Version Changes
Revised. Amendments from Version 1
Changes from the previous version 1. Modification of the PPI section to provide a detailed explanation of PPI activities. 2. Addition of a sentence in the consent process section to include consent for storage of samples for future use. 3. Addition of a small paragraph in the intervention section to explain study procedures and risk mitigation. 4. Introduction of additional sentences to include data availability after study completion and full protocol availability.
Abstract
Background
MecROX is a mechanistic sub-study of the UK-ROX trial which was designed to evaluate the clinical and cost-effectiveness of a conservative approach to oxygen therapy for invasively ventilated adults in intensive care. This is based on the scientific rationale that excess oxygen is harmful. Epithelial cell damage with alveolar surfactant deficiency is characteristic of hyperoxic acute lung injury. Additionally, hyperoxaemia (excess blood oxygen levels) may exacerbate whole-body oxidative stress leading to cell death, autophagy, mitochondrial dysfunction, bioenergetic failure and multi-organ failure resulting in poor clinical outcomes. However, there is a lack of in-vivo human models evaluating the mechanisms that underpin oxygen-induced organ damage in mechanically ventilated patients.
Aim
The aim of the MecROX mechanistic sub-study is to assess lung surfactant composition and global systemic redox status to provide a mechanistic and complementary scientific rationale to the UK-ROX trial findings. The objectives are to quantify in-vivo surfactant composition, synthesis, and metabolism with markers of oxidative stress and systemic redox disequilibrium (as evidenced by alterations in the ‘reactive species interactome’) to differentiate between groups of conservative and usual oxygen targets.
Methods and design
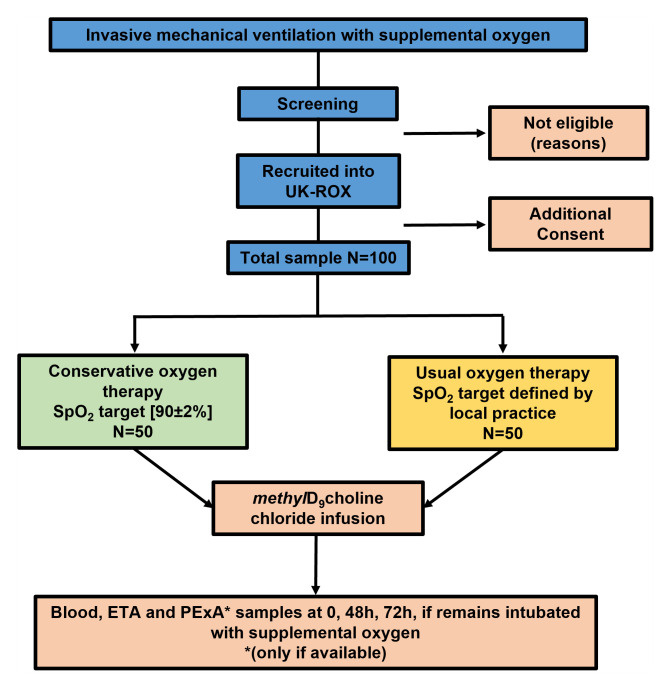
After randomisation into the UK-ROX trial, 100 adult participants (50 in the conservative and 50 in usual care group) will be recruited at two trial sites. Blood and endotracheal samples will be taken at 0, 48 and 72 hours following an infusion of 3 mg/kg methyl-D 9-choline chloride. This is a non-radioactive, stable isotope of choline (vitamin), which has been extensively used to study surfactant phospholipid kinetics in humans. This study will mechanistically evaluate the in-vivo surfactant synthesis and breakdown (by hydrolysis and oxidation), oxidative stress and redox disequilibrium from sequential plasma and bronchial samples using an array of analytical platforms. We will compare conservative and usual oxygenation groups according to the amount of oxygen administered.
Trial registration: ISRCTN
ISRCTN61929838, 27/03/2023 https://doi.org/10.1186/ISRCTN61929838.
Keywords: Oxygen, Hyperoxia, Mechanical ventilation, Surfactant, Redox, Oxidative stress
Introduction
Oxygen therapy is the most commonly used medical intervention in the intensive care unit (ICU). Most mechanically ventilated patients require supplementary oxygen, yet the optimal therapeutic oxygen levels are not known. The UK-ROX randomised controlled trial is an NIHR HTA funded study that aims to evaluate the clinical and cost-effectiveness of a conservative approach to oxygen therapy to achieve a low oxygen saturation target [SpO 2 90±2%] compared with standard therapy, determined by local practices in mechanically ventilated patients 1 (Trial registration ISRCTN 13384956, 08/12/2020, https://doi.org/10.1186/ISRCTN13384956). The primary aim of this study (MecROX) is to provide a mechanistic evaluation of systemic, alveolar redox status and dynamic surfactant biology following different oxygen therapeutic strategies in mechanically ventilated patients enrolled into the UK-ROX study. This is an observational sub-study embedded within the UK-ROX and all patients will be co-enrolled with UK-ROX interventional study.
Hyperoxia has been used to induce acute lung injury in animal models. Following exposure, animals develop significant alveolar cellular damage with capillary leak, resulting in pulmonary oedema and development of acute respiratory distress syndrome 2– 4 . Hyperoxic challenge studies of humans are limited. High concentrations of inspired oxygen for short periods in healthy humans can lead to substernal distress, pleuritic chest pain, cough, progressive dyspnoea, decline in vital capacity and carbon monoxide diffusion (DLCO) capacity and abnormalities of tracheal mucociliary movement 5– 7 . Moreover, inspired oxygen of >95% for 17 hours can lead to significant alveolar-capillary leak with increased fibroblast recruitment and proliferation 8 . These limited human and animal studies, support the notion that oxygen has the potential to cause acute lung injury and lethality in a normal uninjured lung 3, 9 . However, more importantly, an injured lung may respond differently to hyperoxic challenges than a normal lung. The implications of combined insults such as a primary lung pathology, critical illness, and mechanical ventilation in combination with oxygen toxicity in the development and progression of acute lung injury are largely unknown.
