Abstract
Background
Extrahepatic biliary neuroendocrine tumors (EBNETs) are rare. We aimed to characterize EBNETs including factors associated with survival.
Methods
The National Cancer Database was queried for patients with EBNETs from 2004 to 2016. Patients who underwent resection were examined using Cox proportional hazards regression and the Kaplan-Meier method. We compared overall survival (OS) among patients with EBNETs to those with NETs from other primary sites.
Results
Overall, 223 patients with EBNETs were identified. Patients were predominantly male (n = 113, 50.7 %), white (n = 177, 79.4 %) and presented without distant metastasis (n = 182, 81.6 %). The majority underwent operation (n = 127, 57.9 %) with resection of the primary tumor (n = 89, 70 %). Among patients who underwent resection (n = 71), multivariable regression demonstrated older age (HR 1.11, 95 % C.I. 1.04–1.17), lymph node metastases (HR 1.19, 95 % C.I. 1.02–1.38) and poorly/undifferentiated tumors [HR 22.3, 95 % C.I. 3.78–131]) were associated with worse overall survival. Patients with EBNETs experienced abbreviated OS compared to patients with small bowel or pancreas NETs (p < 0.001), but improved OS when compared to patients with gallbladder NETs (p = 0.001).
Conclusions
Tumor differentiation and lymph node status significantly impact overall survival.
Highlights
-
•
Characterization of extrahepatic biliary neuroendocrine tumors, EBNETs, including factors associated with survival.
-
•
Older age, lymph node metastases, and poorly/undifferentiated tumors were associated with worse OS.
-
•
Patients with EBNETs experienced abbreviated OS compared to patients with small bowel or pancreas NETs, but improved OS when compared to patients with gallbladder NETs.
1. Introduction
With less than 200 cases reported in the literature, extrahepatic bile duct neuroendocrine tumors (EBNETs) are an exceedingly rare subtype of cancer arising from the proximal or distal biliary tree [[1], [2], [3]]. Distinguishing these lesions from adenocarcinoma can be challenging clinically, as EBNETs are predominantly non-functioning tumors that commonly present with signs and symptoms of biliary obstruction, much like classical extrahepatic cholangiocarcinoma [3,4]. However, in contrast to patients who typically develop biliary tree adenocarcinoma in the Western world, those who develop EBNETs are more likely to be younger, female, and to have a familial syndrome [1,3,5].
Due to the rarity of EBNETs, there are limited data for prognostication and defining best practices [[1], [2], [3],[6], [7], [8], [9], [10], [11], [12]]. For instance, tumor differentiation has a significant impact on survival in patients with NETs that originate from other anatomic sites whereas little is known of the importance of tumor differentiation on survival in patients with EBNETs [3]. Treatment patterns for patients with EBNETs appear to parallel those with distal bile duct adenocarcinoma and often involve extirpation of the primary tumor by extrahepatic bile duct resection and regional lymphadenectomy, with the occasional use of adjuvant therapy [[6], [7], [8], [9], [10], [11], [12], [13]]. Although patients with EBNETs collectively seem to fare better than their counterparts with classical distal cholangiocarcinoma, these outcomes are poorly defined and are derived mostly from heterogeneous historical cohorts [3].
Due to the limited prognostic and treatment-specific data alongside the lack of defined guidelines for EBNETs, we aimed to characterize EBNETs using a large national database, identify national practice patterns, and determine factors associated with overall survival.
2. Methods
2.1. Data source
The National Cancer Database (NCDB) participant user files were the source of all data in our study. The NCDB is a nationwide repository of de-identified patient data related to cancer metrics and outcomes in the United States derived from the submissions of over 1500 Commission on Cancer (CoC)-accredited programs. The NCDB captures over 70 % of new cancer diagnoses in the United States per year. The CoC is a multidisciplinary association maintained by the American College of Surgeons and the American Cancer Society that accredits US hospitals based on various aspects of cancer care. Due to our study's inclusion of only de-identified data, it was exempt from institutional review board review.
