Abstract
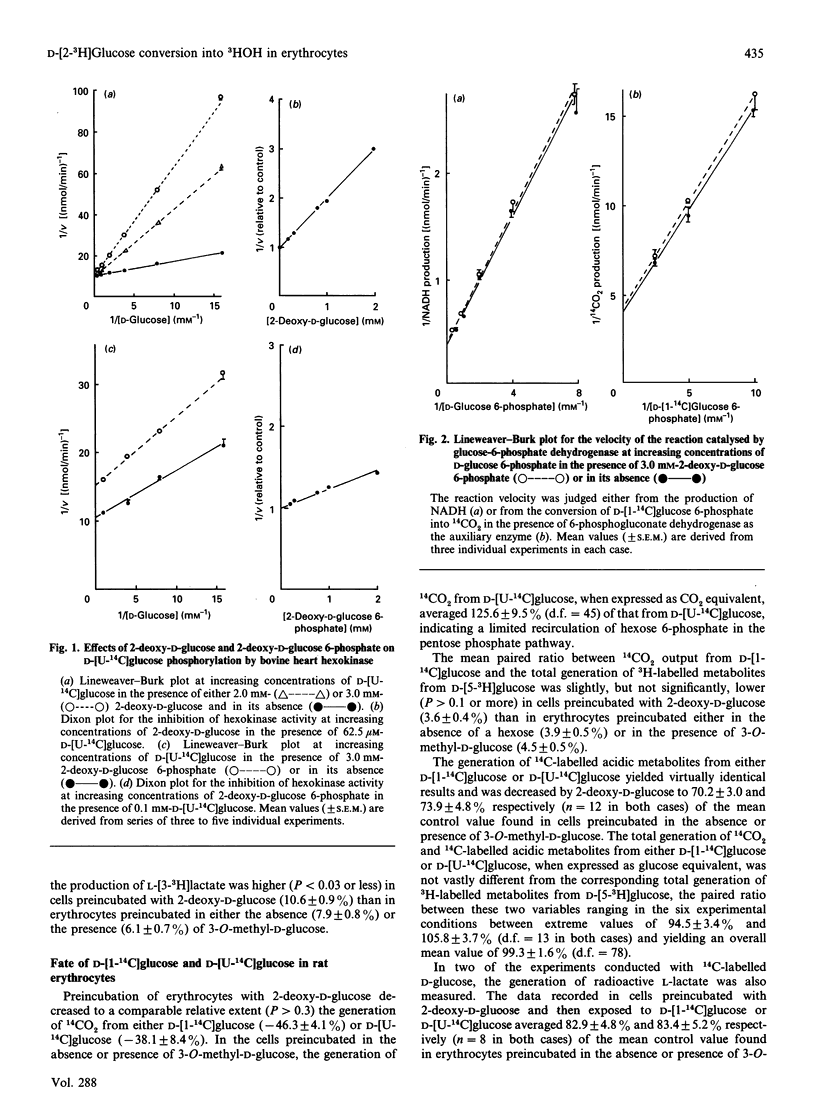
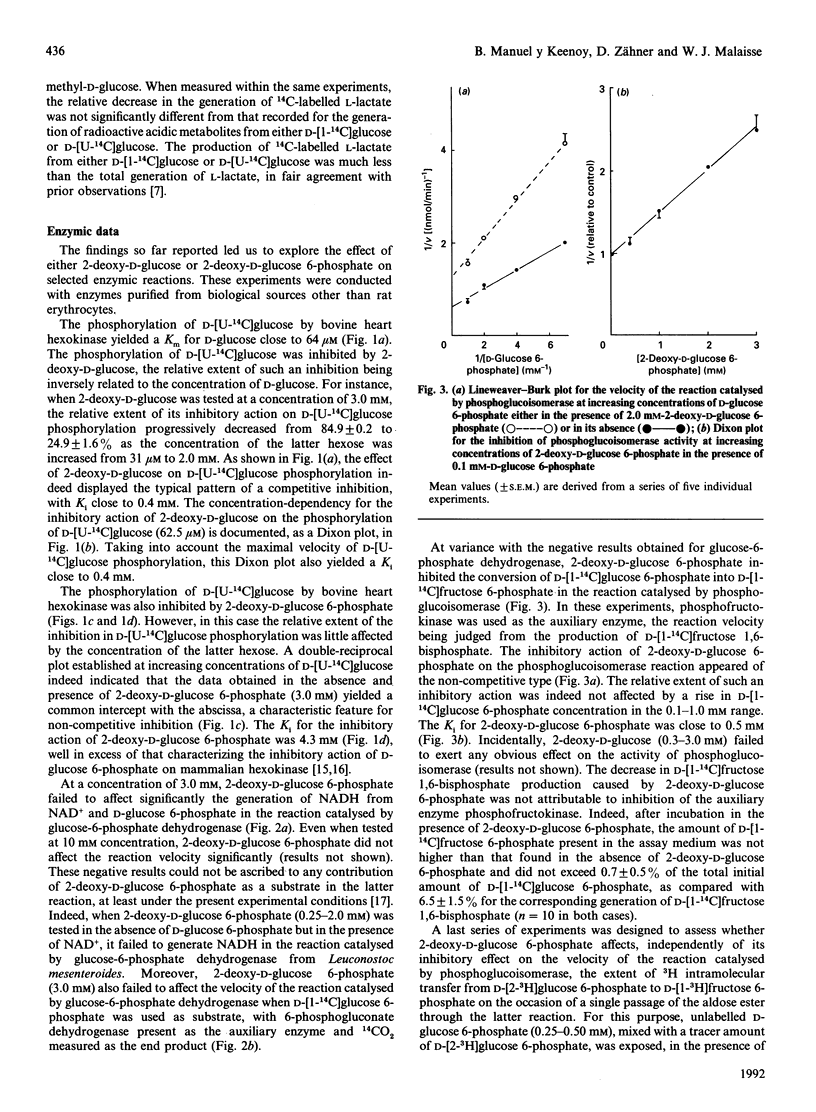
When rat erythrocytes were preincubated with 2-deoxy-D-glucose, the generation of both 3H-labelled acidic metabolites and 3HOH from D-[5-3H]glucose, the total production of L-lactate, and the generation of 14CO2, 14C-labelled acidic metabolites and 14C-labelled lactate from D-[1-14C]glucose or D-[U-14C]glucose were all lower than in erythrocytes preincubated in the absence of a hexose or in the presence of 3-O-methyl-D-glucose. However, preincubation with 2-deoxy-D-glucose failed to decrease the generation of 3H-labelled acidic metabolites and L-[3-3H]lactate from D-[2-3H]glucose, while decreasing the production of 3HOH more severely from D-[2-3H]glucose than from D-[5-3H]glucose. This may be attributable not solely to inhibition of D-glucose phosphorylation by 2-deoxy-D-glucose and 2-deoxy-D-glucose 6-phosphate, but also to inhibition by 2-deoxy-D-glucose 6-phosphate of hexose 6-phosphate interconversion in the reaction catalysed by phosphoglucoisomerase, as also observed with the purified enzyme. The generation of 3HOH from D-[2-3H]glucose should therefore be considered as a tool to assess the efficiency of interconversion of hexose 6-phosphates in the reaction catalysed by phosphoglucoisomerase, rather than to estimate D-glucose phosphorylation rate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chi M. M., Pusateri M. E., Carter J. G., Norris B. J., McDougal D. B., Jr, Lowry O. H. Enzymatic assays for 2-deoxyglucose and 2-deoxyglucose 6-phosphate. Anal Biochem. 1987 Mar;161(2):508–513. doi: 10.1016/0003-2697(87)90481-7. [DOI] [PubMed] [Google Scholar]
- Horton R. W., Meldrum B. S., Bachelard H. S. Enzymic and cerebral metabolic effects of 2-deoxy-D-glucose. J Neurochem. 1973 Sep;21(3):507–520. doi: 10.1111/j.1471-4159.1973.tb05996.x. [DOI] [PubMed] [Google Scholar]