Pulmonary surfactant
Pulmonary surfactant is essential for the maintenance of alveolar integrity and consists primarily of phospholipids, of which 80–85% is phosphatidylcholine (PC), with dipalmitoyl-PC (DPPC) or PC32:0 accounting for 40–60% and proteins 10 . Anionic phospholipids [such as phosphatidylglycerol (PG), phosphatidylinositol (PI), phosphatidylethanolamine (PE), and sphingomyelin] account for the remainder. DPPC (PC32:0) is the primary essential PC molecule required for the surface reduction property of surfactant. Surfactant is synthesised and secreted by alveolar type II (AT-II) cells. Surfactant deficiency from impaired synthesis/secretion, increased breakdown (either by hydrolysis or oxidation), or inactivation/inhibition by biophysical inhibitors can lead to compromised alveolar surface tension 11 . In mechanically ventilated patients, surfactant deficiency can exacerbate the initial lung pathology and impair lung compliance, thus worsening the pre-existing systemic hypoxaemia 12 .
Lungs are the primary target for direct oxygen toxicity, and animal studies have consistently demonstrated that lungs exposed to high oxygen concentrations exhibited quantitative and qualitative alterations in surfactant composition and function 13 . In in-vivo animal models, exposure to sub-lethal doses of oxygen results in decreased lung compliance, increased pulmonary leak, and inflammation with neutrophil migration. This is accompanied by hyaline membrane formation, alveolar septal oedema, fibrosis, and diffuse hyperplasia of alveolar epithelial cells 14 . These changes mimic neonatal respiratory distress syndrome (nRDS) due to primary surfactant deficiency, implying that surfactant deficiency may contribute to acute hyperoxic lung injury.
Surfactant synthesis can be compromised due to hyperoxia. In isolated alveolar type-II cells, from rabbits exposed to hyperoxia, the surfactant phospholipid synthesis is compromised and there was a progressive development of acute lung injury 15 . Moreover, exogenous surfactant replacement can significantly ameliorate hyperoxia-induced acute lung injury and improve alveolar phospholipid concentration 16 . Besides the total surfactant pool size, hyperoxia can induce alterations in the surfactant composition with reductions in DPPC (PC32:0) and the PG/PI ratio, leading to compromised surfactant function 17 . These in-vitro and in-vivo animal studies suggest that surfactant synthesis and function are significantly altered during hyperoxic conditions and likely contribute to adverse clinical outcomes; this concept has never been evaluated in humans.
Oxidative stress and redox balance
The balance of oxidants and antioxidants in healthy physiological states is tightly regulated. Oxidative stress occurs when there is an imbalance of this equilibrium with increased oxidants. Alterations in this equilibrium can lead to a pro-inflammatory state with an influx of inflammatory cells, activation of cytokine cascades and increased vascular permeability 18 . Hyperoxia related perturbations of this homeostatic balance results in the release of highly reactive mitochondrial mediators called reactive oxygen species (ROS) resulting in cellular damage 19 . This imbalance also results in exhaustion of antioxidant mechanisms, exacerbating further tissue damage. Beyond ROS, other reactive nitrogen (RNS) and sulphur-based species (RSS) exist. The conceptual framework of the “reactive species interactome (RSI)” describes the complex chemical interaction between these reactive molecules and their downstream intracellular targets and metabolites contributing to organ dysfunction 20 .
In hyperoxic states in critical illness, the balance between oxidative stress and endogenous antioxidants is altered, leading to a shift in extracellular redox status with a compromised ability to achieve whole-body redox balance and remove toxic molecules. This results in changes in the redox signalling and modulation of secondary messengers, causing mitochondrial dysfunction and cellular bioenergetic failure 21 . Consequently, the main feature of hyperoxia demonstrated in in-vivo models and isolated cell cultures is cell death through apoptosis or necrosis 22, 23 . Importantly, as redox status is an interconnected complex system, using single biomarkers from readouts of oxidative stress is challenging.
There remains a lack of understanding of oxygen related organ damage from in-vivo studies. Therefore, this mechanistic study (MecROX) will aim to address the mechanisms of hyperoxia, giving vital information for clinical management of mechanically ventilated patients requiring oxygen.
Study hypotheses
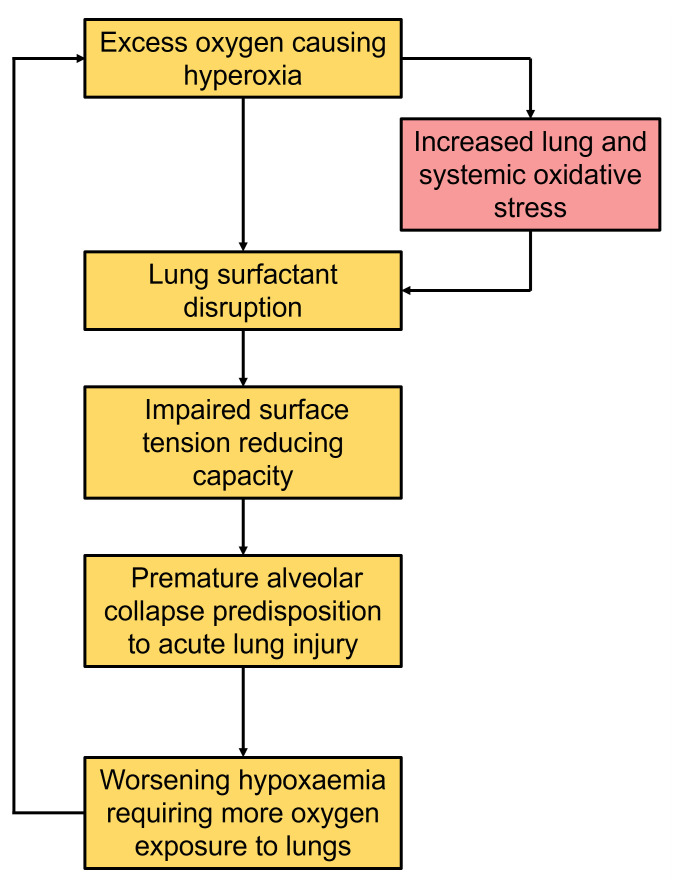
We hypothesise that hyperoxia may increase alveolar and systemic oxidative stress and adversely impact surfactant metabolism. Specifically, in mechanically ventilated patients: ( i) administration of high inspired oxygen concentrations will contribute to increased alveolar and systemic oxidative stress; (ii) increased alveolar and systemic oxidative stress will result in adverse changes in surfactant metabolism. We will characterise these metabolic phenotypes according to surfactant metabolism, alveolar and systemic oxidative stress. Stratification of these phenotypes may help to identify select groups that may benefit from targeted exogenous surfactant replacement, personalised therapeutic oxygen therapy and/or co-administration of targeted candidate therapeutic agents such as antioxidants to minimise surfactant inhibition and breakdown ( Figure 1).