2.2. Cohort selection
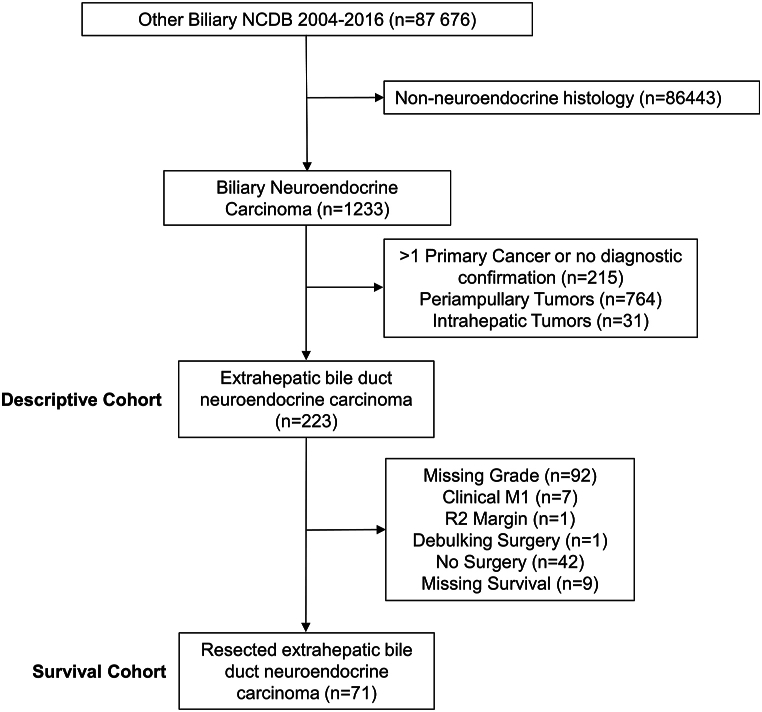
We selected patients diagnosed with EBNETs from the NCDB from 2004 to 2016. Patients were identified using the International Classification of Disease for Oncology, 3rd edition (ICD-O-3) topography code for the extrahepatic bile duct (C24.0), overlapping biliary tract (C24.8) and biliary tract not otherwise specified (NOS) (C24.9). ICD-O-3 morphology codes for neuroendocrine histology (8013, 8041, 8240, 8246) were used to select patients. Individual patients with more than one primary cancer were excluded, as were patients with intrahepatic (C22.1) and periampullary tumors (C24.1). The elimination of intrahepatic and periampullary tumors served to remove the ambiguity associated with evaluating potentially metastatic lesions or pancreatic NETs (Fig. 1).
Fig. 1.
Selection of the study cohort. NCDB National Cancer Database, NET neuroendocrine tumor.
2.3. Survival analysis
In order to determine factors associated with survival in patients who underwent resection of EBNETs (n = 71), we first excluded patients with missing information regarding tumor differentiation (n = 92) and then excluded those whose data suggested the presence of distant metastasis, including those with clinical M1 disease, grossly positive resection margins, documentation of having undergone a debulking surgical procedure, or documentation of not having undergone any surgical procedure (n = 60, Fig. 1). Unadjusted OS was estimated using the Kaplan-Meier method, and comparisons between groups were made using the log-rank test. Additionally, using the same selection criteria and time frame, we compared unadjusted survival of patients with EBNETs to those with NETs from primary sites in the small bowel, pancreas, and gallbladder. Covariate effects on OS were estimated in an adjusted model using bivariable and multivariable Cox proportional hazards regression.
2.4. Statistical analysis
Univariable and bivariable descriptive statistics were calculated for variables of interest. Continuous variables were reported as medians and interquartile range (IQR). Categorical variables were described using frequencies and percentages. Comparisons between groups were performed using chi-squared or Fisher's exact test for categorical variables and Mann Whitney-U test for continuous variables, as appropriate. A multivariable Cox proportional hazards model including all variables with p < 0.10 on bivariable analysis was used to determine adjusted covariate effects on OS. Statistical significance was defined as a two-tailed p-value <0.05. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, N.Y., USA).
