Abstract
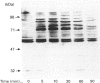
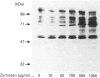
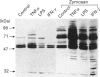
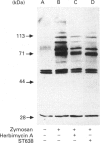
We have investigated the relationship between tyrosine phosphorylation and respiratory-burst activity in mouse bone-marrow-derived macrophages (BMM). We demonstrate that zymosan, an agent known to trigger the macrophage respiratory burst, also triggers the activation of tyrosine kinase activity, resulting in rapid tyrosine phosphorylation on numerous proteins, and provide evidence for the role of tyrosine phosphorylation in the triggering of the BMM respiratory burst. Agents, such as tumour necrosis factor alpha (TNF alpha), interferon-gamma (IFN-gamma) or lipopolysaccharide (LPS), which prime the macrophage for an enhanced zymosan-triggered respiratory burst, increase tyrosine phosphorylation triggered by zymosan. The zymosan-triggered tyrosine phosphorylation and respiratory-burst activity were partially suppressed by the tyrosine kinase inhibitors alpha-cyano-3-ethoxy-4-hydroxy-5-phenylmethylcinnamide (ST638) and herbimycin A. In addition, pre-exposure of BMM to vanadate, a phosphotyrosine phosphatase inhibitor, greatly enhanced the ability of zymosan to induce tyrosine phosphorylation and trigger the respiratory burst. These data highlight the importance of the balance between tyrosine kinase and phosphotyrosine phosphatase activity in determining the ultimate level of tyrosine phosphorylation in BMM and suggest that zymosan-triggered tyrosine phosphorylation is an important biochemical signal for triggering of the respiratory burst.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkow R. L., Dodson M. R. Biochemical mechanisms involved in the priming of neutrophils by tumor necrosis factor. J Leukoc Biol. 1988 Nov;44(5):345–352. doi: 10.1002/jlb.44.5.345. [DOI] [PubMed] [Google Scholar]
- Berkow R. L., Dodson R. W., Kraft A. S. Human neutrophils contain distinct cytosolic and particulate tyrosine kinase activities: possible role in neutrophil activation. Biochim Biophys Acta. 1989 Aug 31;997(3):292–301. doi: 10.1016/0167-4838(89)90200-8. [DOI] [PubMed] [Google Scholar]
- Boulet I., Ralph S., Stanley E., Lock P., Dunn A. R., Green S. P., Phillips W. A. Lipopolysaccharide- and interferon-gamma-induced expression of hck and lyn tyrosine kinases in murine bone marrow-derived macrophages. Oncogene. 1992 Apr;7(4):703–710. [PubMed] [Google Scholar]
- Davila D. R., Edwards C. K., 3rd, Arkins S., Simon J., Kelley K. W. Interferon-gamma-induced priming for secretion of superoxide anion and tumor necrosis factor-alpha declines in macrophages from aged rats. FASEB J. 1990 Aug;4(11):2906–2911. doi: 10.1096/fasebj.4.11.2165948. [DOI] [PubMed] [Google Scholar]
- Evans J. P., Mire-Sluis A. R., Hoffbrand A. V., Wickremasinghe R. G. Binding of G-CSF, GM-CSF, tumor necrosis factor-alpha, and gamma-interferon to cell surface receptors on human myeloid leukemia cells triggers rapid tyrosine and serine phosphorylation of a 75-Kd protein. Blood. 1990 Jan 1;75(1):88–95. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L., Kamps M. P. Monoclonal antibodies to phosphotyrosine. J Immunol Methods. 1988 May 9;109(2):277–285. doi: 10.1016/0022-1759(88)90253-0. [DOI] [PubMed] [Google Scholar]
- Gomez-Cambronero J., Huang C. K., Bonak V. A., Wang E., Casnellie J. E., Shiraishi T., Sha'afi R. I. Tyrosine phosphorylation in human neutrophil. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1478–1485. doi: 10.1016/0006-291x(89)90841-3. [DOI] [PubMed] [Google Scholar]
- Gomez-Cambronero J., Yamazaki M., Metwally F., Molski T. F., Bonak V. A., Huang C. K., Becker E. L., Sha'afi R. I. Granulocyte-macrophage colony-stimulating factor and human neutrophils: role of guanine nucleotide regulatory proteins. Proc Natl Acad Sci U S A. 1989 May;86(10):3569–3573. doi: 10.1073/pnas.86.10.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W., Lu D. J., Mills G. B. Vanadate stimulates oxygen consumption and tyrosine phosphorylation in electropermeabilized human neutrophils. J Biol Chem. 1990 Jan 5;265(1):318–327. [PubMed] [Google Scholar]
- Hamilton J. A., Veis N., Bordun A. M., Vairo G., Gonda T. J., Phillips W. A. Activation and proliferation signals in murine macrophages: relationships among c-fos and c-myc expression, phosphoinositide hydrolysis, superoxide formation, and DNA synthesis. J Cell Physiol. 1989 Dec;141(3):618–626. doi: 10.1002/jcp.1041410321. [DOI] [PubMed] [Google Scholar]
- Heidel J. R., Taylor S. M., Silflow R. M., Laegreid W. W., Leid R. W. Characterization of arachidonic acid metabolism, superoxide production, and bacterial killing by bovine alveolar neutrophils elicited with leukotriene B4 and zymosan-activated plasma. Inflammation. 1991 Feb;15(1):31–42. doi: 10.1007/BF00917907. [DOI] [PubMed] [Google Scholar]