Figure 1. Pathophysiological process of the contribution of hyperoxia in the development of acute lung injury.
Aim and objectives
The primary aim of this study is to characterise in-depth the alveolar surfactant biology, oxidative stress and whole-body redox status in mechanically ventilated adult patients receiving two different oxygen therapy strategies.
Study objectives
Objective 1: Quantify dynamic surfactant phospholipid composition, synthesis, and oxidative catabolism in-vivo and compare between the conservative and usual oxygen therapy group in mechanically ventilated patients.
Objective 2: Quantify lung and systemic oxidative stress and redox status by measuring the ‘reactive species interactome’ and compare between the conservative and usual oxygen therapy group mechanically ventilated patients.
Objective 3: Exploratory assessment of surfactant phenotype, lung and systemic oxidative stress and redox status, in relation to clinical correlates of oxygenation, ventilation, and clinical outcomes.
Protocol
Patient and Public Involvement
The Patient and Public Involvement (PPI) team consisted of 6 members led by Mr. William Jones (PPI Lead) and Mr. Mark Lamond (PPI member). The members of the PPI team had prior experience of hospitalisation and admission to the ICU. The members identified the study concept as a research priority. The review from PPI members contributed enormously to the conception and development of the protocol. They helped modify the protocol to prioritise a patient-centred approach, offering valuable suggestions to improve the consent process, while minimising additional distress. While the initial meetings were helpful for the conception of the study and securing funding, the second stage meetings helped design and finalise the patient-facing documents, ensuring that they were easily accessible and understandable by patients and their families. The focus was to ensure that the concept of oxygen therapy and the importance of lung surfactants were communicated clearly. The PPI team meets with the broader research team twice a year to assess the study's progress, providing suggestions to improve ongoing study recruitment. During study dissemination, we will continue working with the PPI team to create a patient-friendly summary of the findings, which will be available to study participants. Once the study is completed, we will work closely with the PPI groups to maximise study dissemination.
Research design
This is a prospective sub-study of the UK-ROX randomised controlled trial. The CONSORT flow diagram for the study is detailed below ( Figure 2). Participants form two centres (University Hospital Southampton and University Hospitals Plymouth) will be co-enrolled with the UK-ROX trial [1]. Following randomisation, patients will be assigned into either the conservative or standard oxygen therapy.
Figure 2. Study flowchart.
Screening
Potential study participants of mechanically ventilated due to hypoxaemic respiratory failure, hospitalised patients aged ≥18 already enrolled into the UK-ROX study will be identified by the research team.
Randomisation and UK-ROX interventions
All MecROX study participants will be already enrolled into the UK-ROX trial randomised to receive either conservative oxygen therapy (intervention) or usual oxygen therapy (control) using a central telephone or web-based randomisation service. Fifty patients from each group will be enrolled into the MecROX study. All interventions related to oxygen targets will comply with the UK-ROX protocol [1].
Conservative oxygen target: For the conservative group, the lowest concentration of oxygen will be administered to maintain the patient’s oxygen saturation (SpO 2) at 90 ±2% (i.e., for patients receiving oxygen this should not rise above 92%).
Usual oxygen target: This is defined as local practice as determined by treating clinicians.
Participants
All consecutive patients admitted will be screened according to the UK-ROX inclusion and exclusion criteria and they will be eligible if they fulfil the following criteria.
Inclusion criteria
• Aged ≥ 18 years
• Receiving invasive mechanical ventilation in the ICU for hypoxaemic respiratory failure
• Receiving supplemental oxygen (fractional inspired concentration of oxygen (FiO 2>0.21 at the time of enrolment)
• Anticipated to be mechanically ventilated for minimum of 72 hours
Exclusion criteria
• Previously randomised into the UK-ROX in the last 90 days
• Currently receiving extra-corporeal membrane oxygenation (ECMO)
• The treating clinician considers that one UK-ROX trial intervention arm is either indicated or contraindicated
Consent process
Due to the nature of the UK-ROX trial, a deferred consent model has been adopted where eligible patients are randomised to receive the assigned treatment as soon as possible (no later than 12 hours after fulfilling the eligibility criteria). For MecROX, we will seek a patient informed deferred consent from a personal consultee opinion within 24–48 hours of UK-ROX randomisation. We anticipate that all patients enrolled into the study will lack the capacity to make decisions about their care or participation (commonly due to sedative medications and/or critical illness) at the time of enrolment. As a result, it will not be possible to discuss the study with the participant. If the participant does not have the capacity to provide informed consent, a personal consultee will be appointed, who may be a relative or close friend with whom to discuss the patient’s participation in the trial. After giving them the personal consultee information sheet, the research staff will seek the personal consultee’s opinion as to whether they think the patient would wish to take part in the study. If the personal consultee agrees that they believe the patient would want to participate, they will be asked to sign a personal consultee declaration form and a member of the research team will then countersign it. If no personal consultee is present or immediately available in person, we will seek an agreement via the telephone. This can occur during restrictions on visitations (in the case of COVID-19). If an agreement is obtained via the telephone, a member of the research team will complete the telephone personal consultee declaration form after giving information about the study from the personal consultee information sheet. When the participant regains the capacity to consent to the study after a personal or nominated consultee has agreed to their inclusion, a retrospective consent form will be completed and signed by the participant as above. The consent encompass the storage of samples for ethically approved future studies.