3. Results
3.1. Characteristics of extrahepatic biliary neuroendocrine tumors
We identified 223 patients with EBNETs who met inclusion for this analysis. Patients had a median age of 61 years (IQR 51–71) and were mostly male (n = 113, 50.7 %), white (n = 177, 79.4 %) and had Charlson-Deyo Comorbidity Score of zero (n = 170, 76.2 %). Forty-one (18.3 %) patients presented with synchronous distant metastases, and the most common metastatic site was the liver (n = 29, 13.0 %). Median tumor size was 17.5 mm (IQR 6.5–30) and 40 patients (17.9 %) presented with at least one pathologically positive lymph node. Most tumors did not have a specified location of origin within the extrahepatic biliary tree (n = 113, 50.7 %), but 53 (23.8 %) were located in the distal bile duct, and the remaining tumors originated in the proximal/perihilar region or cystic ducts (n = 29, 13 % and n = 28, 13 %, respectively). While most patients had neuroendocrine histology (n = 177, 73.6 %), other represented subtypes included small and large cell carcinoma (n = 42, 19 % and n = 8, 4 %, respectively). Additionally, although many patients were missing information regarding tumor differentiation (n = 92, 41 %), most were well (n = 62, 27.8 %) or poorly differentiated (n = 56, 25 %) (Table 1).
Table 1.
Patient and disease characteristics.
| n = 223 | N | % |
|---|---|---|
| Age at Diagnosis (median, IQR) | 61 | (51,71) |
| Sex | ||
| Male | 113 | 50.7 % |
| Female | 110 | 49.3 % |
| Race | ||
| White | 177 | 79.4 % |
| African-American | 30 | 13.5 % |
| Asian | 7 | 3.1 % |
| Other/Unknown | 9 | 4.0 % |
| Charlson-Deyo Score | ||
| 0 | 170 | 76.2 % |
| 1 | 38 | 17.0 % |
| ≥2 | 15 | 6.7 % |
| Positive Lymph Node(s) | ||
| No Positive Lymph Nodes | 54 | 24.2 % |
| ≥1 Positive Lymph Node | 40 | 17.9 % |
| No Nodes Examined | 129 | 57.8 % |
| Tumor Size (mm), (median, IQR) | 17.5 | (6.5,30) |
| Number of Positive Lymph Nodes, (median, IQR) | 0 | (0,1) |
| Tumor Location | ||
| Distal Bile Duct | 53 | 23.8 % |
| Proximal Bile Duct/Perihilar | 29 | 13.0 % |
| Cystic Duct | 28 | 12.6 % |
| Other NOS | 113 | 50.7 % |
| Histology | ||
| Neuroendocrine Tumor | 173 | 77.6 % |
| Small Cell Carcinoma | 42 | 18.8 % |
| Large Cell Carcinoma | 8 | 3.6 % |
| Tumor Morphology | ||
| Well Differentiated | 62 | 27.8 % |
| Moderately Differentiated | 13 | 5.8 % |
| Poorly Differentiated/Undifferentiated | 56 | 25.1 % |
| Unknown | 92 | 41.3 % |
| Bone Metastasis at Diagnosis | 2 | 0.9 % |
| Brain Metastasis at Diagnosis | 1 | 0.4 % |
| Liver Metastasis at Diagnosis | 29 | 13.0 % |
| Lung Metastasis at Diagnosis | 7 | 3.1 % |
| Other Metastasis at Diagnosis | 2 | 0.9 % |
4. Treatment of extrahepatic biliary neuroendocrine tumors
There were 127 patients (57.9 %) who underwent resection. Among these patients, most underwent removal of the primary tumor (n = 89, 70 %), while 23 (18 %) had a radical resection that included part of an adjacent organ. Most patients (n = 88, 69 %) who underwent surgical resection had specimens with negative margins. Overall, the use of chemotherapy or radiation was more common in patients who did not undergo operative intervention (Table 2).