- Hue L. The role of futile cycles in the regulation of carbohydrate metabolism in the liver. Adv Enzymol Relat Areas Mol Biol. 1981;52:247–331. doi: 10.1002/9780470122976.ch4. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. The metabolism of glucose-2-T by adipose tissue. J Biol Chem. 1969 Jan 10;244(1):99–106. [PubMed] [Google Scholar]
- Katz J., Wals P. A., Rognstad R. Glucose phosphorylation, glucose-6-phosphatase, and recycling in rat hepatocytes. J Biol Chem. 1978 Jul 10;253(13):4530–4536. [PubMed] [Google Scholar]
- Liemans V., Malaisse-Lagae F., Willem R., Malaisse W. J. Phosphoglucoisomerase-catalyzed interconversion of hexose phosphates; diastereotopic specificity, isotopic discrimination and intramolecular hydrogen transfer. Biochim Biophys Acta. 1989 Oct 5;998(2):111–117. doi: 10.1016/0167-4838(89)90261-6. [DOI] [PubMed] [Google Scholar]
- Malaisse-Lagae F., Liemans V., Malaisse W. J. Phosphoglucoisomerase-catalyzed interconversion of hexose-phosphates. Isotopic discrimination between hydrogen and tritium. Mol Cell Biochem. 1989 Aug 15;89(1):57–67. doi: 10.1007/BF00228280. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Bodur H. Hexose metabolism in pancreatic islets: enzyme-to-enzyme tunnelling of hexose 6-phosphates. Int J Biochem. 1991;23(12):1471–1481. doi: 10.1016/0020-711x(91)90290-4. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J. Environmental alteration of 3HOH production from D-[1-3H]glucose, D-[2-3H]glucose and D-[5-3H]glucose by rat erythrocytes. Diabetes Res. 1990 Dec;15(4):191–194. [PubMed] [Google Scholar]
- Malaisse W. J., Giroix M. H., Malaisse-Lagae F., Sener A. 3-O-methyl-D-glucose transport in tumoral insulin-producing cells. Am J Physiol. 1986 Dec;251(6 Pt 1):C841–C846. doi: 10.1152/ajpcell.1986.251.6.C841. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Rasschaert J., Zähner D., Sener A. Hexose metabolism in pancreatic islets: the Pasteur effect. Diabetes Res. 1988 Feb;7(2):53–58. [PubMed] [Google Scholar]