Interventions
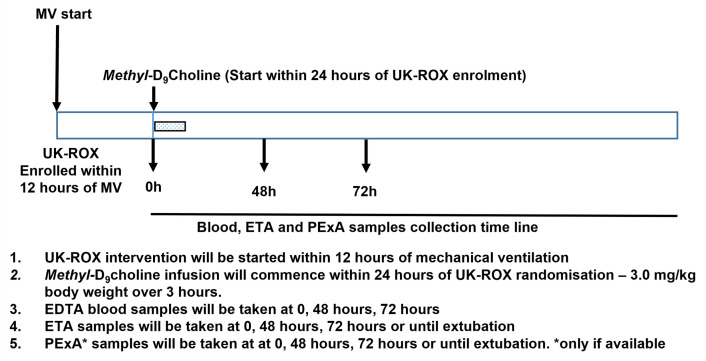
Study interventions and sampling schedule are presented in Figure 3. All samples will be collected in accordance with the standard operating procedures by trained staff experience in the procedure to minimise any discomfort. The labelled vitamin choline has no known side effects and has been used successfully in healthy adults and children. The tracheal aspirate is a well-established technique to improve secretions in ventilated patients. This is routinely done in intensive care units. Blood sampling is taken via an indwelling arterial line placed for routine clinical reasons and should not cause discomfort or distress. Breath samples are collected via the PExA device non-invasively.
Figure 3. Sampling schedule and planned study interventions.
Methyl-D 9 choline infusion
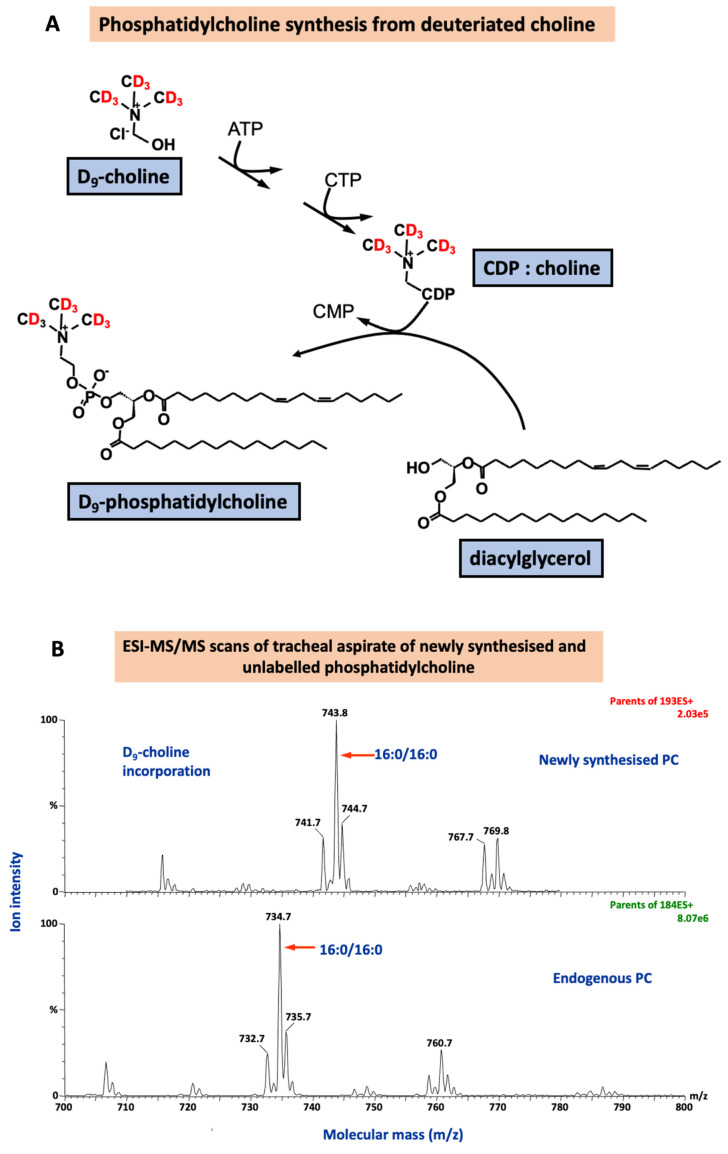
Choline is an essential nutrient grouped within the vitamin B complex and is required as part of a normal healthy diet. Choline is a crucial component of the lung surfactant. Labelling naturally occurring choline ( methyl-D 9 choline chloride) with a non-toxic, non-radioactive, stable isotope of hydrogen (deuterium, D) helps measure the rate of surfactant synthesis and breakdown in different diseases. This is a validated method to assess surfactant synthesis and has been used extensively in several clinical studies of healthy adult volunteers and in adult and neonate patients with lung problems to characterise surfactant and phospholipid dynamics 24, 25 . Choline is rich in several dietary sources and has no known side effects. The dose given is lower than the recommended daily requirement. Methyl-D 9-choline chloride will be dissolved in water at 10 mg/ml and infused at a dose of 3 mg/kg body weight over 3 hours, will enable dynamic assessment of surfactant synthesis and turnover. Methyl-D 9-choline incorporation into lung surfactant DPPC, the major surface-active component, will measure surfactant phospholipid synthesis ( Figure 4). It will answer the mechanistic question:
Figure 4.
