Abstract
Background
Hepatic encephalopathy is a common and serious complication of decompensated cirrhosis. It can considerably contribute to economic burden and impaired quality of life. However, its pathogenesis remains unclear.
Method
In this study, we aimed to visually analyse the research status and development trends in hepatic encephalopathy pathogenesis using bibliometrics and knowledge mapping. Information regarding publications between 1978 and 2022 were obtained from the Web of Science Core Collection. CiteSpace was used to analyse and present data by year, author, institution, country, journal, reference, and keyword.
Results
A total of 1578 publications on hepatic encephalopathy pathogenesis in patients with cirrhosis were retrieved from Web of Science Core Collection. A gradual increasing trend in annual publications has occurred. The collaborative network analysis results suggest the United States of America, the University of London, and Bajaj, Jasmohan S as the most influential country, institution, and author, respectively, in this research field. Notably, China appeariiuis to be the most promising country. Research on ‘hepatology’ garners the most significant papers in the field. Combined with reference co-citation and keyword co-occurrence analyses, we found that ammonia metabolism, gut microbiota, sarcopenia, and trace elements will become future research frontiers that are likely to be explored for a considerable length of time.
Conclusion
Future research directions in HE pathogenesis may target modulating the ammonia metabolism, the gut microbiota, sarcopenia, and trace elements.
Keywords: Hepatic encephalopathy, Ammonia, Gut microbiota, Sarcopenia, Bibliometric analysis, Liver cirrhosis
1. Introduction
Hepatic encephalopathy (HE) is a brain dysfunction caused by hepatic insufficiency and/or portal-systemic shunting. It presents as a broad and complex spectrum of neurologic or psychiatric abnormalities [1]. HE has an insidious onset and is difficult to recognise in its early stages. Minimal HE (MHE), i.e., the earliest form of HE, has a prevalence of approximately 80% among patients with cirrhosis [[2], [3], [4]]. MHE impairs daily function and health-related quality of life, increasing driving risks, hospitalisation, and readmission [[5], [6], [7], [8]]. Moreover, over one-third of patients with cirrhosis develop HE, and approximately 40% of patients with HE die within one year [9]. Cognitive impairment may persist after liver transplantation in patients with decompensated cirrhosis. Compared with other complications, HE imposes a multifaceted burden on patients and the healthcare system [10,11]. Accordingly, researchers aim to identify the most effective treatment targets for HE.
Recent progress in basic and clinical research has advanced the understanding of HE. Although HE pathogenesis has not been fully elucidated, several theories have been proposed. Specifically, inflammation, oxidative stress, bile acids, amino acids, neurotransmission, manganese, and gut microbiota have been reported as being associated with HE onset, causing severe neurological impairment and providing potential therapeutic targets [1]. Additionally, from a pathophysiological perspective, studies have indicated that the ammonia hypothesis has a central role in HE pathogenesis. Given the high morbidity and mortality rates associated with HE, its pathogenesis and therapeutic targets have become important research topics [10]. To date, no bibliometric studies on HE pathogenesis have been published. Hence, in the present study, we aimed to visually analyse the research trends using bibliometrics and knowledge mapping by analysing and presenting the available data on HE pathogenesis from the Web of Science Core Collection (WOSCC) using CiteSpace (version 6.3. R2). The findings of this study can provide researchers with useful guidance when designing and implementing related studies.
2. Materials and methods
2.1. Data sources and search strategies
To improve data quality and accessibility, targeted articles and reviews were selected from the WOSCC. Data were retrieved on September 7, 2022, with the time span from 1978 to September 7, 2022. The following terms related to the topic were searched: #1 topic: cirrhosis OR liver cirrhosis OR cirrhosis of liver AND DT = Article OR Review; #2 topic: hepatic encephalopathy OR HE OR hepatic coma AND DT = Article OR Review; #3 topic: ammonia OR hyperammonemia OR inflammation OR oxidative stress OR bile acids OR amino acids imbalance OR neurotransmission OR manganese OR bacterial translocation OR microbiota OR gut microbiota OR bacterial flora OR electrolyte imbalance OR electrolyte disorder AND DT = Article OR Review; #4, #1 AND #2 AND #3. Fig. 1 depicts the retrieval process.
Fig. 1.
Retrieval process from the Web of Science Core Collection.
2.2. Analysis tool
CiteSpace (version 6.3. R2) was used to perform bibliometric analysis of studies on HE pathogenesis in patients with liver cirrhosis. According to the search strategy, the dataset was extracted from the WOSCC in tag-delimited plain text files with complete records and references and was designated ‘download_XXX.txt.’ Subsequently, data were imported into CiteSpace for visual analysis. The CiteSpace parameters were as follows: period from 1978 to September 7, 2022 (the period that can be identified by CiteSpace is 1996–2022), time slice of 1 year, a cosine link strength, and threshold of k = 15 for the reference co-citation analysis or threshold of k = 25 for other analyses. The remaining parameters were set to the default values of CiteSpace.
Betweenness centrality (BC) is a measure of the extent to which a node in a network is located in the middle of the network and is associated with the connectivity of other nodes [12,13]. When the BC is > 0.1, nodes with purple trims are those that act as a ‘bridge’ and ‘intermediary’ in the network. High BC values can be used to identify high-quality and potential publications [14]. The network timeline view identified research trends and their evolution over different periods. Burst detection was used to determine the statistical significance of frequency fluctuations using a function over a specific time period, relatively reflecting the latest dynamic trends for cited references and keywords in a surge [15]. Red nodes indicated a burst of highly cited references or a burst keyword. Each cluster was named using the log-likelihood ratio algorithm. Among the cluster mapping parameters, the two important indicators were Modularity Q (Q) and Mean Silhouette (S). The Q score of a network measures the extent to which it can be reasonably classified into modules or clusters, whereas the S score is used to interpret and validate homogeneity within data clusters [16]. Q > 0.3 was considered significant, with higher values indicating a well-structured clustering network. S > 0.5 was considered reasonable, whereas S > 0.7 suggested that the clustering result was highly credible. Moreover, the H-index is a valuable bibliometric measure frequently used to assess an individual's scholarly achievements; a higher H-index indicates that their articles have a greater impact [17].
3. Results
3.1. Annual analysis of document volume
Based on our search strategy, 1578 articles related to HE pathogenesis in patients with liver cirrhosis, published between 1978 and September 7, 2022, were retrieved. After de-duplication, the final 1578 publications, including 1243 articles and 335 reviews, were included in CiteSpace for analysis. The number of publications increased from 25 in 2005 to 153 in 2021, with approximately 38% of the publications published in the last five years. Overall, the annual publication number has increased over time. The polynomial trend line is a curve applied to the collection of increasing data at a specific rate. To understand the trends in annual publications, we obtained a significant power correlation with satisfactory explicability (R = 0.9972; Fig. 2).
Fig. 2.
Annual publication volume, accumulated annual publication volume, and trend line of HE pathogenesis in patients with liver cirrhosis.
3.2. Analysis of the author collaboration network
In the author collaboration network analysis, we obtained 798 nodes and 1961 links. Fig. 3 shows the author collaboration network; the top 10 active authors are presented in Table 1. Eight of the top 10 contributing authors are from the United States of America (USA), one is from the UK, and one is from Spain (Table 1). Bajaj, Jasmohan S is the first author of the most co-authored articles (n = 73), with the highest H-index (42) and total number of citations (6693). In the author collaboration network, three main collaborative groups were formed; the largest was centred on Bajaj, Jasmohan S, followed by those centred on Jalan, Rajiv and, Felipo, Vicente. The red nodes indicated a surge in author publications; many authors, including Bajaj, Jasmohan S (USA; n = 73), Jalan, Rajiv (UK; n = 51), Vilstrup, Hendrik (Denmark; n = 13), Fagan, Andrew (USA; n = 28), White, Melanie B (USA; n = 23), Gavis, Edith A (USA; n = 16), and others, are trending towards this growth in publications (Fig. 3).
Fig. 3.
Visualization of author collaboration network. Each node represents an author, the size of the node represents the publication volume, and the connecting line between two nodes represents the author's cooperative relationship; blue indicates earlier published documents; yellow indicates later published documents; red nodes indicate a burst in publications by an author.
Table 1.
