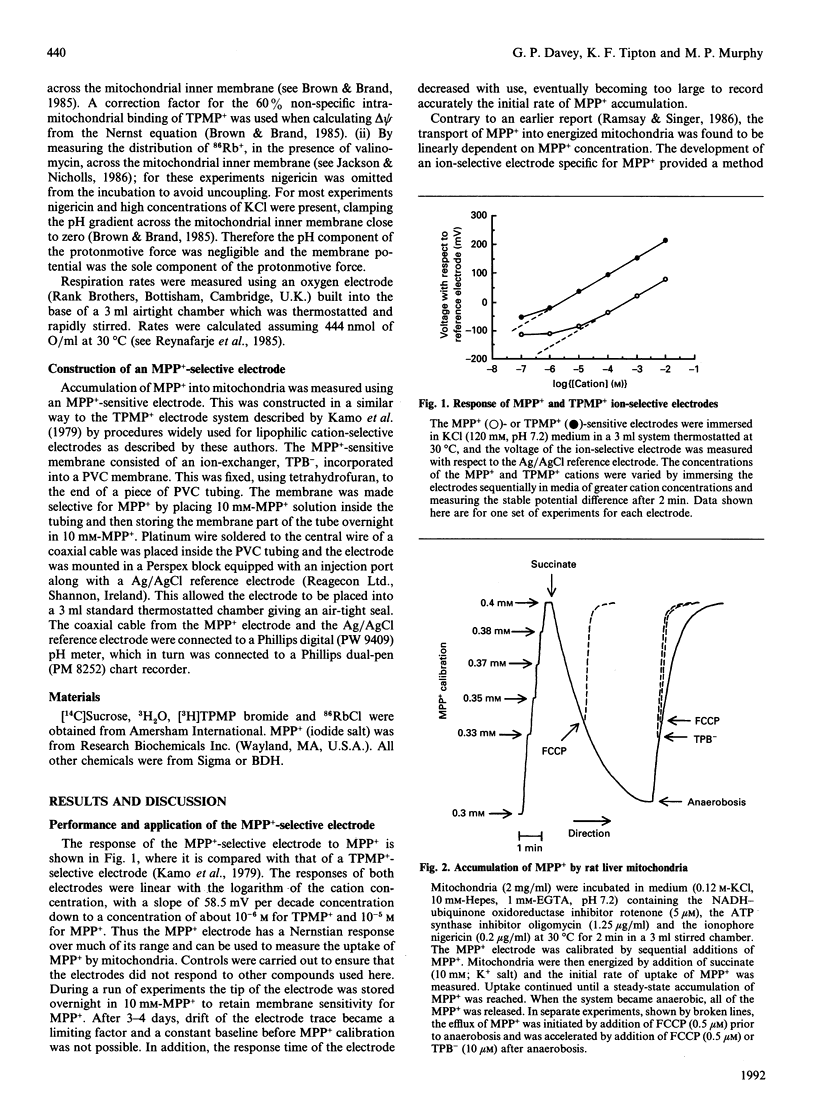
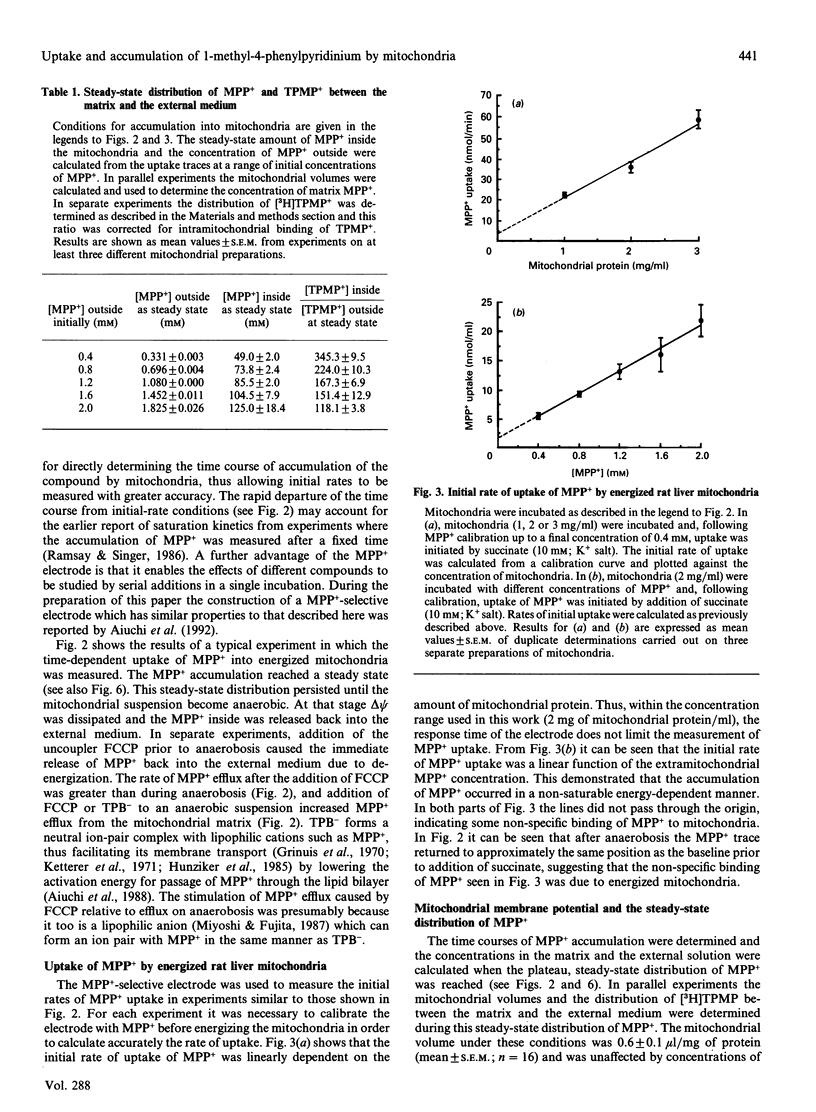
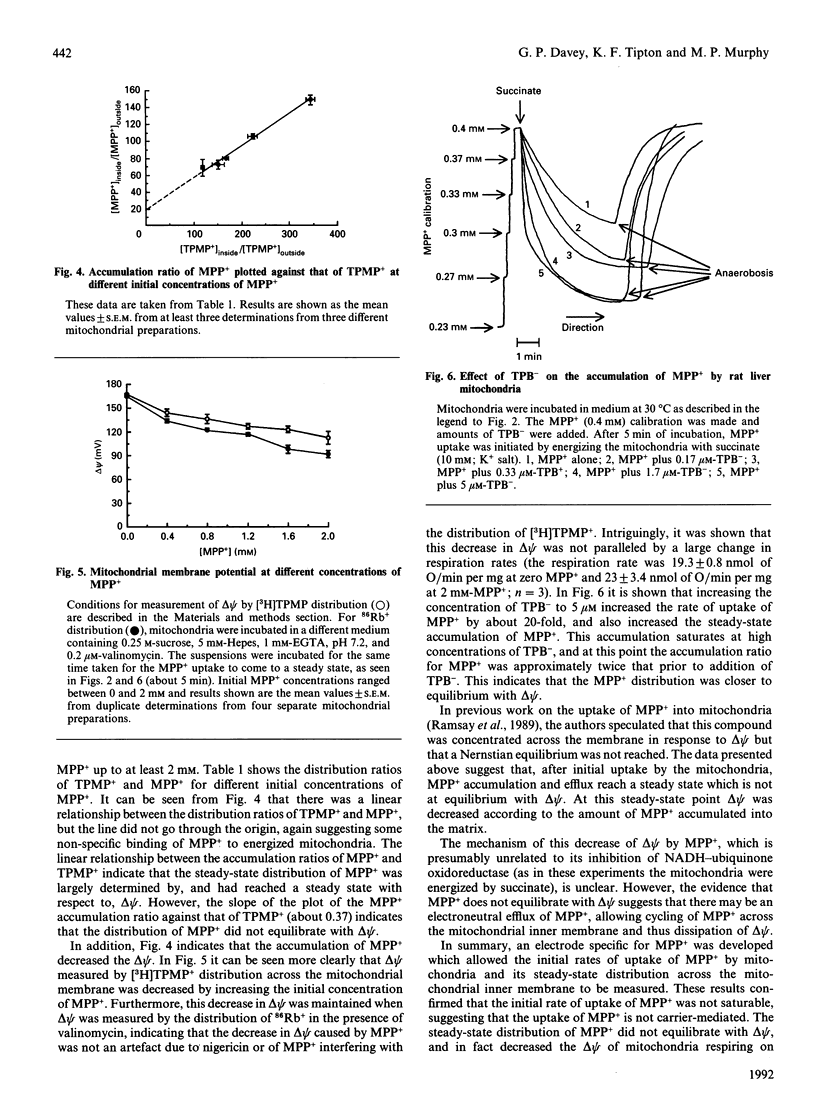
Abstract
The compound 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) causes selective destruction of nigrostriatal dopaminergic neurons in primates, giving rise to a condition resembling Parkinson's disease. The toxicity of MPTP is believed to be due to its metabolite 1-methyl-4-phenylpyridinium (MPP+). MPP+ is an inhibitor of mitochondrial respiration at the NADH-ubiquinone oxidoreductase site and this, together with its selective transport into dopaminergic nerve terminals, accounts for its neurotoxicity. In this paper an electrode selective for MPP+ was developed and used to measure the rate of uptake and the steady-state accumulation of MPP+ in rat liver mitochondria. The initial rates of MPP+ uptake were not saturable, confirming previous work that the transport of MPP+ is not carrier-mediated. The membrane potential of mitochondria respiring on succinate was decreased by MPP+ and the steady-state accumulation ratio of MPP+ did not come to equilibrium with the mitochondrial transmembrane potential gradient (delta psi). The effect of the cation exchanger tetraphenylboron (5 microM) was to increase the initial rate of MPP+ uptake by about 20-fold and the steady-state accumulation by about 2-fold. This suggests that there may be a mechanism of efflux of MPP+ from mitochondria which allows MPP+ to cycle across the membrane and thus decrease delta psi. These data indicate that MPP+ interacts with mitochondria independently of its inhibition of NADH-ubiquinone oxidoreductase, and these alternative interactions may be of relevance for its mechanism of neurotoxicity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiuchi T., Syou M., Matsunaga M., Kinemuchi H., Nakaya K., Nakamura Y. Enhancement of the uptake of 1-methyl-4-phenylpyridinium ion (MPP+) in mitochondria by tetraphenylboron. Biochim Biophys Acta. 1992 Jan 31;1103(2):233–238. doi: 10.1016/0005-2736(92)90092-z. [DOI] [PubMed] [Google Scholar]
