Abstract
This review describes the latest achievements in the development of encapsulated controlled-release fertilizers, which encompasses sustainability issues in agriculture. The research community's interest in this particular area of science has doubled over the last couple of years due to the yearly increasing complexity of the food and supply situation, as well as maintaining the development of modern society in the era of population outbreak. This review covers demand in timely systematization and comprehensive analysis of emerging research in so-called “smart fertilizers” that release mineral components in accordance with the needs for nutrients classified into controlled- and slow-release fertilizers (CRFs and SRFs). Along with the thoroughly selected fundamental studies published in this area, the review specially focuses on the materials-based classification, emphasizing the importance of the host matrix in the time-controlled release of dopant. This substantially differentiates our review and renders scientific novelty and relevancy to it. The review is divided into sections, dealing with the types of slow- and controlled-release fertilizers each, and supplemented with the critical view on their usage. All data regarding encapsulated fertilizers in this review are systematized for the convenience of the readership when becoming familiarized with the latest achievements in this area. Perspectives and potential pathways are also described to recommend and guide researchers working on the related academic fields.
Keywords: Slow-release fertilizers, Controlled-release fertilizers, Supergranules, Superabsorbents, Smart fertilizers, Release profile, Coating
Acronyms and abbreviations
- CRF –
controlled-release fertilizer
- SRF –
slow-release fertilizer
- NUE –
nutrient utilization efficiency
- AAPFCO –
Association of American Plant Food Control Officials
- PVDC –
polyvinylidene chloride
- NPK fertilizers –
complex nitrogen-phosphorus-potassium (NPK) fertilizers contain the key nutrients for plants, with some macro- and microelements added
- BCRF –
bio-based controlled-release fertilizer
- DCRF –
multifunctional bio-based bilayer CRF
- PESOA –
poly-epoxidized soybean oil acrylate
- PSM –
PESOA modified with stearic acid @ Mg (OH)2
- PSMP –
PESOA modified with stearic acid @ Mg (OH)2 immobilized by paraffin
- PSF –
slow-release PESOA
- PSF-SM –
PSM cured by UV radiation
- PSF-SMP –
paraffin-wax PSM cured by UV radiation
- SHT –
superhydrophobic halloysite nanotube
- HNT –
halloysite nanotube
- SHPU –
modifications of castor-oil polyurethane, or superhydrophobic polyurethane
- PUC –
polyurethane coating
- SHPUC –
superhydrophobic polyurethane-coated (fertilizer)
- PHU –
polyhydrophobic urethane, a nanocomposite film made from unprocessed CNTs and polyurethane was also made using the same process
- PHU 1, 2, 3 or SHPU 1, 2, 3 –
numbers in abbreviations indicate the percentage of superhydrophobic coating as related to polyurethane in percents
- PHUС –
PHU coating
- PMDI –
polymeric methylene diphenyl diisocyanate
- Nano-CB –
nanocarbon black
- CSNP –
chitosan nanoparticle
1. Introduction
Global growth of the human population increases the demand for high-quality alimentary products. To maintain the high productivity of agriculture and stimulate the sustainable development of crops, many fertilizers are used. However, their efficiency remains at quite a low level. The role of any agricultural fertilizer is to supply plants with the essential macro- and micronutrients needed for their growth and development. However, in most cases, for crops, it is too hard to sufficiently assimilate nutrients from fertilizer due to high nutrient losses in the soil, water and air, which also leads to environmental problems. According to some estimates, only 30–55 % of nutrients are fixed by crops, while the others are lost resulting from leaching, draining, and volatility [[1], [2], [3]]. These considerable losses of fertilizers both lead to significant wastes of resources and increases in farming costs, and contribute to severe environmental pollution, such as eutrophication, as well as to global warming [4].