Top 10 author with the most publications.
| Authors | Country | Count | Centrality | Year | H-index | Total citations |
|---|---|---|---|---|---|---|
| Bajaj, Jasmohan S | USA | 73 | 0.05 | 2010 | 42 | 6693 |
| Jalan, Rajiv | UK | 51 | 0.09 | 2003 | 35 | 4710 |
| Gillevet, Patrick M | USA | 37 | 0 | 2012 | 28 | 4574 |
| Sikaroodi, Masoumeh | USA | 36 | 0 | 2012 | 27 | 4509 |
| Felipo, Vicente | SPAIN | 32 | 0.03 | 2006 | 20 | 1700 |
| Fagan, Andrew | USA | 28 | 0 | 2016 | 17 | 1563 |
| White, Melanie B | USA | 23 | 0 | 2013 | 20 | 3655 |
| Heuman, Douglas M | USA | 23 | 0 | 2012 | 19 | 2921 |
| Hylemon, Phillip B | USA | 20 | 0 | 2012 | 18 | 3506 |
| Fuchs, Michael | USA | 18 | 0 | 2013 | 14 | 1939 |
3.3. Analysis of the institutional collaboration network
In the institutional collaboration network analysis, approximately 490 institutions participated in research on HE pathogenesis in patients with liver cirrhosis. The top 10 institutions with the highest number of publications are presented in Table 2. Among them, the University of London published the most publications (n = 107), accounting for 4.56% of the total articles published worldwide, with a total of 7945 citations (average: 74.25 citations per paper) and an H-index of 49. Meanwhile, the most citations were from Virginia Commonwealth University (n = 81), accounting for 3.45% of the total articles published worldwide, with a total of 8131 citations (average: 100.38 citations per paper) and an H-index of 44. The highest average number of citations per paper was achieved by George Mason University (n = 39), accounting for 1.66% of the total articles worldwide, a total of 4901 citations (average: 125.67 citations per paper), and an H-index of 29. Of the top 10 most published institutions, most are in the USA (four institutions). Fig. 4 shows the institutional collaboration network in this field of research. Of the top 35 contributing institutions, only Zhejiang University (n = 13) and Nanjing University (n = 12) were from China.
Table 2.
Top 10 Institutions with the most publications.
| Institutions | Country | Count | Centrality | Year | H-index | Total citations |
|---|---|---|---|---|---|---|
| University of London | UK | 107 | 0.07 | 1998 | 49 | 7945 |
| University College London | UK | 92 | 0.07 | 1998 | 44 | 6942 |
| Virginia Commonwealth University | USA | 81 | 0.03 | 2010 | 44 | 8131 |
| Ge Hunter Holmes McGuire Veterinary Affairs Medical Center orge Mason Univ | USA | 67 | 0.03 | 2001 | 40 | 6526 |
| CIBER - Centro de Investigacion Biomedica en Red | SPAIN | 57 | 0.04 | 2008 | 29 | 3453 |
| George Mason University | USA | 39 | 0.02 | 1998 | 29 | 4901 |
| University of Padua | ITALY | 38 | 0.03 | 1998 | 21 | 2251 |
| University of California System | USA | 36 | 0.11 | 2007 | 20 | 2729 |
| Universite de Montreal | CANADA | 35 | 0.04 | 1997 | 23 | 2175 |
| Autonomous University of Barcelona | SPAIN | 34 | 0.01 | 2001 | 20 | 2585 |
Fig. 4.
Visualization of the institutional collaboration network. Each node represents an institution, the size of the node represents the number of documents published, and the connecting line between two nodes represents the institution's cooperative relationship; blue indicates earlier published documents; yellow indicates later published documents; red nodes indicate a burst of publication by an institution.
3.4. Analysis of the country collaboration network
A total of 64 countries were included in the country collaboration network analysis. The 10 most productive countries are presented in Table 3. The USA has the most publications (347), accounting for 17.32% of all publications worldwide, garnering a total of 18,628 citations (average: 53.68 citations per paper), with an H-index of 67. Following the USA, Peoples R China published 165 articles (8.23% of the total) with 4339 citations (average: 29.30 citations per paper) and an H-index of 31. Next, Spain published 162 articles, accounting for 8.10% of the total, with 9308 citations (average: 57.46 citations per paper) and an H-index of 52. The total number of citations to papers in the USA is much higher than in other countries. Moreover, although Germany, Spain, England, Italy, India, Canada, and Denmark published fewer articles than Peoples R China, they garnered higher average numbers of citations per paper. Although England ranked sixth in the total number of published papers (average: 66.16 citations per paper), it ranked first among the top 10 countries with the most published papers. The country collaboration network mapping revealed that the USA and Spain cooperated closely with other countries, while Peoples R China had less cooperation (Fig. 5). This map also facilitated the visualization of the BC and burst strengths of the countries in the network. The USA (BC = 0.26), England (BC = 0.29), Denmark (BC = 0.17), Spain (BC = 0.20), and Italy (BC = 0.11) had nodes with purple trims, indicating high BCs that acted as bridges.
Table 3.
Top 10 country with the most publications.
| Country | Count | Centrality | Year | H-index | Total citations |
|---|---|---|---|---|---|
| USA | 347 | 0.26 | 1996 | 67 | 18628 |
| PEOPLES R CHINA | 165 | 0.10 | 2000 | 31 | 4339 |
| SPAIN | 162 | 0.20 | 1999 | 52 | 9308 |
| JAPAN | 156 | 0 | 2000 | 32 | 3614 |
| ITALY | 145 | 0.11 | 1996 | 48 | 7422 |
| ENGLAND | 140 | 0.29 | 1996 | 54 | 9262 |
| GERMANY | 134 | 0.04 | 1996 | 43 | 6273 |
| INDIA | 96 | 0.09 | 2000 | 33 | 3446 |
| CANADA | 65 | 0.04 | 1997 | 31 | 3503 |
| DENMARK | 56 | 0.17 | 2001 | 31 | 2946 |
Fig. 5.
Visualization of the country collaboration network. Each node represents a country, the size of the node represents publication volume, and the connecting line between two nodes represents the national cooperative relationship; blue indicates earlier published documents; yellow indicates later published documents; red nodes indicate a burst in publications by a country.
3.5. Cited-journal analysis
Table 4 presents the top 10 most influential co-cited journals in this field. Hepatology (n = 1404; IF = 13.5), J Hepatol (n = 1254; IF = 25.7), and Gastroenterology (n = 1111; IF = 29.4) are the top co-cited journals, with impact factors exceeding 10. Five of the top 10 most cited journals are published in the USA. The co-cited journal mapping revealed that Hepatology-led journals closely collaborate with J Hepatol, Gastroenterology, Metab Brain Dis, Am J Gastroenterol, Gut, Lancet, New Engl J Med, Liver Int, and World J Gastroentero (Fig. 6). Additionally, this map revealed three red nodes: PLoS One (n = 363; IF = 3.7), J Clin Gastroenterol (n = 314; IF = 2.9), and Liver Int (n = 558; IF = 6.7), all of which have high bursts.
Table 4.
Top 10 most frequently cited-journal.
| Journal | Count | Centrality | country | Year | IF (2022) |
|---|---|---|---|---|---|
| HEPATOLOGY | 1404 | 0 | USA | 1996 | 13.5 |
| J HEPATOL | 1254 | 0 | NETHERLANDS | 1996 | 25.7 |
| GASTROENTEROLOGY | 1111 | 0 | USA | 1996 | 29.4 |
| AM J GASTROENTEROL | 830 | 0 | USA | 1996 | 9.8 |
| METAB BRAIN DIS | 823 | 0.01 | USA | 1996 | 3.6 |
| GUT | 674 | 0.01 | UK | 1996 | 24.5 |
| NEW ENGL J MED | 615 | 0.01 | USA | 1996 | 158.5 |
| LANCET | 612 | 0.01 | UK | 1996 | 168.9 |
| LIVER INT | 578 | 0.01 | DENMARK | 2005 | 6.7 |
| WORLD J GASTROENTERO | 546 | 0 | PEOPLES R CHINA | 2005 | 4.3 |
IF: Impact factor.
Fig. 6.
Visualization of cited-journal mapping. Each node represents a journal, the size of the node represents publication volume, and the connecting line between two nodes represents the journal's cooperative relationship; blue indicates earlier published documents; yellow indicates later published documents; red nodes indicate a burst in publication by a journal.