Table 2.
Type(s) of treatment received.
| Surgical Management |
||||
|---|---|---|---|---|
| n = 223 |
Yes (n = 127) |
No (n = 96) |
||
| Received Chemotherapy | N | % | N | % |
| No | 98 | 77.20 % | 57 | 59.40 % |
| Yes | 29 | 22.80 % | 39 | 40.60 % |
| Received Radiation | ||||
| No | 117 | 92.10 % | 92 | 95.80 % |
| Yes | 10 | 7.90 % | 4 | 4.20 % |
| Surgical Procedure | ||||
| Local Therapy Only | 11 | 8.70 % | ||
| Removal of Primary Site | 89 | 70.10 % | ||
| Debulking | 1 | 0.80 % | ||
| Radical Resection | 23 | 18.10 % | ||
| Surgery, NOS | 3 | 2.40 % | ||
| Margin Status | ||||
| R0 | 88 | 69.30 % | ||
| R1/R2 | 27 | 21.30 % | ||
| Unknown | 12 | 9.40 % | ||
5. Factors associated with survival
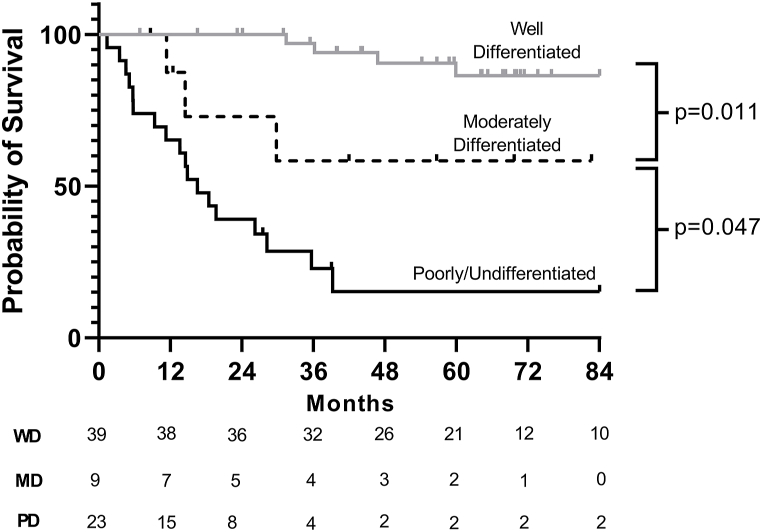
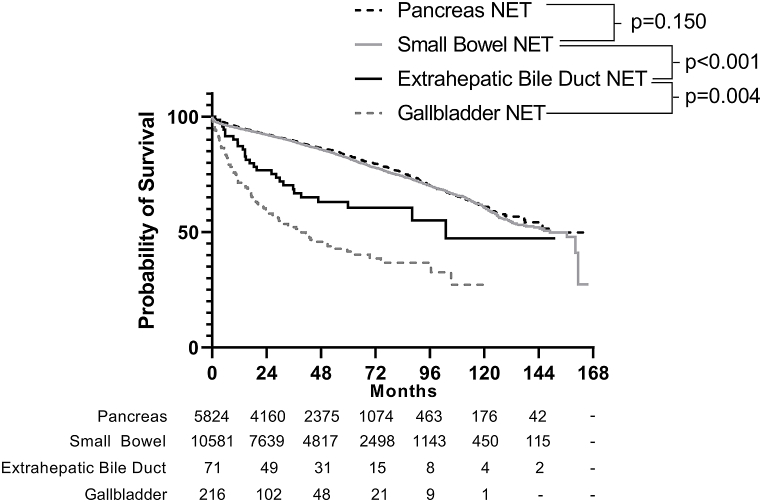
Among patients who underwent resection, multivariable regression demonstrated that older age (HR 1.11, 95 % C.I. 1.04–1.17) and lymph node metastasis (HR 1.19, 95 % C.I. 1.02–1.38) were associated with worse survival on a per-unit basis. Additionally, in this model, tumor differentiation was the strongest independent predictor of decreased survival (moderately-differentiated [HR 38.8, 95 % C.I. 4.11–367] and poorly/undifferentiated [HR 22.3, 95 % C.I. 3.78–131]). Notably, tumor location within the bile duct, receipt of chemotherapy, small or large cell histology, and tumor size were not associated with survival (Table 3). Median survival was not reached for all resected EBNETs, but mean survival was 93.1 months (95 % C.I. 76.1–110.0). The differences in survival stratified by degree of tumor differentiation that were seen in the Cox model were also demonstrated in the unadjusted model (Fig. 2), which demonstrates that median overall survival is reached only in patients with poorly/undifferentiated histology (16.6 months, 95 % C.I. 10.4–22.7) (Fig. 2). Furthermore, when compared to those with NETs originating from other primary sites, survival among patients with EBNETs who undergo resection is worse than those with NET originating from the pancreas or small bowel (p < 0.001), but better than those with NET arising from the gallbladder (p = 0.001, Fig. 3).