It is vital to note that mineral fertilizers not only provide nutrients to crops and help growth, but also play an important role in regulating pH and soil fertility [5,5]. Improving the efficiency of plant nutrition can be achieved through the production and application of “smart fertilizers” that release mineral components over a longer period, thereby saving human and natural resources [6,7]. On the other hand, smart fertilizers improve nutrient utilization efficiency (NUE) not only by controlling the release of minerals but also by reducing their removal from the soil by natural conditions, which also reduces environmental risks. These two approaches allow reducing the recommended amount of fertilizer by 20–30 %, saving the same yield results [[8], [9], [10], [11], [12], [13]]. Controlled- and slow-release fertilizers (CRFs and SRFs) stand as the main classes of materials in this field. Slow-release fertilizers are represented mainly by compounds with low solubility and a complex/high molecular weight chemical structure that release nutrients through it's degradation period. The term “controlled-release fertilizers” refers more to compounds of the encapsulated type, where the control of the release of water-soluble nutrients provides the surface of such micro- or nano-containers [14].
However, the following problems occur in the practice of manufacturing fertilizers. First, the coefficient of using nutrients from fertilizers is low in the contemporary agriculture, which leads to accumulating wastes and considerably contaminating the non-point sources [15]. Therefore, to efficiently increase the coefficient of using fertilizers (such as N or P), various fertilizers were developed with a low-release coating [7,16]. It should be noted that for CRFs and SRFs, coating materials are basically made of petrochemical products that represent non-renewable resources and have a high value. It essentially limits a large-scaled advancement of CRFs [[17], [18], [19]]. Accumulating metals in soil become an issue, as well. Some metals, such as Zn, Fe, and Cu, can be a source of microelements for agricultural crops. However, their accumulation in soil cannot be efficiently fixed by crops, while their excess affects the entire soil ecology and potentially contaminates water via drainages [20]. Moreover, the main soil heavy-metal remediation methods include coating the surfaces, leaching operations, electroextraction, hardening, vitrification, and phytoremediation [21]. These techniques require much manpower and many resources and become a considerable economic burden in agricultural practice [22]. Therefore, there is currently an acute need for stable and cost-effective functional materials for creating “smart fertilizers” [23].
This review provides details on CRFs and SRFs, their types, advantages and disadvantages, and a special section deals with studies that have been made in the area of encapsulated fertilizers as of today.
2. Slow- and controlled-release fertilizers
The Association of American Plant Food Control Officials (AAPFCO) defines slow-release fertilizers as materials with a high molecular weight, a complex structure, and a low water-soluble content. Chemical-controlled-release fertilizers (CRFs) are materials where mineral components are released through a polymeric layer or membrane [5].
There are mandatory criteria by which a slow-release fertilizer can be considered as such. The requirements state that at 25 °C the release of nutrients must be lower than 15 % in 24 h and no more than 75 % in 28 days, at least about 75 % must go into the fertilizer in the specified time (Fig. 1a). It is worth noting that there are currently different types of slow-release and controlled-release fertilizers, which can be classified as supergranules, slow release fertilizers, controlled release fertilizers [24].
Fig. 1.
Description of the fertilizer release criteria for SRF over 1 day, 28 days and longer period (a); materials used as coatings in controlled-release fertilizer production (b).
2.1. Condensation products of urea and urea-Aldehydes (slow-release fertilizers)
Urea-formaldehyde (UF), isobutyraldehyde urea (IBDU), croton-aldehyde urea (CDU) are most often used as nitrogen-containing fertilizer compounds in the spheres of professional grass plots, seeding nurseries, greenhouses, lawns, and for garden and landscape design,
Let us consider one of the types of presented fertilizers, namely urea-formaldehyde, as an example. It should be noted that the introduction of nitrogen from UF-fertilizer is a specific multi-step process, which consists of 2 main parts: dissolution, when capsulated or leaved on the surface urea dissolves at the process of moisture, and decomposition, when the decomposing polymer releases the remaining amount of nitrogen. This is correlating with the stages of fertilizers release.
-
1.
Slow release of one part of the nitrogen;
-
2.
More gradual nitrogen release over several (3–4) months.
However, not only the type of product, but also temperature, moisture, and soil organism activity have their influence on the nature of nitrogen release. Due to the low concentration in the soil, UF-fertilizer does not allow the plant to wilt quickly and does not interfere with crop growth. In general, urea-formaldehyde products can show great results in process of slow nitrogen release if there is good compatibility of the fertilizer with the crops, as well as a warm climate, because their effectiveness increases at higher temperatures.