3.6. Reference co-citation analysis
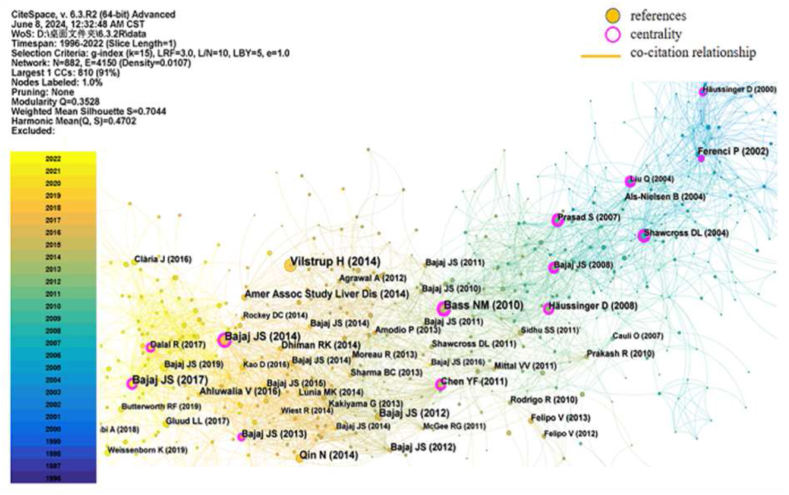
Co-citation networks reflect co-citation relationships involving the simultaneous co-reference of two documents to a third document [18]. Reference co-citation analysis is an effective way to analyse the research knowledge base in a specific field. Among the 1578 articles analysed using CiteSpace, the k = 15 threshold was set, with 882 nodes and 4150 links. The cumulative total number of citations for publications was 6322 times, with an average of ∼7.17 per document and an H-index of 28. The top 10 most co-cited references between 1978 and 2022 are presented in Table 5, and the reference co-citation mapping is shown in Fig. 7. The most-cited article was published by Vilstrup H from Aarhus University Hospital (a Danish institution), with 104 citations. The second most-cited documents were published by Bajaj, JS from an American institution and were cited 72 times. The third most-cited documents were published by Bass NM, also from an American institution, and were cited 68 times. Of the top 10 most-cited articles, five belonged to Bajaj, JS and were cited 271 times.
Table 5.
The top 10 most frequently cited references.
| Title | Author | Journal | Centrality | Year | Document type | Citation |
|---|---|---|---|---|---|---|
| Hepatic Encephalopathy in Chronic Liver Disease:2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver | Vilstrup H | HEPATOLOGY | 0.01 | 2014 | guideline | 104 |
| Altered profile of human gut microbiome is associated with cirrhosis and its complications | Bajaj JS | J HEPATOL | 0.11 | 2014 | article | 72 |
| Rifaximin Treatment in hepatic encephalopathy | Bass NM | NEW ENGL J MED | 0.28 | 2010 | article | 68 |
| Fecal Microbiota Transplant From a Rational Stool Donor Improves hepatic encephalopathy: A Randomized Clinical Trial | Bajaj JS | HEPATOLOGY | 0.11 | 2017 | article | 66 |
| Alterations of the human gut microbiome in liver cirrhosis | Qin N | NATURE | 0.02 | 2014 | article | 51 |
| Linkage of gut microbiome with cognition in hepatic encephalopathy | Bajaj JS | AM J PHYSIOL-GASTR L | 0.02 | 2012 | article | 50 |
| Hepatic encephalopathy in Chronic Liver Disease: 2014 Practice Guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases | Amer ASSOCSTUDYLIVERDIS | J HEPATOL | 0.05 | 2014 | guideline | 47 |
| Pathogenetic mechanisms of hepatic encephalopathy | Häussinger D | GUT | 0.19 | 2008 | review | 42 |
| Modulation of the Metabiome by Rifaximin in Patients with Cirrhosis and Minimal hepatic encephalopathy | Bajaj JS | PLOS ONE | 0.15 | 2013 | article | 42 |
| Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation | Bajaj JS | AM J PHYSIOL-GASTR L | 0.01 | 2012 | article | 41 |
Fig. 7.
Visualization of reference co-citation mapping. Each node represents a reference, the size of the node represents the publication frequency, and the connecting line between two nodes represents the co-citation relationships; blue indicates earlier appearance of the reference and co-citation relationship; yellow indicates later appearance of the reference and co-citation relationship; nodes with purple trims represent reference of high BC, acting as bridges.
Reference co-citation cluster mapping (Fig. 8) and the reference timeline view (Fig. 9) identified 12 unique clusters and revealed two major research trends. The first trend was related to early pathogenesis from 1991 to 2005 with eight research clusters: clusters #1, #6–10, and #11. The second trend involved emerging pathogenesis from 2004 to 2022 with five research clusters: clusters #0, #2–5. Among these, clusters #0, #1, #2, #4, and #5 had strong citation bursts, indicating that they had a major and active influence between 2000 and 2016, particularly clusters #0, #2, and #4 (Fig. 9). In the last five years, clusters #2, #3, and #5 have remained active. In the burst detection of the reference co-citation analysis, we set the minimum duration of citation bursts to 5 years. The top 32 references with the strongest citation bursts are shown in Fig. 10, indicating that the contribution of each paper was relatively significant.
Fig. 8.
Visualization of reference co-citation cluster mapping. Each cluster is represented by a different coloured area.
Fig. 9.
Visualization of reference co-citation timeline view. The later nodes appear on the right side of the timeline view, and the earlier nodes appear on the left side of the node.
Fig. 10.
Top 32 references with strongest citation bursts, sorted by the beginning year of the burst. Each bar represents one year; red bar represents strong citation bursts.
Papers with a high citation number are considered influential in the research field. Thus, we selected the top 50 highly cited articles for retrospective analysis to investigate research trends and scientific progress (Supplemental Table 1). These articles were published between 2002 and 2019, with most published in 2014 (n = 9), followed by 2011 (n = 8), 2013 (n = 6), 2016 (n = 5), 2010 (n = 4), 2004, 2012, 2017 and 2019 (n = 3 each), 2007 and 2008 (n = 2 each), and 2015 and 2002 (n = 1 each). The total number of citations for the top 50 highly cited papers is 1686. These articles were published in 19 journals, with the most in Hepatology (n = 12 articles), followed by the Journal of Hepatology (n = 6). Articles can be categorised into the following groups based on their publication format: article (n = 29), review (n = 18, with 1 meta-analysis), practice guideline (n = 2), and case report (n = 1). Of the 29 articles, 15 reported on microbial therapy of HE, 12 discussed HE-associated mechanisms, and 2 focused on acute-on-chronic liver failure. Of the 18 reviews, 8 discussed the therapeutic strategies of HE, while 7 evaluated HE pathogenesis, 2 reported on the definition, clinical grading, and diagnostic principles of HE, and 1 discussed the design of clinical trials in HE. The two practice guidelines discussed the definition, diagnosis, and management of HE, while the one case report reported on faecal microbial transplant (FMT).
3.7. Keyword co-occurrence analysis
The construct co-occurrence networks analysed the imported data, identifying keywords in the text, calculating the frequency of keyword co-occurrences, and finding keyword clusters in the network [19]. In the keyword co-occurrence analysis, 724 nodes and 5875 links were obtained. The top 10 keyword frequency rankings included ‘hepatic encephalopathy,’ ‘cirrhosis,’ ‘ammonia,’ ‘liver disease,’ ‘gut microbiota,’ ‘double blind,’ ‘cirrhotic patient,’ ‘hyperammonemia,’ ‘diagnosis,’ and ‘brain’ (Table 6). Fig. 11 shows the keyword co-occurrence mapping, with ‘cirrhosis’ and ‘liver disease’ playing an ‘intermediary’ role. Fig. 12, Fig. 13 show the keyword co-occurrence cluster mapping and keyword timeline views, respectively (Q = 0.3528 and S = 0.7030), indicating that this clustering result is reasonable.
Table 6.
Top 10 keywords with the most co-occurrences.
| Count | Centrality | Year | Keywords |
|---|---|---|---|
| 1057 | 0.01 | 1996 | hepatic encephalopathy |
| 725 | 0.05 | 1996 | cirrhosis |
| 258 | 0.07 | 1996 | ammonia |
| 203 | 0.07 | 1999 | liver disease |
| 199 | 0.04 | 2004 | gut microbiota |
| 184 | 0.06 | 2000 | double blind |
| 170 | 0.08 | 1996 | cirrhotic patient |
| 160 | 0.06 | 1998 | hyperammonemia |
| 148 | 0.03 | 1996 | diagnosis |
| 137 | 0.08 | 1996 | brain |
Fig. 11.
Visualization of keyword co-occurrence mapping. Each node represents a keyword, the size of the node represents the keyword frequency, the connecting line between two nodes represents the co-occurrence relationship; blue represents earlier appearance of a keyword and co-occurrence relationship; yellow represents later appearance of the keyword and co-occurrence relationship. Nodes with purple trims represent keywords with high BC, acting as bridges.
Fig. 12.
Visualization of keyword co-occurrence cluster mapping. Each cluster is represented by a different coloured area.
Fig. 13.
Visualization of keyword co-occurrence timeline view. The later nodes appear on the right side of the timeline view, and the earlier nodes appear on the left side of the node.