The incorporation of deuterated choline ( methyl-D 9 choline chloride) to quantify surfactant phosphatidylcholine (PC) synthesis via the CDP-choline pathway ( A) and the corresponding mass spectra for endogenous PC composition and newly synthesised PC fraction. ATP: adenosine triphosphate; CDP: cytidine diphosphate; CMP: cytidine monophosphate; CTP: cytidine triphosphate.
1. Does excess oxygen affect surfactant DPPC (PC32:0) synthesis and turnover?
A metabolic pathway for methyl-D 9-choline incorporation into phospholipids is shown below, together with an example of the diagnostic mass spectrometry scans used for detection and quantification of unlabelled and deuterium-labelled PC.
We will aim to recruit patients and take the initial samples within 24 hours of randomisation into the UK-ROX study groups. The earliest time point will enable baseline assessment and subsequent 48 hours and 72 hours sampling times will assess the dynamic changes resulting from initiation of the intervention. The peak incorporation of methyl-D 9 choline is between 48–72 hours after infusion, which will provide a measure of the maximal surfactant PC and LysoPC enrichment patterns monitoring both PC synthesis via the CDP choline pathway and the extent of enhanced breakdown through hydrolysis.
Blood samples collection
EDTA (10 ml) blood samples will be taken at baseline, 48, and 72 hours, (while hospitalised) after the methyl-D 9-choline infusion. These samples will be taken only if the patient is still hospitalised. While in ICU, these samples are usually taken from pre-existing venous or arterial access lines. The collected blood samples will be stored at +4°C until transfer to the laboratory for processing. Processed samples taken at University Hospitals Plymouth will be transferred in frozen form to University Hospital Southampton for further analysis.
Endotracheal tracheal aspirate
Endotracheal aspirate (ETA) is a commonly performed procedure for secretion clearance in patients in the intensive care unit. Tracheal samples can be used to assess airway surfactant composition and metabolism. We have used tracheal aspirates to successfully isolate surfactant material in previous studies of healthy human volunteers and COVID-19 patients 26 . Tracheal aspirate samples will be taken at 0, 48, and 72 hours, after the methyl-D 9-choline infusion. The ETA samples will consist of blind suctioning by a cannula introduced through a port into the endotracheal tube. A volume of 20–50 ml saline will be administered, with an estimated recovery of 8–10 ml. This procedure will not interrupt the ventilator circuit and will not result in desaturation and is usually performed during physiotherapy sessions for mucus clearance. ETA samples will be taken into pre-labelled tissue culture (Falcon) tubes and stored at +4 °C in the ICU sample fridge until transfer to the laboratory for processing.
Particles of exhaled air (PExA)
Small airway samples are the gold-standard for surfactant measurements. However, sampling from small airway is often very difficult as bronchoscopy and lavage is the only option. While safe and a routine procedure, bronchoscopy is an invasive method for airway sampling which require medical expertise and have potential for desaturations during the procedure. Moreover, repeated sampling will likely to require preoxygenation with high inspired oxygen which will interfere with trial interventions. To avoid this, PExA from patients will be measured by PExA device (Gothenberg, Sweden) 27 . This will enable repeated small airway sampling for surfactant assessments without the use of bronchoscopy. This PExA device contains an optical particle counter (OPC) connected to an impactor for collection of samples at a diameter range of 0.41–4.55 µm. The PExA instrument will be connected to the ventilator outflow circuit to capture expiratory samples. The samples will be taken for an hour at 0, 48 and 72. The device will measure number of particles (count) and total accumulated mass (ng) of particles which will be collected onto a membrane for further biochemical analysis.
Sample analysis
Surfactant lipid composition and dynamic turnover
Phospholipids, lysophospholipids and oxidised phospholipids molecular species will be analysed in tracheal fluid samples and from PExA. Mass spectrometric analysis of molecular species compositions of phosphatidylcholine (PC), phosphatidylglycerol (PG) and phosphatidylinositol (PI) in small volume ETA samples. Lysophosphatidylcholine products of PLA2-mediated hydrolysis of surfactant phospholipid will be determined by diagnostic precursor scans, together with molecular species compositions of intact surfactant phospholipid. Oxidation of unsaturated phospholipid initially generates higher mass peroxides, which then undergo chemical degradation to form truncated lower mass lipid aldehydes and hydroxyls. Intact and high and low mass oxidised phospholipids will be determined by specific MRM scans using electrospray ionization tandem mass spectrometry (ESI-MS/MS). An estimate of surfactant concentration will be determined by urea dilution analysis in parallel samples of bronchial fluid and plasma samples.
Quantification of redox biology
The extent of local and systemic oxidative stress with associated modifications of surfactant composition, alterations in cell signalling (due to interference with nitric oxide and hydrogen sulfide-related cell function) and shifts in redox status following the increased production of reactive oxygen species will be characterised in aliquots of pulmonary secretions and blood. Oxidative stress, nitric oxide, hydrogen sulfide and other redox related metabolites and products of ‘reactive species’ interactions will be quantified by an array of analytical platforms including ELISA, gas-phase chemiluminescence, HPLC, IC-MS, and LC-MS/MS to determine the following readouts: 8-isoprostanes, malondialdehyde (TBARS), nitrite, nitrate, total nitroso species, thiosulfate, sulfate, total free thiols, free and bound low-molecular weight thiols (including cysteine, homocysteine, glutathione), sulfide and polysulfide species according to local standard operating procedures.