Table 3.
Cox proportional hazards regression.
| Univariable |
Multivariable |
||||||
|---|---|---|---|---|---|---|---|
| n = 71 | N | HR | 95 % C.I. | p-value | HR | 95 % C.I. | p-value |
| Age at Diagnosis | 71 | 1.06 | (1.03–1.09) | <0.001 | 1.11 | (1.04–1.17) | 0.001 |
| Sex | |||||||
| Male | 38 | ref | – | – | ref | – | – |
| Female | 33 | 0.44 | (0.19–1.01) | 0.051 | 0.31 | (0.07–1.27) | 0.102 |
| Race | |||||||
| White | 59 | ref | – | ||||
| African-American | 8 | 0.23 | (0.03–1.71) | 0.152 | |||
| Other/Unknown | 4 | 1.29 | (0.30–5.52) | 0.729 | |||
| Charlson-Deyo Score | |||||||
| 0 | 57 | ref | – | – | |||
| 1 | 11 | 0.62 | (0.19–2.08) | 0.442 | |||
| ≥2 | 3 | 1.05 | (0.14–7.90) | 0.963 | |||
| Lymphovascular Invasion | |||||||
| Absent | 19 | ref | – | – | Excluded from MVA due to missing values | ||
| Present | 14 | 4.88 | (1.24–19.24) | 0.023 | |||
| Unknown | 12 | 0.97 | (0.16–5.83) | 0.974 | |||
| Tumor Size (mm), (median, IQR) | 62 | 1.02 | (1.00–1.05) | 0.040 | 0.98 | (0.92–1.04) | 0.495 |
| Number of Positive Lymph Nodes | 51 | 1.19 | (1.09–1.30) | <0.001 | 1.19 | (1.02–1.38) | 0.025 |
| Tumor Location | |||||||
| Distal Bile Duct | 20 | ref | – | – | |||
| Proximal Bile Duct/Perihilar | 10 | 1.50 | (0.42–5.33) | 0.528 | |||
| Cystic Duct | 12 | 0.48 | (0.10–2.38) | 0.369 | |||
| Other NOS | 29 | 1.44 | (0.55–3.79) | 0.463 | |||
| Histology | |||||||
| Neuroendocrine Tumor | 62 | ref | – | – | ref | – | – |
| Small/Large Cell Tumor | 9 | 4.76 | (1.97–11.47) | 0.001 | 1.32 | (0.35–4.97) | 0.683 |
| Tumor Morphology | |||||||
| Well Differentiated | 39 | ref | – | – | ref | – | – |
| Moderately Differentiated | 9 | 4.52 | (1.07–19.10) | 0.040 | 38.84 | (4.11–366.99) | 0.001 |
| Poorly Differentiated/Undifferentiated | 23 | 15.58 | (5.66–42.91) | <0.001 | 22.30 | (3.78–131.49) | 0.001 |
| Chemotherapy | |||||||
| No | 53 | ref | – | – | ref | – | – |
| Yes | 12 | 2.66 | (1.14–6.17) | 0.023 | 0.32 | (0.05–2.13) | 0.236 |
| Unknown | 6 | 0.67 | (0.09–5.04) | 0.696 | 1.69 | (0.16–17.60) | 0.661 |
| Radiation | |||||||
| No | 63 | ref | – | – | |||
| Yes | 8 | 2.27 | (0.85–6.02) | 0.101 | |||
Fig. 2.