Keyword clustering also obtained nine cluster IDs (with indication of the label, size, silhouette score, and average year of the cluster members' keyword): cluster #0 (‘hyperammonemia,’ 140, S = 0.649, 2006), #1 (‘bacterial translocation,’ 131, S = 0.635, 2013), #2 (‘manganese,’ 111, S = 0.709, 2003), #3 (‘lactulose,’ 91, S = 0.697, 2009), #4 (‘helicobacter pylori,’ 66, S = 0.713, 2011), #5 (‘diagnosis,’ 61, S = 0.790, 2001), #6 (‘liver fibrosis,’ 54, S = 0.790, 2009), #7 (‘mars,’ 48, S = 0.810, 2007), and #8 (‘sleep fragmentation,’ 6, S = 0.980, 2012).
Keyword burst detection can identify changes in research trends and visualise recent research hotspots. When performing keyword burst detection, the minimum duration of burst detection was set to 5 years. The top 25 keywords with the strongest citation bursts are shown in Fig. 14. Large-scale keyword citation bursts exploded between 2016 and 2022. Therefore, we can conclude that research on ‘portal systemic encephalopathy,’ ‘amino acids,’ ‘basal ganglia,’ ‘globus pallidus,’ ‘manganese,’ ‘helicobacter pylori,’ ‘cerebral blood flow,’ and ‘soluble guanylate cyclase’ has stabilised in this field (1996–2015). Since then, ‘sarcopenia,’ ‘gut microbiota,’ ‘mortality,’ ‘association,’ ‘risk,’ ‘survival,’ and ‘probiotics’ have emerged in the last five years and are gradually becoming hot research topics related to HE pathogenesis.
Fig. 14.
Top 25 keywords with strongest citation bursts, sorted by the beginning year of the burst. Each bar represents one year; red bar represents strong citation bursts.
4. Discussion
4.1. General information
In the present study, 1578 publications on HE pathogenesis published between 1978 and 2022 were visualised using CiteSpace to help researchers understand the current global research status and predict future research hotspots. Based on the collaborative network analysis, gaining insight into the active countries, authors, and institutions in this field and tracking international research hotspots over time is advantageous. The increase in the number of articles published annually shows that HE pathogenesis is an active research field that has attracted widespread attention. The collaborative network analysis revealed that the USA had the highest number of publications, total citations, and H-index; moreover, four of the top 10 institutions with the highest number of publications are within the USA. Hepatology is the most influential journal, also published in the USA, which may be related to the country's economic strength, research capacity, science and technology, and more robust healthcare system. In the author collaborative network analysis, Bajaj, Jasmohan S, was a prominent author from the USA with the most publications, including five of the 10 most cited papers, and total citations and the highest H-index. Their research is dedicated to exploring the role of gut microbiome in HE [20]. Given the similar research focuses of Gillevet, Patrick M and Sikaroodi, Masoumeh, they have formed a collaborative group with Bajaj, Jasmohan S to evaluate changes in the gut microbiome in cirrhosis and the associated complications. Key findings that have come out of this collaboration include describing the improved cognitive dysfunction and gut microbiome dysbiosis associated with microbiome therapeutics (including rifaximin and faecal microbiota transplant) in cirrhosis complicated with HE [21,22]. Meanwhile, Jalan, Rajiv from the UK has extensively researched the liver–gut axis and ammonia metabolic pathways. The collaborative group centred on them focuses on ammonia-lowering strategies [23,24]. Felipo, Vicente, from Spain, focuses on the central role of hyperammonemia and inflammation in HE and has formed a collaborative group to conduct more in-depth research in this field [25,26]. None of the top 10 largest contributing authors were Chinese, and only Zhejiang University and Nanjing University in China were among the top 35 contributing institutions; however, these two institutions lack international collaborations, potentially accounting for the low quality of the papers published by Chinese researchers. Burst detection further revealed that China published several early-stage articles, which received international attention and ranked second among the total number of published articles, making a non-negligible contribution to the field within a limited period. Therefore, China must increase its investment in research resources, learn advanced technologies from developed countries, and strengthen international cooperation to promote domestic academic progress.
4.2. Historiography analysis
The highly cited references were analysed to identify the key knowledge base in this field and explain its origins and evolution. The top 10 highly cited articles reveal the link between gut microbiota and HE, offering new insights into targeted gut therapy for HE. In reference co-citation cluster mapping (Fig. 8), we categorised the references into two different trends. The first is the study of early HE pathogenesis. Ammonia toxicity occupies a central place in HE pathogenesis [27]. In 1995, a study found that Manganese deposition in the basal ganglia promotes HE development [28]. Further research [29] noted that the accumulation of false neurotransmitters’ inhibitory neurotransmitter system and activation of the γ-aminobutyric acid adversely affect brain enzyme metabolism [30]. In 1991, Vanderrijt et al. [31] identified an association between OHE and zinc deficiency based on successive zinc depletion and supplementation regimens. Meanwhile, in the last decade, researchers have focused extensively on trace elements, revealing that reduced manganese intake, oral zinc supplementation, and oral administration of magnesium can improve the cognitive status of patients with HE [[32], [33], [34]]. However, current data have not elaborated on the specific roles of these metals in HE development. Hyponatremia—a common complication in patients with advanced liver disease—affects brain cell metabolism and may exacerbate cognitive dysfunction [7]. Bossen [35] also reported that serum sodium concentrations are associated with the risk of developing HE. Hence, the role of hyponatremia in HE pathogenesis cannot be underestimated; nevertheless, its management in patients with cirrhosis remains challenging. Moreover, cerebellar neurodegeneration has been observed in a rat model of episodic HE [36]; however, none of these mechanisms are thoroughly understood. Therefore, while ammonia is thought to be the primary mechanism triggering HE, systemic inflammation is also a key factor in HE induction and exacerbation [23].
The top 50 highly cited papers published from 2002 to 2019 nearly coincide with the second phase of HE pathogenesis research in the timeline view. The second trend focused on the relationship between gut microbiota, sarcopenia, and HE, proposing microbiome therapies. For instance, using a systems biology approach, one study found that specific bacterial families are strongly associated with cognition and inflammation in HE [20]. Meanwhile, Qin et al. [29] revealed alterations in the gut microbiome in patients with liver cirrhosis by establishing a novel gut gene catalogue (liver cirrhosis catalogue). Ahluwalia et al. [37] used multimodal MRI to link the gut–liver–brain axis and found that specific gut microbial changes are associated with ammonia, systemic inflammation, and neuronal and astrocyte dysfunction. Similarly, reviews have detailed a model of impaired gut–liver–brain axis in HE [38,39]. A connection between gut microbiota and HE has clearly been reported, sparking interest in microbiome-based therapies. Indeed, microbiome therapeutics for HE have achieved some success. In 2004, synbiotic modulation of the gut microbiota was reported as being associated with significantly reduced blood ammonia levels, endotoxemia, and reversal of MHE [40]. Moreover, a growing number of studies have found that lactulose, probiotics, and rifaximin significantly improve MHE and HRQoL [21,41]. In 2017, Bajaj et al. [22] found that faecal FMT improved cognitive and ecological dysregulation in patients with HE and cirrhosis. Additionally, a prospective study found that muscle depletion—a common issue encountered in patients with cirrhosis—increases the risk of HE [42]. Sarcopenia can be regarded as a predictor of MHE, and its amelioration may improve MHE [43]. EASL stated that patients with HE should be assessed for nutritional status and sarcopenia [44].
Overall, the historiography analysis described four themes: (1) ammonia toxicity occupies a central place in HE pathogenesis; (2) trace element levels are associated with HE, but a lack of data has confirmed this association; (3) gut microbiota is associated with HE, and targeted gut therapy is becoming recognised as an emerging treatment for HE; and (4) sarcopenia is associated with HE and nutritional management should be provided to HE patients.