It is worth noting that UF fertilizers are more common in the slow-release fertilizer market, other products are less relevant due to the fact that they are not widely used and are not economically viable [24].
2.2. Coated or encapsulated fertilizers (controlled-release fertilizers)
In the case of CRFs the main role in the control of slow nutrient release plays encapsulating/covering material standing as a physical barrier. Most often such a protective membrane (insoluble in water) is obtained by granulation, instillation or crystallization, which prevents water penetration and regulates the rate of dissolution and release of nutrients.
There are a variety of forms of slow release/controlled release fertilizer (Fig. 1b) [24,25].
Let us take a closer look at some of the examples mentioned above.
2.3. Inorganic mineral fertilizers
Inorganic minerals, such as sulfur, zeolite, gypsum, dolomite, diatomite, and bentonite, are often used as coating materials within controlled-release fertilizers [25]. Pure sulfur is the most conventional coating material used in synthesizing controlled-release fertilizers, because a sulfur-coated fertilizer can reduce the solubility degree of a normal fertilizer. Being the most available coating for fertilizer granules, sulfur can also cause negative effects associated with its reduction to the sulfide form, in which sulfur binds with heavy metals [26], reducing the amount of available microelements and seed survival in general, or sulfur oxidation to the sulfate form, in which it can cause soil acidification [27]. These processes occur mainly due to the activity of bacteria, and their study over time will make it possible to avoid negative consequences and benefit from greater digestibility of sulfur when used in elemental form [28]. as demonstrated by the group of Rousk J, who in their study showed the possibility of conversion up to 90 % when maintaining pH at 6.0–6.5 [29].
In one of studies, the quality of sulfur-coated urea was determined by the urea dissolution rate and analyzed using electronic microscopy. Elemental sulfur was liquidized and dispersed in particles. Experiments were planned to check how the flow rate of sulfur and atomizing air, as well as the temperature of the air used in atomizing layer, affect the surface quality of coated particles. Supply air temperature and the atomizing air flow rate affected the coating quality considerably [30].
Sulfur coating could prolongate the release of nutrients. However, it cracked easily because it was very breakable. To overcome this limitation and improve the coating quality, a sealer and a sulfur modification were used. In the study, organic resin dicyclopentadiene (DCPD) was used to modify the sulfide coating [31].
One of studies reported that combining sulfur with gypsum, starch, or bentonite used as coating material reduces the urea release rate. It turned out that the sulfur-/gypsum-coated urea release rate was lower than that of the sulfur-/bentonite- or sulfur-/starch-coated urea. Sulfur-gypsum coating appears to be more uniform over the urea granules and had minimum number of pores. In this form urea had better resistance to water. Moreover, sulfur-/gypsum-coated urea also showed good water retention property, which makes this coating even more promising relative to comparable analogues [30,32].
2.4. Polymer-based fertilizers
To overcome the disadvantages of using inorganic mineral basis, a polymer is used instead of sulfur coating, since it is impregnable against microorganisms. Polymers that are commonly used as protective membranes include polyurethane [33], polysulfone, polyacrylonitrile, cellulose acetate [34,35], polyolefin [36], and polyvinylchloride [37]. The use of biodegradable polymers is considered more promising when creating encapsulated fertilizers, due to the absence of their accumulation in the soil and gradual conversion into nutrients [38]. However, the decisive factor in determining the possibility of using a particular polymer is its biocompatibility and low toxicity to environment. In this connection, fertilizers based on polysulfones, polyacrylonitriles, etc., exhibiting high resistance to decomposition, are mentioned as potential encapsulating agents [[39], [40], [41]]. However, it is also worth considering the cost of the polymers used, since the possibility of recycling and reuse requires additional ones, as a rule, they are not provided or require additional modifications of the shell [42,43].
The use of encapsulated fertilizers in small amounts does not lead to any significant changes in the soil, however, in some cases, with the constant use of encapsulated fertilizers, the content of undecomposed polymer capsules in the soil can reach 10 %, as demonstrated in rice fields in Japan [44]. Such amounts of residual microplastic can significantly affect the soil's ability to retain moisture, microelements and bacteria, which significantly affects the amount of moisture consumed and the quality of the resulting crop [45,46].