Survival in resected patients stratified by degree of tumor differentiation (n = 71). Median overall survival is reached only in patients with poorly/undifferentiated histology (16.6 months, 95 % C.I. 10.4–22.7).
Fig. 3.
When comparing survival in patients with NETs from different primary sites, survival is worse in patients with EBNETs than in patients with NETs originating from the pancreas or small bowel (p < 0.001), but better than those with NETs arising from the gallbladder (p = 0.001).
6. Discussion
Here we report a large cohort of patients with EBNETs using a national database. Our findings demonstrate survival stratification is governed by tumor differentiation, akin to NETs arising from other primary sites. Most patients in this study were treated with resection alone for this rare tumor. Our findings suggest that EBNETs generally carry a better prognosis than the classical adenocarcinoma subtype of bile duct cancer, however poorly differentiated EBNETs portend a substantially worse overall survival than more differentiated variants. Interestingly, while prognosis for patients with EBNETs is generally worse than that of pancreatic or small bowel NET, it appears to be better than that of gallbladder NETs.
Cancer prognostication and the formulation of consensus recommendations must involve the consideration of the best available evidence. Thus, while prior data consisted of case reports and small series, we believe that our study offers the largest and most comprehensive cohort to evaluate the importance of tumor differentiation and lymph node metastasis on long-term survival, as well as highlights modern, national treatment trends for EBNETs [3]. Our data demonstrate that EBNET survival clearly stratifies based on the degree of tumor differentiation, even when including only a modest sample size. It is important to recognize that while we do not have these tumors' complete grade-specific data, which would include a mitotic count and Ki-67 proliferative index in addition to each tumor's degree of differentiation, our findings are largely consistent with those of other visceral NETs [[14], [15], [16], [17]]. Specifically, while well-differentiated NETs can be associated with a spectrum of low-to high-grade histopathologic features, poorly-differentiated NETs are uniformly high-grade and aggressive cancers that draw morphologic and survival comparisons to small cell lung carcinoma [14,18]. Our data also suggest that the presence of metastases in lymph nodes does carry prognostic significance, but the effect size is lower than that of poorer tumor differentiation. Once again, this finding is consistent with suggested conventions for poorly-differentiated (and thus, high-grade) bronchopulmonary and gastroenteropancreatic NETs, for which the conventional TNM staging systems have been called into question [18]. Overall, we believe that while lymph node positivity is important in EBNET staging, tumor differentiation should hold more weight in an optimal system, as it does for poorly-differentiated NETs from other primary sites.
Additionally, this study re-emphasizes the utility of large national databases in studying rare tumors. The NCDB has been used to evaluate prognosis and treatment patterns for patients with NETs of various primary sites, and we demonstrate that these insights can now be extended to patients with EBNETs [[19], [20], [21]]. Accurately evaluating national trends in treatment strategies is challenging when using the aggregation of case series and reports due to the heterogeneity of patients, tumors, and reporting quality of historical cohorts. While not without flaws, the NCDB provides a more uniform survey of this and other tumor subtypes and allows for a broad evaluation of a modern cohort of EBNET patients. While we concede that a dedicated, multi-institutional collaborative likely would have the potential to generate more granular and potentially more reliable data, the overall challenges that would be associated with such an endeavor are perhaps the reason why such an enterprise has not been undertaken to date.