4.3. Research hotspots
4.3.1. Ammonia and HE
The pathogenesis of HE is complex and multifactorial and remains poorly understood. However, there is a consensus that ammonia plays a key role in cirrhosis-related brain dysfunction in patients with HE and has been indicated as a therapeutic target [27]. When a patient has cirrhosis, ammonia metabolism in the extrahepatic organs is altered due to the decreased ability of the liver to remove ammonia, disrupting the balance between organs that produce ammonia and those that expel it [45]. The primary event in HE occurrence could be the inhibition of cerebral energy metabolism by increased blood ammonia [46]. Ammonia exerts deleterious effects through myriad pathways associated with cell swelling, inflammation, oxidative stress, and mitochondrial dysfunction [27]. The duration of hyperammonemia and the blood ammonia level may affect the survival of neuronal cells, a notion that is difficult to further substantiate due to the difficulty in dynamically detecting blood ammonia [47]. In addition, ammonia neurotoxicity induces astrocyte senescence, leading to neuronal death [48]. However, the underlying mechanisms remain unclear. Collectively, we have recognised the critical role of hyperammonemia and maintained that ammonia-lowering therapy remains the most central treatment for HE; however, limited information is available regarding ammonia production inhibition and enhanced ammonia removal. Systemic inflammation is also a key factor in HE induction and exacerbation [23], and together with ammonia, enhances neurotoxicity through increased blood–brain barrier permeability and oxidative stress [49]. Ammonia interferes with mitochondrial energy metabolism. Heidari et al. [50] suggested the potential therapeutic value of mitochondrial protectors in managing hyperammonemia and that brain mitochondria may serve as a potential therapeutic target for HE treatment. Astrocyte swelling is a central neuromarker in HE pathology; however, the precise molecular pathways are not fully understood. The ‘Trojan horse’ hypothesis has been proposed to explain astrocyte swelling, in which glutamine serves as a carrier of ammonia into the mitochondria, and the accumulation of ammonia in the mitochondria leads to oxidative stress, resulting in astrocyte swelling [51]. In animal models, HE-related astrocyte oedema may be associated with the dysregulation of tumour necrosis factor-alpha (TNFα), glial fibrillary acidic protein (GFAP), and aquaporin-4 (AQP4), as well as the inwardly rectifying potassium channels (Kir 4.1) [52].
4.3.2. Gut microbiota and HE
In patients with cirrhosis, decreased intestinal motility, reduced gastric acid and pancreatic biliary secretions, and portal hypertensive enteropathy (or colopathy) contribute to intestinal barrier impairment. This contributes to dysbiosis and possible bacterial translocation (BT) [35,53]. Dysbiosis-induced dysregulation of the intestinal immune system promotes barrier disruption and BT [54]. HE episodes are characterised by alterations in the gut microbiota composition and microbiota-mediated changes in ammonia metabolism [55]. With the recent development of non-culture techniques, the understanding of the gut microbiota in patients with HE has advanced. Deep stool microbiome analysis in patients with cirrhosis revealed that OHE is associated with a wide range of faecal bacterial species and lower concentrations of short-chain fatty acids (SCFA). SCFAs are essential energy sources for the host colonic epithelium. SCFA deficiency is detrimental to the maintenance of intestinal barrier function [56]. 16S rRNA-based pyrosequencing has revealed that MHE patients have higher levels of Streptococcus salivariu, an ammonia-producing gut bacteria [57]. Specific bacterial families belonging to the phylum Proteobacteria (Escherichia coli and Klebsiella pneumoniae) are potentially harmful. In contrast, certain bacterial families belonging to gram-positive clostridia (Lachnospiraceae and Ruminococcaceae) are potentially beneficial [58]. The inflammatory markers interleukin (IL)-23, IL-2, IL-1b, IL-13, and IL-4 are strongly correlated with gut microbiome composition, which may indicate a synergistic effect between inflammation and the gut microbiome in promoting cognitive impairment [59,60]. In particular, the IL-23/IL-17 cytokine pathway may be a potential mechanism underlying intestinal inflammation in patients with HE [61,62].
Two forms of gut microbiota disease have been described in patients with cirrhosis: small intestinal bacterial overgrowth (SIBO) and dysbiosis intestinalis [63,64]. The incidence of SIBO in patients with cirrhosis is as high as 73% and is particularly common in those with a history of HE [53]. In patients with cirrhosis, SIBO is one of the major factors promoting BT and a risk factor for HE [63,65]. PPIs might promote the development of SIBO [66,67]. The desired effect of PPIs is to reduce gastric acid production and increase gastric pH. However, eliminating the gastric acid barrier promotes SIBO, increasing the risk of intestinal bacterial metastasis to the mesenteric lymph nodes and to the blood and lymph [65,68]. This can lead to systemic inflammation, an important factor in the development of HE in patients with cirrhosis. However, other studies disagree with this notion. In an adequately powered equivalence study, PPI was found to not be significantly associated with SIBO [69]. Through our investigations, we found that this difference in experimental results was likely due to the test type, geographical location, and age confounding the association between PPIs. Silvia et al. [70] have also proposed that cognitive impairment may be reversible after PPI withdrawal. PPIs are widely used to prevent peptic complications in patients with cirrhosis [71]. However, most patients hospitalised for cirrhosis are prescribed PPIs with unclear indications [72,73].
4.3.3. Sarcopenia and HE
Sarcopenia, a common complication of end-stage liver disease, is broadly defined as loss of muscle mass and function. It is a disorder of skeletal muscle with multifactorial pathogenesis that results from an imbalance between protein synthesis and breakdown [[74], [75], [76]]. The prevalence of sarcopenia is as high as 70% among patients with cirrhosis, whereas that of MHE is higher among patients with sarcopenia than those without [43,[77], [78], [79]]. The mechanisms by which blood ammonia and sarcopenia mutually promote each other are relatively well-defined. Muscle catabolism during muscle wasting promotes an increased release of glutamine, produces ammonia through glutaminase, and elevates blood ammonia levels [80]. Hyperammonemia contributes to sarcopenia in several ways, including increasing serum myostatin levels, inhibiting muscle protein anabolism, and increasing autophagy [[81], [82], [83]]. Therefore, the amelioration of sarcopenia may improve cognitive function [42]. Lowering the ammonia levels reverses sarcopenia by restoring skeletal muscle proteostasis, which may reduce the risk of HE [80]. Therefore, targeted ammonia-lowering therapy remains the key treatment for improving HE and sarcopenia. Notably, the gut microbiota influences the development of sarcopenia in patients with cirrhosis. Although the concept of the gut–muscle axis has been raised, its causal relationship remains unclear. BT-induced systemic inflammation in the gut leads to proteolytic metabolism and reduced muscle mass [84,85]. Excess bacteria in SIBO compete with the body for nutrients in the gut, contributing to malnutrition in cirrhotic patients [86]. The gut microbiota pathology in patients with cirrhosis and sarcopenia is associated with increased myostatin, a protein that promotes protein catabolism in muscles and inhibits growth [87]. The relationship between gut microbiota and sarcopenia is not fully understood. However, studies have shown that microbiome therapeutics can effectively address the impaired gut-muscle axis in cirrhotic patients [84].
4.3.4. Microbiome therapeutics and HE
Recent research suggests promising microbiome-targeted HE therapies, such as prebiotics, probiotics, FMT, antibiotics, postbiotics, and absorbents [63,88,89]. Microbiome therapeutics aim to increase beneficial and decrease harmful taxa. Microbiome therapeutics for HE, particularly lactulose and rifaximin, have achieved some success, with probiotics and FMT showing potential [90]. Probiotics may improve HE by improving intestinal barrier function, decreasing portal hypertension, and immune modulation [[91], [92], [93]]. While probiotics may improve cognitive status, reverse MHE, and reduce OHE incidence, the evidence quality is low or moderate. Meanwhile, FMT is a medical procedure that involves transferring processed stool from a healthy donor to a recipient to help restore a healthy balance of gut bacteria. FMT affects HE through various potential mechanisms, such as altering the microbiome community structure, producing SCFAs, reducing ammonia production, and regulating bile acid metabolism [22,94,95]. FMT enemas have been shown to reduce hospitalisation rates and improve cognitive and ecological dysregulation in patients with recurrent HE and cirrhosis [22]. FMT capsules have also been proven safe in patients with HE [96]. Microbiome therapy is associated with several challenges. Currently, we have neither mastered the method to prepare FMT, found an ideal donor for FMT, nor established the optimal FMT dosing regimen or whether the patient will require repeat dosing. Therefore, future therapies may target microbiota-host interactions, intestinal permeability, and host immune regulation. The human gut microbiota is constantly challenged by pharmacological, dietary, and host factors. Future research in this area should focus on more rigorous trial designs, selection of microbiome therapeutics, and personalised approaches to HE. This will help optimise the effectiveness of treatments and improve patient outcomes.
4.4. Limitations
First, the data in this study were collected only from the WOSCC, causing a lack of data to a certain extent, as data from PubMed, MEDLINE, Google Scholar, and Scope were not included, possibly due to CiteSpace being incompatible with other databases. Future studies are required to verify the findings in other databases. Second, combining synonyms cannot rule out bias in the results owing to subjective factors.
5. Conclusions
This study summarises and analyses the global research trends in HE pathogenesis using bibliometric methods. The overall number of publications has shown an increasing trend annually, and the USA has made the greatest contribution to this field, suggesting the need for greater academic collaboration. Sarcopenia and PPIs can also contribute to HE development by affecting the gut microbiota; therefore, acid-inhibiting drugs should be used with caution. Ammonia-lowering therapy and microbiome therapeutics are two important initiatives in HE treatment. Despite the success of microbiome therapeutics, many challenges remain, and additional studies are needed to validate their safety and efficacy.