Outcome measures
Primary outcome
The difference of percentage of DPPC (PC32:0) in relation to total phosphatidylcholine composition (% of total PC in surfactant) at 48 hours between conservative and usual oxygen target groups.
Secondary outcomes
1. Surfactant index: This is a composite PC surfactant molecular index calculated from surfactant specific PC molecules (PC32:0, PC32:1 and PC30:0) and unsaturated surfactant PC34:1. This index will give a composite measure of surfactant PC alterations, which will provide a measure of surfactant PC status for the two different targets after 48 hours of oxygen therapy. This outcome is a measure of surfactant specific PC composition.
Surfactant index =
2. Surfactant phosphatidylcholine concentration (urea corrected) at 48 hours. This outcome is a measure of endogenous surfactant level.
3. Systemic oxidative stress: Total free thiols, lipid peroxides and total surfactant oxidation products. This outcome will measure whole-body oxidative stress.
Secondary explanatory outcomes
4. Surfactant total phosphatidylcholine and PC32:0 methyl-D 9choline enrichment at 48 hours. Measure of endogenous surfactant synthesis. This will measure the surfactant PC synthesis via the CDP-Choline pathway.
5. Surfactant total lysoPC and lysoPC16:0 concentrations, composition and methyl-D 9 choline enrichment at 48 hours. This outcome is a measure of endogenous surfactant breakdown. This will help to assess dynamic surfactant PC breakdown through hydrolysis.
6. Surfactant oxidised PC composition and concentrations at 48 hours. Measure of endogenous surfactant breakdown. This will help to assess dynamic surfactant breakdown by oxidation.
7. Whole-body redox balance by quantifying stable products of ROS (e.g., isoprostanes), RNS (e.g., nitrite, nitrate, nitrosation products) and RSS (e.g., total free thiols, thiosulfate, low molecular weight thiols including sulfide) at 48 hours from tracheal aspirates and plasma. Measure of lung and systemic redox status.
Secondary exploratory outcomes
8. Exploratory outcomes: Comparison of clinical outcomes (ICU mortality, hospital mortality, 90-day mortality, ICU, and hospital length of stay) in relation to surfactant abnormalities.
9. Exploratory outcomes: Comparison of clinical outcomes (ICU mortality, hospital mortality, 90-day mortality, ICU, and hospital length of stay) in relation to specific markers of oxidative stress.
Sample size calculation
There is no previous index of surfactant damage documented in patients or animal models of acute hyperoxic lung injury. From ARDS and healthy volunteer studies, estimates of the mean (SD) for DPPC (PC32:0) composition are patients with ARDS 35.6% (SD 12.1%) and healthy controls 53.1% (SD 4.3%) 10, 24 . Excess exposure will likely have alterations in DPPC composition from healthy volunteers but not significantly similar to the patients with ARDS, so we estimate that the standard deviation will be between 4.3 and 12.1 ( Table 1). With the higher estimate of SD, a sample size of 90 patients will achieve 90% power to detect a difference of 8.4% with a significance level of 0.05 using a two-sided two-sample t-test. Using the lower estimate, a sample size of 90 patients will achieve 90% power to detect a difference of 3.0% between groups with a significance level of 0.05 using a two-sided two-sample t-test. Allowing for an estimated 10% drop out we will recruit 100 patients.
Table 1. Surfactant DPPC compositions a from healthy volunteers and ARDS patients used for the power calculations.
| Variable | Timepoint | Group | Mean (SD) | Minimum absolute
difference which can be detected at 90% power, N=90 |
|---|---|---|---|---|
| DPPC or PC32: 0 | 48 | ARDS | 35.6 (12.1) | 8.4 |
| 48 | Healthy control | 53.1 (4.3) | 3.0 |
Statistical analysis
The research hypothesis is that there is a difference in DPPC (PC32:0) fractional concentration between the conservative and usual oxygen groups. The primary endpoint is percentage DPPC (PC32:0) relative to total PC composition (% of total PC in surfactant) at 48 hours. For the primary analysis, we will use a multiple regression model adjusted for baseline to investigate the difference between the conservative and usual oxygen groups. If data are not normally distributed, we will investigate whether a log transformation improves normality. We will also perform an adjusted analysis to address clinical heterogeneity, using multiple regression, with up to 10 variables including the following baseline variables: age, gender, body mass index, percentage of PC32:0 in relation to total PC composition at admission, clinical conditions (e.g., sepsis, pneumonia) and concentration of inspired oxygen required at admission. We will also perform descriptive subgroup analysis for group differences and present them as box and whisker plots and scatter plots. As samples are collected from time points T=0, T=48 and T=72 hours as a secondary analysis, we will also investigate the use of mixed models to look at the difference between groups over time.
For the secondary outcomes of surfactant index; surfactant PC32:0, PC32:1, PC30:0; surfactant total phosphatidylcholine and PC32:0 methyl-D 9 choline enrichment; surfactant total lysoPC and lysoPC16:0 concentrations, composition and methyl-D 9 choline enrichment and surfactant oxidised PC composition and concentrations (all measured at 48 hours), we will investigate the difference between groups using a two-sample t-test or Wilcoxon- Mann-Whitney test depending on the normality of data. If appropriate, adjusted analyses will also be performed as specified for the primary endpoint. The above will be repeated for the analysis of individual redox/oxidative stress markers listed above.
For the exploratory clinical outcomes of ICU mortality, hospital mortality and all mortality censored at 90 days, we will use Cox regression with time-varying covariates to investigate the effect of surfactant abnormalities and specific markers of oxidative stress on survival. For ICU and hospital length of stay, we will use cause-specific Cox regression model to account for the fact that patients may die before being discharged. As an exploratory analysis, we will illustrate the relationship between surfactant markers and oxidative stress markers using scatter plots and will quantify these relationships using regression models.