Despite the strengths that we believe our analysis offers, it is not without limitations. The retrospective nature of our study renders it susceptible to selection bias, potential effects on survival from unmeasured confounders, shortcomings related to non-standardized follow-up, inconsistent recording of data, and a lack of granularity among histopathologic characteristics. Specifically, the NCDB lacks details regarding mitotic count and proliferative index, subsequently preventing tumor grade designation. Interestingly, although conventional staging of NETs strongly considers grade in prognostication, studies aimed specifically at delineating the importance of this finding have shown that even high-grade well-differentiated pancreatic NETs seem to have considerably better survival than poorly differentiated tumors [14,18]. Furthermore, the NCDB does not report data on recurrence, and we are also not able to ascertain the details of the respective surgical operation or systemic therapy regimen that each patient underwent. Another limitation of the study is exclusion of lymphovascular invasion. With respect to the multivariable Cox model, there was a high proportion of patients (35, 43.8 %) with missing values among other covariates in patients with available lymphovascular invasion status (LVI). The inclusion of only patients with documented LVI status in this model would have excluded many patients who were missing values of several other covariates, therefore, in order to preserve the maximum number of patients, LVI was excluded from the model. Additionally, survival analysis is limited to patients with available follow up. A significant number of patients are lost to follow up and censored in the survival analysis during the longer study period times, therefore the far right of the curves must be interpreted with caution. Nevertheless, the use of this large database offers insight into a rare tumor subtype that otherwise is difficult to evaluate even when approaching this uncommon entity through single-institution experiences or even a large, multi-institutional collaborative.
In conclusion, EBNETs are a rare variant of extrahepatic bile duct tumors associated with worse overall survival than pancreatic and small bowel NETs. Older age, lymph node positivity, and poor tumor differentiation were independent predictors of abbreviated survival in patients who underwent surgical intervention. These findings suggest that patients with locoregional EBNETs may benefit from resection of their primary tumors and regional lymphadenectomy for local tumor control and adequate staging. Advances in systemic options for patients with NETs from all primary sites may augment the benefit of primary tumor resection in EBNETs.
CRediT authorship contribution statement
Dominguez Da: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Eade Av: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis. Aversa Jg: Writing – original draft, Formal analysis, Conceptualization. Hagerty Bl: Writing – original draft, Methodology, Conceptualization. A.M. Blakely: Writing – review & editing, Supervision, Conceptualization. Davis Jl: Writing – review & editing, Supervision, Conceptualization. Melstrom Lg: Writing – review & editing, Supervision, Conceptualization. Hernandez Jm: Writing – review & editing, Writing – original draft, Supervision, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e34714.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Modlin I.M., Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;79:813–829. doi: 10.1002/(sici)1097-0142(19970215)79:4<813::aid-cncr19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Chandrakanth A., Gonen M., D'Angelica M., DeMatteo R., Fong Y., Blumgart L., et al. Differential diagnosis of proximal biliary obstruction. Surgery. 2006;140:756–763. doi: 10.1016/j.surg.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Michalopoulos N., Papavramidis T.S., Karayannopoulou G., Pilakos I., Papavramidis S., Kanellos I. Neuroendocrine tumors of extrahepatic biliary tract. Pathol. Oncol. Res. 2014;20:765–775. doi: 10.1007/s12253-014-9808-4. [DOI] [PubMed] [Google Scholar]
- 4.Esnaola N.F., Meyer J.E., Karachristos A., Maranki J., Camp E., Delinger C. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer. 2016;122:1349–1369. doi: 10.1002/cncr.29692. [DOI] [PubMed] [Google Scholar]