Ethics and consent statement
Review and/or approval by an ethics committee was not needed for this study because no humans or animals were involved.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81700467), the Foundation of Science and Technology Department of Sichuan Province (2022YFS0340), the Chengdu Science and Technology Bureau (2021-YF05-00585-SN) and the Foundation of Medical Association of Sichuan Province (S22085).
CRediT authorship contribution statement
Shiyan Wu: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Lu Li: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Data curation. Heng Xi: Writing – original draft, Validation, Methodology, Conceptualization. Xiaoping Wu: Writing – original draft, Validation, Software. Yumei He: Writing – review & editing, Validation. Xiaobin Sun: Validation, Supervision, Conceptualization. Liping Wu: Writing – review & editing, Writing – original draft, Validation, Supervision, Data curation.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Liping Wu reports financial support was provided by the National Natural Science Foundation of China, the Foundation of Science and Technology Department of Sichuan Province, the Chengdu Science and Technology Bureau and the Foundation of Medical Association of Sichuan Province. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e34330.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Rose C.F., Amodio P., Bajaj J.S., et al. Hepatic encephalopathy: novel insights into classification, pathophysiology and therapy[J/OL] J. Hepatol. 2020;73(6):1526–1547. doi: 10.1016/j.jhep.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Lauridsen M.M., Jepsen P., Vilstrup H. Critical flicker frequency and continuous reaction times for the diagnosis of minimal hepatic encephalopathy: a comparative study of 154 patients with liver disease[J/OL] Metab. Brain Dis. 2011;26(2):135–139. doi: 10.1007/s11011-011-9242-1. [DOI] [PubMed] [Google Scholar]
- 3.Allampati S., Duarte-Rojo A., Thacker L.R., et al. Diagnosis of minimal hepatic encephalopathy using stroop EncephalApp: a multicenter US-based, norm-based study[J/OL] Am. J. Gastroenterol. 2016;111(1):78–86. doi: 10.1038/ajg.2015.377. [DOI] [PubMed] [Google Scholar]
- 4.Romero-Gómez M., Córdoba J., Jover R., et al. Value of the critical flicker frequency in patients with minimal hepatic encephalopathy[J/OL] Hepatol. (Baltimore, Md) 2007;45(4):879–885. doi: 10.1002/hep.21586. [DOI] [PubMed] [Google Scholar]
- 5.Bajaj J.S., Pinkerton S.D., Sanyal A.J., et al. Diagnosis and treatment of minimal hepatic encephalopathy to prevent motor vehicle accidents: a cost-effectiveness analysis[J/OL] Hepatol. (Baltimore, Md) 2012;55(4):1164–1171. doi: 10.1002/hep.25507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero-Gómez M., Córdoba J., Jover R., et al. Quality of life in patients with minimal hepatic encephalopathy[J/OL] World J. Gastroenterol. 2018;24(48):5446–5453. doi: 10.3748/wjg.v24.i48.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tapper E.B., Halbert B., Mellinger J. Rates of and reasons for hospital readmissions in patients with cirrhosis: a multistate population-based cohort study[J/OL] Clin. Gastroenterol. Hepatol.: The Off. Clin. Pract. J. The Am. Gastroenterol. Assoc. 2016;14(8):1181–1188.e2. doi: 10.1016/j.cgh.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj J.S., Reddy K.R., Tandon P., et al. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis[J/OL] Hepatol. (Baltimore, Md) 2016;64(1):200–208. doi: 10.1002/hep.28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gustot T., Fernandez J., Garcia E., et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis[J/OL] Hepatol. (Baltimore, Md) 2015;62(1):243–252. doi: 10.1002/hep.27849. [DOI] [PubMed] [Google Scholar]
- 10.Cordoba J., Ventura-Cots M., Simón-Talero M., Amorós À., et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF)[J/OL] J. Hepatol. 2014;60(2):275–281. doi: 10.1016/j.jhep.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Tapper E.B. Challenge accepted: confronting readmissions for our patients with cirrhosis[J/OL] Hepatol. (Baltimore, Md) 2016;64(1):26–28. doi: 10.1002/hep.28471. [DOI] [PubMed] [Google Scholar]
- 12.Freeman L.C. A set of measures of centrality based on betweenness[J/OL] Sociometry. 1977;40(1):35. doi: 10.2307/3033543. [DOI] [Google Scholar]
- 13.Brandes U. A faster algorithm for betweenness centrality*[J/OL] J. Math. Sociol. 2001;25(2):163–177. doi: 10.1080/0022250X.2001.9990249. [DOI] [Google Scholar]
- 14.Chen C. The centrality of pivotal points in the evolution of scientific networks[C/OL]//Proceedings of the 10th international conference on Intelligent user interfaces. San Diego California USA: ACM. 2005:98–105. doi: 10.1145/1040830.1040859. https://dl.acm.org/doi/10.1145/1040830.1040859 [2022-10-24] [DOI] [Google Scholar]
- 15.Chen C., Ibekwe-SanJuan F., Hou J. The structure and dynamics of cocitation clusters: a multiple-perspective cocitation analysis[J/OL] J. Am. Soc. Inf. Sci. Technol. 2010;61(7):1386–1409. doi: 10.1002/asi.21309. [DOI] [Google Scholar]
- 16.Shibata N., Kajikawa Y., Takeda Y., et al. Detecting emerging research fronts based on topological measures in citation networks of scientific publications[J/OL] Technovation. 2008;28(11):758–775. doi: 10.1016/j.technovation.2008.03.009. [DOI] [Google Scholar]
- 17.Wu K., Liu Y., Liu L., et al. Emerging trends and research foci in tumor microenvironment of pancreatic cancer: a bibliometric and visualized study[J/OL] Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.810774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Small H. Co-citation in the scientific literature: a new measure of the relationship between two documents[J/OL] J. Am. Soc. Inf. Sci. 1973;24(4):265–269. doi: 10.1002/asi.4630240406. [DOI] [Google Scholar]
- 19.Synnestvedt M.B., Chen C., Holmes J.H. CiteSpace II: visualization and knowledge discovery in bibliographic databases[J] AMIA … Annu. Symp. Proc. AMIA Symp. 2005:724–728. [PMC free article] [PubMed] [Google Scholar]
- 20.Bajaj J.S., Ridlon J.M., Hylemon P.B., et al. Linkage of gut microbiome with cognition in hepatic encephalopathy[J/OL] Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302(1):G168–G175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajaj J.S., Heuman D.M., Sanyal A.J., et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy[J/OL] PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajaj J.S., Kassam Z., Fagan A., et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial[J/OL] Hepatol. (Baltimore, Md) 2017;66(6):1727–1738. doi: 10.1002/hep.29306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shawcross D.L., Davies N.A., Williams R., et al. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis[J/OL] J. Hepatol. 2004;40(2):247–254. doi: 10.1016/j.jhep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Olde Damink S.W.M., Jalan R., et al. The kidney plays a major role in the hyperammonemia seen after simulated or actual GI bleeding in patients with cirrhosis[J/OL] Hepatol. (Baltimore, Md) 2003;37(6):1277–1285. doi: 10.1053/jhep.2003.50221. [DOI] [PubMed] [Google Scholar]
- 25.Hernández-Rabaza V., Cabrera-Pastor A., Taoro-González L., et al. Hyperammonemia induces glial activation, neuroinflammation and alters neurotransmitter receptors in hippocampus, impairing spatial learning: reversal by sulforaphane[J/OL] J. Neuroinflammation. 2016;13:41. doi: 10.1186/s12974-016-0505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabrera-Pastor A., Llansola M., Montoliu C., et al. Peripheral inflammation induces neuroinflammation that alters neurotransmission and cognitive and motor function in hepatic encephalopathy: underlying mechanisms and therapeutic implications[J/OL] Acta Physiol. 2019;226(2) doi: 10.1111/apha.13270. [DOI] [PubMed] [Google Scholar]
- 27.Bosoi C.R., Rose C.F. Identifying the direct effects of ammonia on the brain[J/OL] Metab. Brain Dis. 2009;24(1):95–102. doi: 10.1007/s11011-008-9112-7. [DOI] [PubMed] [Google Scholar]