Three types of coated NPK-fertilizers were developed using polyacrylonitrile, polysulfone, and cellulose acetate. It is found that increasing the coating thickness and polymer concentration reduces the coating porosity. A higher porosity was observed in cellulose acetate as compared to polysulfone and polyacrylonitrile. NPK release rate from a polysulfone-coated fertilizer was the lowest, while that from a fertilizer coated with cellulose acetate was the highest after 5 h of dissolution. Low NPK release rate from polysulfone and polyacrylonitrile is explained by their low porosity and high hydrophobic properties [34].
2.5. Superabsorbent hydrogel-based fertilizer
Superabsorbent hydrogel represents a three-dimensional matrix formed by a cross-linked hydrophilic polymer. This coating can also be used as a carrier/matrix. There are several types of hydrogel-based fertilizers: synthetic fertilizer, a combination of synthetic and natural and a pure natural hydrogel-based fertilizer. Synthetic hydrogel is usually made based on acrylic acid and acrylamide. A prime example of this approach is provided by the group of Klinpituksa P., who obtained slow nitrogen release material based on polyacrylamide and urea [47]. It is found that urea is released in distilled water gradually, for up to 40 days [30,48].
2.6. Supergranules and others
-
•
As a rule, fertilizers from this group do not contain special substances in their contents, and provide unusual properties with the help of appropriate regulation of the granules size and density. They have a relatively small surface-to-volume ratio, which reduces the speed of nutrient release and helps to stay on the surface of the soil for a long time. The simplest example of supergranules are urea granules with a size of about 10 mm or more. The scientific group of M. I. Khalil compared the effectiveness of using 1–2 mm urea granules with 10 mm super granules. They demonstrated a significant increase in fertilizer consumption by spring wheat when using super granules (up to 78 %) relative to conventional granules (56.6 %), however, only when they were placed at a depth of 5–7 cm. They attribute this effect to the slower dissolution of super granules and the plant's consumption of fertilizer during later stages of growth [49]. Depending on the culture of plants, such fertilizers can contain different proportion of microelements. In Western Europe, such UF-based supergranules, briquets, pellets, or sticks are preferred to fertilize trees and shrubs, as well as some vegetables, while in tropical regions such fertilizers are preferred to cultivate lowland rice [24].
2.7. Examples of slow- and controlled-release fertilizers
This section gives the examples of slow- and controlled-release fertilizers with several types of nutrients (macro- and microelements) and various efficient prolonging shells obtained using different synthesis methods.
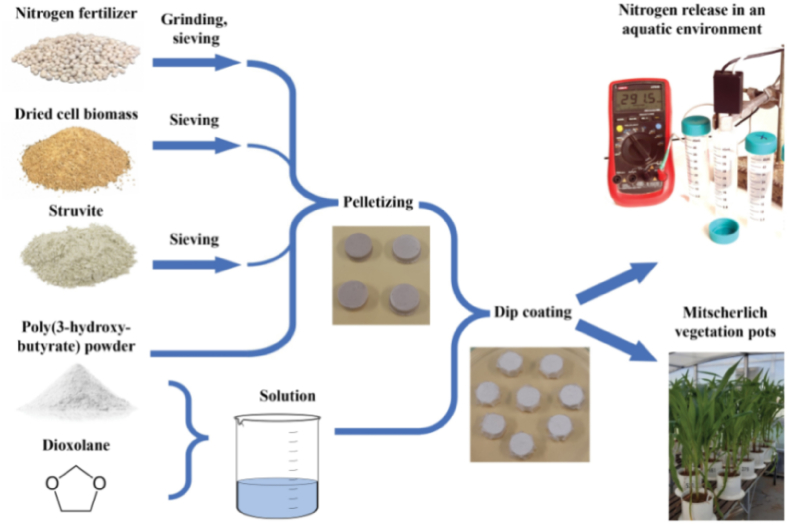
A recent example of a slow-release fertilizer is presented by nitrogen rich granules coated with poly (3-hydroxy-butirate) (Fig. 2). There nitrogen fertilizer (calcium-ammonium nitrate) have been granulated with the selected fillers, such as poly (3-hydroxy-butirate), struvite, or dry biomass. The poly (3-hydroxy-butirate) solution in dioxolane was applied onto the granules, which formed a high-quality and thin polymer coating [14].