Data management
The clinical data collection will be enhanced within the ICNARC CMP research platform. Additional data collection will be obtained beyond the standard data collection by the UK-ROX clinical trial. The additional data will include baseline demographic variables, ventilation, and oxygenation parameters. The principal investigator will oversee and be responsible for data collection, quality, and recording. The nested CMP research platform enables data collection to be incorporated within the ICNARC routine CMP data collection process, streamlining data linkage. Study data will be made available online at the time study results dissemination.
Ethics approval and study management
The study is sponsored by the University Hospital Southampton NHS Foundation Trust. The study is approved by HRA and the London Bromley Research Ethics Committee, REC: 22/LO/0877 and IRAS: 320671. The study is registered on ISRCTN registry, ISRCTN61929838 ( https://doi.org/10.1186/ISRCTN61929838). The MecROX study is managed by the Southampton Clinical Trials Unit (CTU) and Sponsored by the University Hospital Southampton. Chief Investigator will ensure all study personnel are appropriately orientated and trained, oversee recruitment and report to the trial safety monitoring committee.
Study dissemination
The findings of this study will be presented locally, nationally, and internationally in intensive care and respiratory conference and society meetings and published through peer review journals.
Study status
As of 19 th of February 2024, both sites are open for recruitment and recruited 25 participants so far.
Strengths of the study
This is the first study to investigate the lung surfactant metabolism and systemic redox markers from mechanically ventilated patients with different oxygen targets providing a mechanistic in-vivo evaluation of oxidative stress and redox biology in critical illness. Results will inform underlying mechanisms of hyperoxia and hyperoxemia.
Discussion
MecROX is a mechanistic study that aims to determine the mechanisms underlying hyperoxia and hyperoxemia induced lung surfactant and whole-body redox changes in-vivo. While oxygen therapy is essential for critically ill mechanically ventilated patients, there are potentially many harmful effects from hyperoxia, as demonstrated by several studies of hospitalised patients with acute conditions. Oxygen toxicity due to hyperoxia/hyperoxaemia is a well-recognised phenomenon in animal studies. Hyperoxaemia may exacerbate whole-body oxidative stress leading to cell death, autophagy, mitochondrial dysfunction, biogenetic failure with eventual multi-organ failure resulting in poor clinical outcomes. However, in-vivo human models evaluating hyperoxia-induced organ damage in mechanically ventilated patients are lacking. Decreased surfactant synthesis due to cellular apoptosis and increased surfactant breakdown by oxidation/hydrolysis can result in surfactant deficiency during states of increased oxidative stress. However, the influence of hyperoxia on direct surfactant damage and whole-body redox status has never been evaluated before in relation to various oxygen targets. It is critical to assess the alveolar environment to quantify the oxidative stress and redox imbalance to minimise surfactant breakdown to prevent worsening lung atelectasis and hypoxaemia.
This proposed study will mechanistically evaluate the in-vivo surfactant metabolism using a novel stable isotope technique and various platforms of mass spectrometry analytical methods. Surfactant phospholipid breakdown through hydrolysis (lysophospholipids) and oxidation (oxidised phospholipids) and redox disequilibrium (‘reactive species interactome’) will be quantified from sequential plasma and bronchial samples using an array of analytical platforms. These assessments will help characterise the alveolar and systemic redox status during different oxygen targets to stratify patients according to evidence of oxidative surfactant damage that may be responsive to targeted administration of therapies to minimise oxidative stress in the future.
Conclusions
This observational study aims to characterise in-depth the molecular mechanisms relating to oxygen therapy in mechanically ventilated patients enrolled in the UK-ROX clinical trial receiving oxygen therapy to two different target targets. The study will collect serial biological samples to evaluate dynamic surfactant phospholipid concentrations, composition, synthesis, catabolism, and global assessment of whole-body oxidative stress and redox status (markers of the “reactive species interactome”). By doing so, the study aims to provide a mechanistic link between pathophysiology and clinical outcomes in critically ill intensive care patients receiving oxygen therapy.
Acknowledgements
We acknowledge our PPI group and the intensive care research nursing teams from University Hospital Southampton and University Hospitals Plymouth. We also thank the ICNARC data management team for the development and management of electronic CRF for this study.
Funding Statement
This project is funded by the National Institute for Health and Care Research (NIHR) under its Efficacy and Mechanism Evaluation (EME) programme (NIHR151287) and supported by the University Hospital Southampton NIHR Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NIHR, NHS or the Department of Health and Social Care.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
Data availability
Underlying data
No data are associated with this article. Once the study analysis is complete and the results of the study are published, the anonymised data will be made available via an online repository according to the NIHR data sharing policy.
The full detailed protocol is available upon request to the corresponding author.