- Brown G. C., Brand M. D. Thermodynamic control of electron flux through mitochondrial cytochrome bc1 complex. Biochem J. 1985 Jan 15;225(2):399–405. doi: 10.1042/bj2250399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnoli N., Jr, Chiba K., Trevor A. J. Potential bioactivation pathways for the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Life Sci. 1985 Jan 21;36(3):225–230. doi: 10.1016/0024-3205(85)90063-3. [DOI] [PubMed] [Google Scholar]
- Chiba K., Trevor A., Castagnoli N., Jr Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem Biophys Res Commun. 1984 Apr 30;120(2):574–578. doi: 10.1016/0006-291x(84)91293-2. [DOI] [PubMed] [Google Scholar]
- Grinius L. L., Jasaitis A. A., Kadziauskas Y. P., Liberman E. A., Skulachev V. P., Topali V. P., Tsofina L. M., Vladimirova M. A. Conversion of biomembrane-produced energy into electric form. I. Submitochondrial particles. Biochim Biophys Acta. 1970 Aug 4;216(1):1–12. doi: 10.1016/0005-2728(70)90153-2. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Hwang J., Ofori S., Geller H. M., Nicklas W. J. Potentiation by the tetraphenylboron anion of the effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and its pyridinium metabolite. J Neurochem. 1990 Mar;54(3):743–750. doi: 10.1111/j.1471-4159.1990.tb02314.x. [DOI] [PubMed] [Google Scholar]
- Hoppel C. L., Grinblatt D., Kwok H. C., Arora P. K., Singh M. P., Sayre L. M., Greenblatt D. Inhibition of mitochondrial respiration by analogs of 4-phenylpyridine and 1-methyl-4-phenylpyridinium cation (MPP+), the neurotoxic metabolite of MPTP. Biochem Biophys Res Commun. 1987 Oct 29;148(2):684–693. doi: 10.1016/0006-291x(87)90931-4. [DOI] [PubMed] [Google Scholar]
- Hunziker A., Orme F. W., Macey R. I. Transport of hydrophobic ions in erythrocyte membrane: I. Zero membrane potential properties. J Membr Biol. 1985;84(2):147–156. doi: 10.1007/BF01872212. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Nicholls D. G. Methods for the determination of membrane potential in bioenergetic systems. Methods Enzymol. 1986;127:557–577. doi: 10.1016/0076-6879(86)27044-5. [DOI] [PubMed] [Google Scholar]