- Kohno M., Nishizawa N., Tsujimoto M., Nomoto H. Mitogenic signalling pathway of tumour necrosis factor involves the rapid tyrosine phosphorylation of 41,000-Mr and 43,000-Mr cytosol proteins. Biochem J. 1990 Apr 1;267(1):91–98. doi: 10.1042/bj2670091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McColl S. R., DiPersio J. F., Caon A. C., Ho P., Naccache P. H. Involvement of tyrosine kinases in the activation of human peripheral blood neutrophils by granulocyte-macrophage colony-stimulating factor. Blood. 1991 Oct 1;78(7):1842–1852. [PubMed] [Google Scholar]
- McColl S. R., Kreis C., DiPersio J. F., Borgeat P., Naccache P. H. Involvement of guanine nucleotide binding proteins in neutrophil activation and priming by GM-CSF. Blood. 1989 Feb;73(2):588–591. [PubMed] [Google Scholar]
- Morel F., Doussiere J., Vignais P. V. The superoxide-generating oxidase of phagocytic cells. Physiological, molecular and pathological aspects. Eur J Biochem. 1991 Nov 1;201(3):523–546. doi: 10.1111/j.1432-1033.1991.tb16312.x. [DOI] [PubMed] [Google Scholar]
- Nasmith P. E., Mills G. B., Grinstein S. Guanine nucleotides induce tyrosine phosphorylation and activation of the respiratory burst in neutrophils. Biochem J. 1989 Feb 1;257(3):893–897. doi: 10.1042/bj2570893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips W. A., Croatto M., Hamilton J. A. Priming the macrophage respiratory burst with IL-4: enhancement with TNF-alpha but inhibition by IFN-gamma. Immunology. 1990 Aug;70(4):498–503. [PMC free article] [PubMed] [Google Scholar]
- Phillips W. A., Croatto M., Veis N., Hamilton J. A. Protein kinase C has both stimulatory and suppressive effects on macrophage superoxide production. J Cell Physiol. 1992 Jul;152(1):64–70. doi: 10.1002/jcp.1041520109. [DOI] [PubMed] [Google Scholar]
- Phillips W. A., Hamilton J. A. Phorbol ester-stimulated superoxide production by murine bone marrow-derived macrophages requires preexposure to cytokines. J Immunol. 1989 Apr 1;142(7):2445–2449. [PubMed] [Google Scholar]
- Pittet D., Krause K. H., Wollheim C. B., Bruzzone R., Lew D. P. Nonselective inhibition of neutrophil functions by sphinganine. J Biol Chem. 1987 Jul 25;262(21):10072–10076. [PubMed] [Google Scholar]
- Richter J., Andersson T., Olsson I. Effect of tumor necrosis factor and granulocyte/macrophage colony-stimulating factor on neutrophil degranulation. J Immunol. 1989 May 1;142(9):3199–3205. [PubMed] [Google Scholar]
- Sako T., Tauber A. I., Jeng A. Y., Yuspa S. H., Blumberg P. M. Contrasting actions of staurosporine, a protein kinase C inhibitor, on human neutrophils and primary mouse epidermal cells. Cancer Res. 1988 Aug 15;48(16):4646–4650. [PubMed] [Google Scholar]
- Shiraishi T., Owada M. K., Tatsuka M., Fuse Y., Watanabe K., Kakunaga T. A tyrosine-specific protein kinase inhibitor, alpha-cyano-3-ethoxy-4-hydroxy-5-phenylthiomethylcinnamamide, blocks the phosphorylation of tyrosine kinase substrate in intact cells. Jpn J Cancer Res. 1990 Jun-Jul;81(6-7):645–652. doi: 10.1111/j.1349-7006.1990.tb02622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup G., Speeg K. V., Jr, Cohen S., Garbers D. L. Phosphotyrosyl-protein phosphatase of TCRC-2 cells. J Biol Chem. 1982 Jul 10;257(13):7298–7301. [PubMed] [Google Scholar]
- Trudel S., Downey G. P., Grinstein S., Pâquet M. R. Activation of permeabilized HL60 cells by vanadate. Evidence for divergent signalling pathways. Biochem J. 1990 Jul 1;269(1):127–131. doi: 10.1042/bj2690127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tushinski R. J., Oliver I. T., Guilbert L. J., Tynan P. W., Warner J. R., Stanley E. R. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982 Jan;28(1):71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- Vairo G., Hamilton J. A. CSF-1 stimulates Na+K+-ATPase mediated 86Rb+ uptake in mouse bone marrow-derived macrophages. Biochem Biophys Res Commun. 1985 Oct 15;132(1):430–437. doi: 10.1016/0006-291x(85)91040-x. [DOI] [PubMed] [Google Scholar]
- Veis N., Hamilton J. A. Colony stimulating factor-1 stimulates diacylglycerol generation in murine bone marrow-derived macrophages, but not in resident peritoneal macrophages. J Cell Physiol. 1991 May;147(2):298–305. doi: 10.1002/jcp.1041470215. [DOI] [PubMed] [Google Scholar]
- Warren J. S., Kunkel S. L., Cunningham T. W., Johnson K. J., Ward P. A. Macrophage-derived cytokines amplify immune complex-triggered O2-. responses by rat alveolar macrophages. Am J Pathol. 1988 Mar;130(3):489–495. [PMC free article] [PubMed] [Google Scholar]
- Weinstein S. L., Gold M. R., DeFranco A. L. Bacterial lipopolysaccharide stimulates protein tyrosine phosphorylation in macrophages. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4148–4152. doi: 10.1073/pnas.88.10.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S. F., Wilson C. B., Perlmutter R. M. Augmented expression of a myeloid-specific protein tyrosine kinase gene (hck) after macrophage activation. J Exp Med. 1988 Nov 1;168(5):1801–1810. doi: 10.1084/jem.168.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]