- Malaisse W. J., Sener A. Hexose metabolism in pancreatic islets. Feedback control of D-glucose oxidation by functional events. Biochim Biophys Acta. 1988 Oct 7;971(3):246–254. doi: 10.1016/0167-4889(88)90139-5. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Yilmaz M. T., Malaisse-Lagae F., Sener A. Underestimation of D-glucose phosphorylation as measured by 3H2O production from D-[2-3H]glucose. Biochem Med Metab Biol. 1988 Aug;40(1):35–41. doi: 10.1016/0885-4505(88)90101-6. [DOI] [PubMed] [Google Scholar]
- Manuel y Keenoy B., Malaisse-Lagae F., Malaisse W. J. Metabolism of tritiated D-glucose in rat erythrocytes. Metabolism. 1991 Sep;40(9):978–985. doi: 10.1016/0026-0495(91)90076-9. [DOI] [PubMed] [Google Scholar]
- Manuel y Keenoy B., Malaisse W. J. Assay of L-[3-3H]lactate generated from D-[1-3H]glucose, D-[2-3H]glucose and D-[6-3H]glucose in rat erythrocytes. Horm Metab Res. 1991 Aug;23(8):395–396. doi: 10.1055/s-2007-1003709. [DOI] [PubMed] [Google Scholar]
- ROSE I. A., O'CONNELL E. L. Intramolecular hydrogen transfer in the phosphoglucose isomerase reaction. J Biol Chem. 1961 Dec;236:3086–3092. [PubMed] [Google Scholar]
- Rijksen G., Staal G. E. Regulation of human erythrocyte hexokinase. The influence of glycolytic intermediates and inorganic phosphate. Biochim Biophys Acta. 1977 Nov 23;485(1):75–86. doi: 10.1016/0005-2744(77)90194-2. [DOI] [PubMed] [Google Scholar]
- Sener A., Malaisse-Lagae F., Malaisse W. J. Fructose metabolism via the pentose cycle in tumoral islet cells. Eur J Biochem. 1987 Dec 30;170(1-2):447–452. doi: 10.1111/j.1432-1033.1987.tb13720.x. [DOI] [PubMed] [Google Scholar]
- Sener A., Malaisse W. J. A sensitive radioisotopic method for the measurement of NAD(P)H: its application to the assay of metabolites and enzymatic activities. Anal Biochem. 1990 May 1;186(2):236–242. doi: 10.1016/0003-2697(90)90073-i. [DOI] [PubMed] [Google Scholar]
- WICK A. N., DRURY D. R., NAKADA H. I., WOLFE J. B. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957 Feb;224(2):963–969. [PubMed] [Google Scholar]
- Zalitis J., Oliver I. T. Inhibition of glucose phosphate isomerase by metabolic intermediates of fructose. Biochem J. 1967 Mar;102(3):753–759. doi: 10.1042/bj1020753. [DOI] [PMC free article] [PubMed] [Google Scholar]