References
- 1. Martin DS, Shahid T, Gould DW, et al. : Evaluating the clinical and cost-effectiveness of a conservative approach to oxygen therapy for invasively ventilated adults in intensive care: protocol for the UK-ROX trial. J Intensive Care Soc. 2024;25(2):223–230. 10.1177/17511437241239880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith JL: The pathological effects due to increase of oxygen tension in the air breathed. J Physiol. 1899;24(1):19–35. 10.1113/jphysiol.1899.sp000746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clark JM, Lambertsen CJ: Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971;23(2):37–133. [PubMed] [Google Scholar]
- 4. Wolfe WG, Robinson LA, Moran JF, et al. : Reversible pulmonary oxygen toxicity in the primate. Ann Surg. 1978;188(4):530–43. 10.1097/00000658-197810000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Comroe JH, Dripps RD, Dumke PR, et al. : Oxygen toxicity: the effect of inhalation of high concentrations of oxygen for twenty-four hours on normal men at sea level and at a simulated altitude of 18,000 feet. JAMA. 1945;128(10):710–717. 10.1001/jama.1945.02860270012004 [DOI] [Google Scholar]
- 6. Caldwell PR, Lee WL, Jr, Schildkraut HS, et al. : Changes in lung volume, diffusing capacity, and blood gases in men breathing oxygen. J Appl Physiol. 1966;21(5):1477–83. 10.1152/jappl.1966.21.5.1477 [DOI] [PubMed] [Google Scholar]
- 7. Sackner MA, Landa J, Hirsch J, et al. : Pulmonary effects of oxygen breathing. A 6-hour study in normal men. Ann Intern Med. 1975;82(1):40–3. 10.7326/0003-4819-82-1-40 [DOI] [PubMed] [Google Scholar]
- 8. Davis WB, Rennard SI, Bitterman PB, et al. : Pulmonary oxygen toxicity. Early reversible changes in human alveolar structures induced by hyperoxia. N Engl J Med. 1983;309(15):878–83. 10.1056/NEJM198310133091502 [DOI] [PubMed] [Google Scholar]
- 9. Kallet RH, Matthay MA: Hyperoxic Acute Lung Injury. Respir Care. 2013;58(1):123–41. 10.4187/respcare.01963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dushianthan A, Goss V, Cusack R, et al. : Phospholipid composition and kinetics in different endobronchial fractions from healthy volunteers. BMC Pulm Med. 2014;14: 10. 10.1186/1471-2466-14-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dushianthan A, Cusack R, Goss V, et al. : Clinical review: exogenous surfactant therapy for Acute Lung Injury/Acute Respiratory Distress Syndrome--where do we go from here? Crit Care. 2012;16(6): 238. 10.1186/cc11512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raghavendran K, Willson D, Notter RH: Surfactant therapy for Acute Lung Injury and Acute Respiratory Distress Syndrome. Crit Care Clin. 2011;27(3):525–59. 10.1016/j.ccc.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Putman E, van Golde LM, Haagsman HP: Toxic oxidant species and their impact on the pulmonary surfactant system. Lung. 1997;175(2):75–103. 10.1007/pl00007561 [DOI] [PubMed] [Google Scholar]
- 14. Jackson RM: Pulmonary oxygen toxicity. Chest. 1985;88(6):900–5. 10.1378/chest.88.6.900 [DOI] [PubMed] [Google Scholar]
- 15. Holm BA, Matalon S, Finkelstein JN, et al. : Type II pneumocyte changes during hyperoxic lung injury and recovery. J Appl Physiol (1985). 1988;65(6):2672–8. 10.1152/jappl.1988.65.6.2672 [DOI] [PubMed] [Google Scholar]
- 16. Matalon S, Holm BA, Loewen GM, et al. : Sublethal hyperoxic injury to the alveolar epithelium and the pulmonary surfactant system. Exp Lung Res. 1988;14 Suppl:1021–33. 10.3109/01902148809064190 [DOI] [PubMed] [Google Scholar]
- 17. King RJ, Coalson JJ, Seidenfeld JJ, et al. : O2- and pneumonia-induced lung injury. II. Properties of pulmonary surfactant. J Appl Physiol (1985). 1989;67(1):357–65. 10.1152/jappl.1989.67.1.357 [DOI] [PubMed] [Google Scholar]
- 18. Cumpstey AF, Clark AD, Santolini J, et al. : COVID-19: a redox disease-what a stress pandemic can teach us about resilience and what we may learn from the Reactive Species Interactome about its treatment. Antioxid Redox Signal. 2021;35(14):1226–1268. 10.1089/ars.2021.0017 [DOI] [PubMed] [Google Scholar]
- 19. Stevens JL, Feelisch M, Martin DS: Perioperative Oxidative Stress: the unseen enemy. Anesth Analg. 2019;129(6):1749–1760. 10.1213/ANE.0000000000004455 [DOI] [PubMed] [Google Scholar]
- 20. Cortese-Krott MM, Koning A, Kuhnle GGC, et al. : The Reactive Species Interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxid Redox Signal. 2017;27(10):684–712. 10.1089/ars.2017.7083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McKenna HT, O'Brien KA, Fernandez BO, et al. : Divergent trajectories of cellular bioenergetics, intermediary metabolism and systemic redox status in survivors and non-survivors of critical illness. Redox Biol. 2021;41: 101907. 10.1016/j.redox.2021.101907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pagano A, Barazzone-Argiroffo C: Alveolar cell death in hyperoxia-induced lung injury. Ann N Y Acad Sci. 2003;1010:405–16. 10.1196/annals.1299.074 [DOI] [PubMed] [Google Scholar]
- 23. Tang PS, Mura M, Seth R, et al. : Acute Lung Injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol. 2008;294(4):L632–41. 10.1152/ajplung.00262.2007 [DOI] [PubMed] [Google Scholar]
- 24. Dushianthan A, Goss V, Cusack R, et al. : Altered molecular specificity of surfactant phosphatidycholine synthesis in patients with Acute Respiratory Distress Syndrome. Respir Res. 2014;15(1): 128. 10.1186/s12931-014-0128-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goss KCW, Goss VM, Townsend JP, et al. : Postnatal adaptations of phosphatidylcholine metabolism in extremely preterm infants: implications for choline and PUFA metabolism. Am J Clin Nutr. 2020;112(6):1438–1447. 10.1093/ajcn/nqaa207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Postle AD, Clark HW, Fink J, et al. : Rapid phospholipid turnover after surfactant nebulization in severe COVID-19 infection: a randomized clinical trial. Am J Respir Crit Care Med. 2022;205(4):471–473. 10.1164/rccm.202110-2279LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Larsson P, Holz O, Koster G, et al. : Exhaled breath particles as a novel tool to study lipid composition of Epithelial Lining Fluid from the distal lung. BMC Pulm Med. 2023;23(1): 423. 10.1186/s12890-023-02718-8 [DOI] [PMC free article] [PubMed] [Google Scholar]