- 28.Krieger D., Krieger S., Jansen O., et al. Manganese and chronic hepatic encephalopathy[J/OL] Lancet (London, England) 1995;346(8970):270–274. doi: 10.1016/s0140-6736(95)92164-8. [DOI] [PubMed] [Google Scholar]
- 29.Qin N., Yang F., Li A., et al. Alterations of the human gut microbiome in liver cirrhosis[J/OL] Nature. 2014;513(7516):59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 30.Jones E.A., Basile A.S., Skolnick P. Hepatic encephalopathy, GABA-ergic neurotransmission and benzodiazepine receptor ligands[J/OL] Adv. Exp. Med. Biol. 1990;272:121–134. doi: 10.1007/978-1-4684-5826-8_7. [DOI] [PubMed] [Google Scholar]
- 31.Bajaj J.S., Salzman N.H., Acharya C., et al. Overt hepatic encephalopathy precipitated by zinc deficiency[J/OL] Gastroenterology. 1991;100(4) doi: 10.1016/0016-5085(91)90290-2. https://pubmed.ncbi.nlm.nih.gov/2001810/ 2023-20210-24. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Mei L.H., Qiang J.W., et al. Reduction of manganese intake improves neuropsychological manifestations in rats with minimal hepatic encephalopathy[J/OL] Neuroscience. 2017;347:148–155. doi: 10.1016/j.neuroscience.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Takuma Y., Nouso K., Makino Y., et al. Clinical trial: oral zinc in hepatic encephalopathy[J/OL] Aliment. Pharmacol. Ther. 2010;32(9):1080–1090. doi: 10.1111/j.1365-2036.2010.04448.x. [DOI] [PubMed] [Google Scholar]
- 34.Li Y., Ji C.X., Mei L.H., et al. Oral administration of trace element magnesium significantly improving the cognition and locomotion in hepatic encephalopathy rats[J/OL] Sci. Rep. 2017;7(1):1817. doi: 10.1038/s41598-017-02101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quigley E.M.M., Stanton C., Murphy E.F. The gut microbiota and the liver. Pathophysiological and clinical implications[J/OL] J. Hepatol. 2013;58(5):1020–1027. doi: 10.1016/j.jhep.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 36.García-Lezana T., Oria M., Romero-Giménez J., et al. Cerebellar neurodegeneration in a new rat model of episodic hepatic encephalopathy[J/OL] J. Cerebr. Blood Flow Metabol.: Off. J. The Int. Soc. of Cereb. Blood Flow and Metabolism. 2017;37(3):927–937. doi: 10.1177/0271678X16649196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahluwalia V., Betrapally N.S., Hylemon P.B., et al. Impaired gut-liver-brain Axis in patients with cirrhosis[J/OL] Sci. Rep. 2016;6 doi: 10.1038/srep26800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bajaj J.S. The role of microbiota in hepatic encephalopathy[J/OL] Gut Microb. 2014;5(3):397–403. doi: 10.4161/gmic.28684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Häussinger D., Schliess F. Pathogenetic mechanisms of hepatic encephalopathy[J/OL] Gut. 2008;57(8):1156–1165. doi: 10.1136/gut.2007.122176. [DOI] [PubMed] [Google Scholar]
- 40.Liu Q., Duan Z.P., Ha D.K., et al. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis[J/OL] Hepatol. (Baltimore, Md) 2004;39(5):1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 41.Mittal V.V., Sharma B.C., Sharma P., et al. A randomized controlled trial comparing lactulose, probiotics, and L-ornithine L-aspartate in treatment of minimal hepatic encephalopathy[J/OL] Eur. J. Gastroenterol. Hepatol. 2011;23(8):725–732. doi: 10.1097/MEG.0b013e32834696f5. [DOI] [PubMed] [Google Scholar]
- 42.Merli M., Giusto M., Lucidi C., et al. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study[J/OL] Metab. Brain Dis. 2013;28(2):281–284. doi: 10.1007/s11011-012-9365-z. [DOI] [PubMed] [Google Scholar]
- 43.Hanai T., Shiraki M., Watanabe S., et al. Sarcopenia predicts minimal hepatic encephalopathy in patients with liver cirrhosis[J/OL] Hepatol. Res.: The Official Journal of the Japan Society of Hepatology. 2017;47(13):1359–1367. doi: 10.1111/hepr.12873. [DOI] [PubMed] [Google Scholar]
- 44.EASL Clinical Practice Guidelines on nutrition in chronic liver disease[J/OL] J. Hepatol. 2019;70(1):172–193. doi: 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rose C.F. Ammonia-lowering strategies for the treatment of hepatic encephalopathy[J/OL] Clin. Pharmacol. Therapeut. 2012;92(3):321–331. doi: 10.1038/clpt.2012.112. [DOI] [PubMed] [Google Scholar]
- 46.Iversen P., Sørensen M., Bak L.K., et al. Low cerebral oxygen consumption and blood flow in patients with cirrhosis and an acute episode of hepatic encephalopathy[J/OL] Gastroenterology. 2009;136(3):863–871. doi: 10.1053/j.gastro.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 47.Braissant O., McLin V.A., Cudalbu C. Ammonia toxicity to the brain[J/OL] J. Inherit. Metab. Dis. 2013;36(4):595–612. doi: 10.1007/s10545-012-9546-2. [DOI] [PubMed] [Google Scholar]
- 48.Görg B., Karababa A., Häussinger D. Hepatic encephalopathy and astrocyte senescence[J/OL] J. Clin. Exp. Hepatol. 2018;8(3):294–300. doi: 10.1016/j.jceh.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aldridge D.R., Tranah E.J., Shawcross D.L. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation[J/OL] J. Clin. Exp. Hepatol. 2015;5(Suppl 1):S7–S20. doi: 10.1016/j.jceh.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heidari R. Brain mitochondria as potential therapeutic targets for managing hepatic encephalopathy[J/OL] Life Sci. 2019;218:65–80. doi: 10.1016/j.lfs.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 51.Albrecht J., Norenberg M.D. Glutamine: a Trojan horse in ammonia neurotoxicity[J/OL] Hepatol. (Baltimore, Md) 2006;44(4):788–794. doi: 10.1002/hep.21357. [DOI] [PubMed] [Google Scholar]
- 52.Elsherbini D.M.A., Ghoneim F.M., El-Mancy E.M., et al. Astrocytes profiling in acute hepatic encephalopathy: possible enrolling of glial fibrillary acidic protein, tumor necrosis factor-alpha, inwardly rectifying potassium channel (Kir 4.1) and aquaporin-4 in rat cerebral cortex[J/OL] Front. Cell. Neurosci. 2022;16 doi: 10.3389/fncel.2022.896172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiest R., Lawson M., Geuking M. Pathological bacterial translocation in liver cirrhosis[J/OL] J. Hepatol. 2014;60(1):197–209. doi: 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 54.Muñoz L., Borrero M.-J., Úbeda M., et al. Intestinal immune dysregulation driven by dysbiosis promotes barrier disruption and bacterial translocation in rats with cirrhosis[J/OL] Hepatol. (Baltimore, Md) 2019;70(3):925–938. doi: 10.1002/hep.30349. [DOI] [PubMed] [Google Scholar]
- 55.Sung C.M., Lin Y.-F., Chen K.-F., et al. Predicting clinical outcomes of cirrhosis patients with hepatic encephalopathy from the fecal microbiome[J/OL] Cell. Mol. Gastroenterol. Hepatol. 2019;8(2):301–318.e2. doi: 10.1016/j.jcmgh.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bloom P.P., Luévano J.M., Miller K.J., et al. Deep stool microbiome analysis in cirrhosis reveals an association between short-chain fatty acids and hepatic encephalopathy[J/OL] Ann. Hepatol. 2021;25 doi: 10.1016/j.aohep.2021.100333. [DOI] [PubMed] [Google Scholar]
- 57.Z Z., H Z., J G., et al. Large-scale survey of gut microbiota associated with MHE via 16S rRNA-based pyrosequencing[J/OL] Am. J. Gastroenterol. 2013;108(10) doi: 10.1038/ajg.2013.221. https://pubmed.ncbi.nlm.nih.gov/23877352/ [2023-11-21] [DOI] [PubMed] [Google Scholar]
- 58.Nava G.M., Stappenbeck T.S. Diversity of the autochthonous colonic microbiota[J/OL] Gut Microb. 2011;2(2):99–104. doi: 10.4161/gmic.2.2.15416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keshavarzian A., Holmes E.W., Patel M., et al. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage[J/OL] Am. J. Gastroenterol. 1999;94(1):200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 60.Mutlu E., Keshavarzian A., Engen P., et al. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats[J/OL] Alcohol Clin. Exp. Res. 2009;33(10):1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huber S., Flavell R.A. Checks and balances: IL-23 in the intestine[J/OL] Immunity. 2010;33(2):150–152. doi: 10.1016/j.immuni.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahern P.P., Schiering C., Buonocore S., et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells[J/OL] Immunity. 2010;33(2):279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bajaj J.S., Heuman D.M., Hylemon P.B., et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications[J/OL] J. Hepatol. 2014;60(5):940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghosh G., Jesudian A.B. Small intestinal bacterial overgrowth in patients with cirrhosis[J/OL] Journal of Clinical and Experimental Hepatology. 2019;9(2):257–267. doi: 10.1016/j.jceh.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gupta A., Dhiman R.K., Kumari S., et al. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy[J/OL] J. Hepatol. 2010;53(5):849–855. doi: 10.1016/j.jhep.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 66.Lombardo L., Foti M., Ruggia O., Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy[J/OL] Clin. Gastroenterol. Hepatol.: The Off. Clin. Pract. J. The Am. Gastroenterol. Assoc. 2010;8(6):504–508. doi: 10.1016/j.cgh.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 67.Compare D., Pica L., Rocco A., et al. Effects of long-term PPI treatment on producing bowel symptoms and SIBO[J/OL] Eur. J. Clin. Invest. 2011;41(4):380–386. doi: 10.1111/j.1365-2362.2010.02419.x. [DOI] [PubMed] [Google Scholar]
