer screening: implementation of an automated hereditary cancer screening program for patients with advanced cancer undergoing tumor sequencing in a large HMO. Fam. Cancer. 2023;22(2):225–235. doi: 10.1007/s10689-022-00317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Richards S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of medical genetics and genomics and the association for molecular pathology. Genet. Med. 2015;17(5):405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Monaghan A., Copson E., Cutress R. Hereditary genetic testing and mainstreaming: a guide for surgeons. Ann. R. Coll. Surg. Engl. 2024;106(4):300–304. doi: 10.1308/rcsann.2024.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burke W., Korngiebel D.M. Closing the gap between knowledge and clinical application: challenges for genomic translation. PLoS Genet. 2015;11(2) doi: 10.1371/journal.pgen.1004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alowais S.A., et al. Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC Med. Educ. 2023;23(1):689. doi: 10.1186/s12909-023-04698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saidak Z., et al. Contribution of genomics to the surgical management and study of oral cancer. Ann. Surg Oncol. 2021;28(11):5842–5854. doi: 10.1245/s10434-021-09904-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hennocq Q., et al. AI-based diagnosis and phenotype – genotype correlations in syndromic craniosynostoses. J. Cranio-Maxillofacial Surg. 2024 [Google Scholar]