- 5.Shaib Y., El-Serag H.B. The epidemiology of cholangiocarcinoma. Semin. Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 6.Kihara Y., Yokomizo H., Urata T., Nagamine M., Toshihiko H. A case report of primary neuroendocrine carcinoma of the perihilar bile duct. BMC Surg. 2015;15:125. doi: 10.1186/s12893-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raspanti C., Falco N., Silvestri V., Rotolo G., Bonventre S., Gulotta G. Neuroendocrine tumor of the common bile duct: case report. G Chir. 2016;37:275–280. doi: 10.11138/gchir/2016.37.6.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Z., Chen C., Li B., Liu H., Zhou L., Zhang H., et al. Biliary neuroendocrine neoplasms: clinical profiles, management, and analysis of prognostic factors. Front. Oncol. 2019;9:38. doi: 10.3389/fonc.2019.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costin A.I., Păun I., Păun M., Constantin V., Varcus F. Primary neuroendocrine tumors - an extremely rare cause of obstruction of extrahepatic bile ducts: a case report. Rom J Morphol Embryol = Rev Roum Morphol Embryol. 2017;58:641–644. [PubMed] [Google Scholar]
- 10.Ishida M., Okano K., Sandoh K., Ito H., Ikeura T., Mitsuyama T., et al. Neuroendocrine carcinoma diagnosis from bile duct cytological specimens: a retrospective single-center study. Diagn. Cytopathol. 2020;48:154–158. doi: 10.1002/dc.24334. [DOI] [PubMed] [Google Scholar]
- 11.Safwan M., Vij M., Govil S., Rela M. Well-differentiated neuroendocrine tumour of the extrahepatic bile duct: a case report with review of literature. J. Gastrointest. Cancer. 2016;47:93–99. doi: 10.1007/s12029-015-9726-z. [DOI] [PubMed] [Google Scholar]
- 12.Hoepfner L., White J.A. Primary extrahepatic bile duct neuroendocrine tumor with obstructive jaundice masquerading as a Klatskin tumor. J. Surg. Case Rep. 2017;2017:rjx104. doi: 10.1093/jscr/rjx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abe T., Nirei A., Suzuki N., Todate Y., Azami A., Waragai M., et al. Neuroendocrine tumor of the extrahepatic bile duct: a case report. Int J Surg Case Rep. 2017;40:6–9. doi: 10.1016/j.ijscr.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang L.H., Basturk O., Sue J.J., Kilmstra D. A practical approach to the classification of WHO grade 3 (G3) well-differentiated neuroendocrine tumor (WD-NET) and poorly differentiated neuroendocrine carcinoma (PD-NEC) of the pancreas. Am. J. Surg. Pathol. 2016;40:1192–1202. doi: 10.1097/PAS.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cattoni M., Vallières E., Brown L.M., Sarkeshik A., Margaritora S., Siciliani A., et al. Improvement in TNM staging of pulmonary neuroendocrine tumors requires histology and regrouping of tumor size. J. Thorac. Cardiovasc. Surg. 2018;155:405–413. doi: 10.1016/j.jtcvs.2017.08.102. [DOI] [PubMed] [Google Scholar]
- 16.Dasari A., Mehta K., Byers L.A., Halfdan S., Yao J. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: a SEER database analysis of 162,983 cases. Cancer. 2018;124:807–815. doi: 10.1002/cncr.31124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basturk O., Tang L., Hruban R.H., Adsay V., Yang Z., Krasinkskas A., et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am. J. Surg. Pathol. 2014;38:437–447. doi: 10.1097/PAS.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eads J.R. Poorly differentiated neuroendocrine tumors. Hematol Oncol Clin North Am. 2016;30:151–162. doi: 10.1016/j.hoc.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Kim M.K., Warner R.R.P., Roayaie S., Harpaz N., Ward S.C., Itzkowitz S., et al. Revised staging classification improves outcome prediction for small intestinal neuroendocrine tumors. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:3776–3781. doi: 10.1200/JCO.2013.51.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landry C.S., Woodall C., Scoggins C.R., McMasters K., Martin R.C.G. Analysis of 900 appendiceal carcinoid tumors for a proposed predictive staging system. Arch. Surg. 2008;143:664–670. doi: 10.1001/archsurg.143.7.664. ; discussion 670. [DOI] [PubMed] [Google Scholar]
- 21.Chagpar R., Chiang Y.-J., Xing Y., Cormier J., Feig B., Rashid A., et al. Neuroendocrine tumors of the colon and rectum: prognostic relevance and comparative performance of current staging systems. Ann. Surg Oncol. 2013;20:1170–1178. doi: 10.1245/s10434-012-2746-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.