- 68.Janarthanan S., Ditah I., Adler D.G., et al. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis[J/OL] Am. J. Gastroenterol. 2012;107(7):1001–1010. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 69.Ratuapli S.K., Ellington T.G., O'Neill M.-T., et al. Proton pump inhibitor therapy use does not predispose to small intestinal bacterial overgrowth[J/OL] Am. J. Gastroenterol. 2012;107(5):730–735. doi: 10.1038/ajg.2012.4. [DOI] [PubMed] [Google Scholar]
- 70.Nardelli S., Gioia S., Ridola L., et al. Proton pump inhibitors are associated with minimal and overt hepatic encephalopathy and increased mortality in patients with cirrhosis[J/OL] Hepatol. (Baltimore, Md) 2019;70(2):640–649. doi: 10.1002/hep.30304. [DOI] [PubMed] [Google Scholar]
- 71.Lodato F., Azzaroli F., Girolamo M.D., et al. Proton pump inhibitors in cirrhosis: tradition or evidence based practice?[J/OL] World J. Gastroenterol. 2008;14(19):2980–2985. doi: 10.3748/wjg.14.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cole H.L., Pennycook S., Hayes P.C. The impact of proton pump inhibitor therapy on patients with liver disease[J/OL] Aliment. Pharmacol. Ther. 2016;44(11–12):1213–1223. doi: 10.1111/apt.13827. [DOI] [PubMed] [Google Scholar]
- 73.De Roza M.A., Kai L., Kam J.W., et al. Proton pump inhibitor use increases mortality and hepatic decompensation in liver cirrhosis[J/OL] World J. Gastroenterol. 2019;25(33):4933–4944. doi: 10.3748/wjg.v25.i33.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cruz-Jentoft A.J., Sayer A.A. Sarcopenia[J/OL] Lancet. 2019;393(10191):2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 75.Tandon P., Montano-Loza A.J., Lai J.C., et al. Sarcopenia and frailty in decompensated cirrhosis[J/OL] J. Hepatol. 2021;75:S147–S162. doi: 10.1016/j.jhep.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vinel C., Lukjanenko L., Batut A., et al. The exerkine apelin reverses age-associated sarcopenia[J/OL] Nat. Med. 2018;24(9):1360–1371. doi: 10.1038/s41591-018-0131-6. [DOI] [PubMed] [Google Scholar]
- 77.Kim H.Y., Jang J.W. Sarcopenia in the prognosis of cirrhosis: going beyond the MELD score[J/OL] World J. Gastroenterol. 2015;21(25):7637–7647. doi: 10.3748/wjg.v21.i25.7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Montano-Loza A.J., Duarte-Rojo A., Meza-Junco J., et al. Inclusion of sarcopenia within MELD (MELD-Sarcopenia) and the prediction of mortality in patients with cirrhosis[J/OL] Clin. Transl. Gastroenterol. 2015;6(7):e102. doi: 10.1038/ctg.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanai T., Shiraki M., Nishimura K., et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. J/OL]. Nutrition (Burbank, Los Angeles County, Calif.) 2015;31(1):193–199. doi: 10.1016/j.nut.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 80.Kumar A., Davuluri G., Silva R.N.E., et al. Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis[J/OL] Hepatol. (Baltimore, Md) 2017;65(6):2045–2058. doi: 10.1002/hep.29107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qiu J., Thapaliya S., Runkana A., et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism[J/OL] Proc. Natl. Acad. Sci. U.S.A. 2013;110(45):18162–18167. doi: 10.1073/pnas.1317049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davuluri G., Krokowski D., Guan B.-J., et al. Metabolic adaptation of skeletal muscle to hyperammonemia drives the beneficial effects of l-leucine in cirrhosis[J/OL] J. Hepatol. 2016;65(5):929–937. doi: 10.1016/j.jhep.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qiu J., Tsien C., Thapalaya S., et al. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis[J/OL] Am. J. Physiol. Endocrinol. Metab. 2012;303(8):E983–E993. doi: 10.1152/ajpendo.00183.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han J.W., Kim D.I., Nam H.C., et al. Association between serum tumor necrosis factor-α and sarcopenia in liver cirrhosis[J/OL] Clin. Mol. Hepatol. 2022;28(2):219–231. doi: 10.3350/cmh.2021.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sato S., Namisaki T., Murata K., et al. The association between sarcopenia and endotoxin in patients with alcoholic cirrhosis[J/OL] Medicine. 2021;100(36) doi: 10.1097/MD.0000000000027212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jia Yao, Chang Le, Yuan Lili, et al. Nutrition status and small intestinal bacterial overgrowth in patients with virus - related cirrhosis[J/OL] Asia Pac. J. Clin. Nutr. 2016;25(2) doi: 10.6133/apjcn.2016.25.2.06. [DOI] [PubMed] [Google Scholar]
- 87.Sharma M., McFarlane C., Kambadur R., et al. Myostatin: expanding horizons[J/OL] IUBMB Life. 2015;67(8):589–600. doi: 10.1002/iub.1392. [DOI] [PubMed] [Google Scholar]
- 88.Victor D.W., Quigley E.M.M. Hepatic encephalopathy involves interactions among the microbiota, gut, brain[J/OL] Clin. Gastroenterol. Hepatol.: The Off. Clin. Pract. J. The. Am. Gastroenterol. Assoc. 2014;12(6):1009–1011. doi: 10.1016/j.cgh.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 89.Chen Yanfei, Yang F., Lu H., et al. Characterization of fecal microbial communities in patients with liver cirrhosis[J/OL] Hepatol. (Baltimore, Md) 2011;54(2):562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 90.Bloom P.P., Tapper E.B., Young V.B., et al. Microbiome therapeutics for hepatic encephalopathy[J/OL] J. Hepatol. 2021;75(6):1452–1464. doi: 10.1016/j.jhep.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mennigen R., Nolte K., Rijcken E., et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis[J/OL] Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296(5):G1140–G1149. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- 92.Scarpignato C., Gatta L., Zullo A., et al. Effective and safe proton pump inhibitor therapy in acid-related diseases - a position paper addressing benefits and potential harms of acid suppression[J/OL] BMC Med. 2016;14(1):179. doi: 10.1186/s12916-016-0718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roselli M., Finamore A., Nuccitelli S., et al. Prevention of TNBS-induced colitis by different Lactobacillus and Bifidobacterium strains is associated with an expansion of gammadeltaT and regulatory T cells of intestinal intraepithelial lymphocytes[J/OL] Inflamm. Bowel Dis. 2009;15(10):1526–1536. doi: 10.1002/ibd.20961. [DOI] [PubMed] [Google Scholar]
- 94.Bajaj J.S., Fagan A., Gavis E.A., et al. Long-term outcomes of fecal microbiota transplantation in patients with cirrhosis[J/OL] Gastroenterology. 2019;156(6):1921–1923.e3. doi: 10.1053/j.gastro.2019.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bajaj J.S., Salzman N., Acharya C., et al. Microbial functional change is linked with clinical outcomes after capsular fecal transplant in cirrhosis[J/OL] JCI insight. 2019;4(24) doi: 10.1172/jci.insight.133410. 133410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bajaj J.S., Salzman N.H., Acharya C., et al. Fecal microbial transplant capsules are safe in hepatic encephalopathy: a phase 1, randomized, placebo-controlled trial[J/OL] Hepatol. (Baltimore, Md) 2019;70(5):1690–1703. doi: 10.1002/hep.30690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.