- Javitch J. A., D'Amato R. J., Strittmatter S. M., Snyder S. H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamo N., Muratsugu M., Hongoh R., Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol. 1979 Aug;49(2):105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- Nicklas W. J., Vyas I., Heikkila R. E. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985 Jul 1;36(26):2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Ramsay R. R., McKeown K. A., Johnson E. A., Booth R. G., Singer T. P. Inhibition of NADH oxidation by pyridine derivatives. Biochem Biophys Res Commun. 1987 Jul 15;146(1):53–60. doi: 10.1016/0006-291x(87)90689-9. [DOI] [PubMed] [Google Scholar]
- Ramsay R. R., Mehlhorn R. J., Singer T. P. Enhancement by tetraphenylboron of the interaction of the 1-methyl-4-phenylpyridinium ion (MPP+) with mitochondria. Biochem Biophys Res Commun. 1989 Mar 31;159(3):983–990. doi: 10.1016/0006-291x(89)92205-5. [DOI] [PubMed] [Google Scholar]
- Ramsay R. R., Singer T. P. Energy-dependent uptake of N-methyl-4-phenylpyridinium, the neurotoxic metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, by mitochondria. J Biol Chem. 1986 Jun 15;261(17):7585–7587. [PubMed] [Google Scholar]
- Reynafarje B., Costa L. E., Lehninger A. L. O2 solubility in aqueous media determined by a kinetic method. Anal Biochem. 1985 Mar;145(2):406–418. doi: 10.1016/0003-2697(85)90381-1. [DOI] [PubMed] [Google Scholar]
- Salach J. I., Singer T. P., Castagnoli N., Jr, Trevor A. Oxidation of the neurotoxic amine 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) by monoamine oxidases A and B and suicide inactivation of the enzymes by MPTP. Biochem Biophys Res Commun. 1984 Dec 14;125(2):831–835. doi: 10.1016/0006-291x(84)90614-4. [DOI] [PubMed] [Google Scholar]