Fig. 2.
Illustrative scheme of forming and analyzing the encapsulated fertilizer based on calcium ammonium nitrate. Reproduced from Ref. [14].
The main feature of this coating is ability for biodegradation, which allows them to gradually release nitrogen and stay in stable form in the soil. Analysis of coated granules in water and soil mediate demonstrated their great stability even upon 76 days in water, when only 20 % of ammonium nitrate were released. The efficiency of this system has also been checked in the process of maize development with Mitscherlich pots. Experiments show that the way of feeding maize with this granule fertilizer provided an appropriate amount of nutrients for the culture in accordance with its needs and the absence of any adverse effects upon the corn growth from coating - poly (3-hydroxy-butirate) [14].
There are scientific studies aimed at using ethyl cellulose in agricultural chemistry. The purpose of the next study was to obtain fertilizers with prolonging properties by the method of dipping of granules of multi-component NPK-fertilizer into biopolymer material - ethyl cellulose (EC).
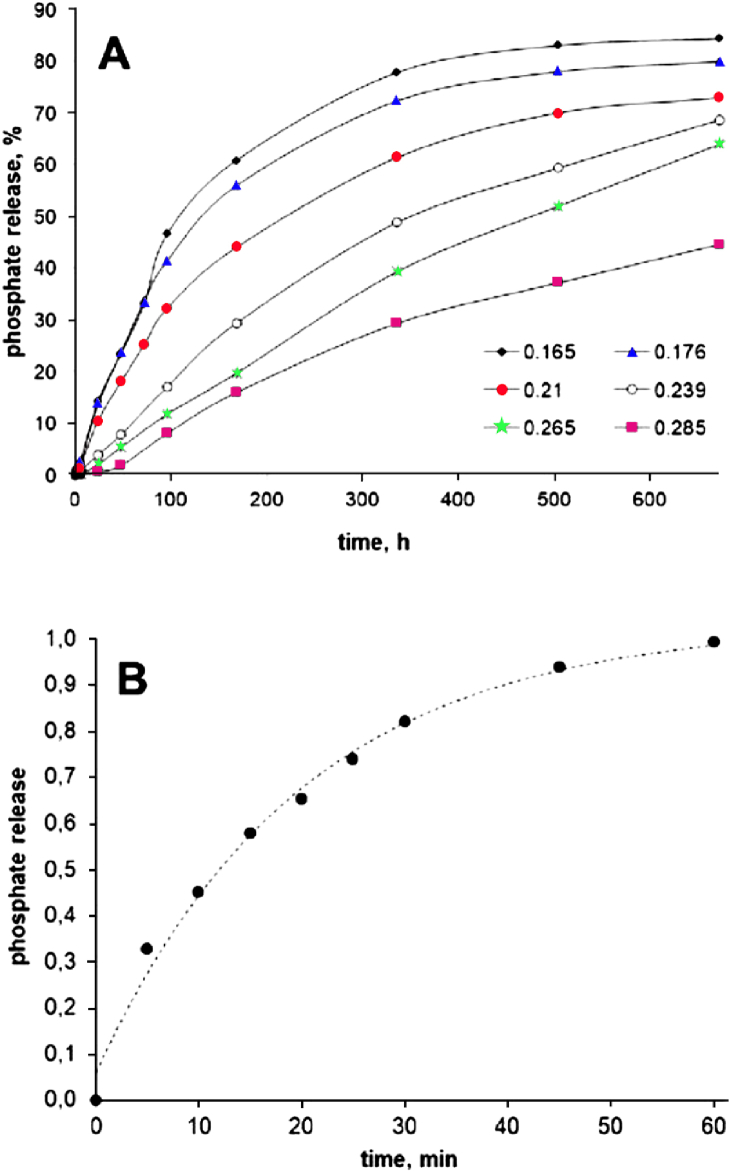
Studies were conducted over 28 days for all materials coated with ethyl cellulose, with mass ratios of polymer to fertilizer ranging from 0.16 to 0.285 (Fig. 3). After conducting a hip of similar experiments, it was concluded, that the mass ratio of biopolymer to fertilizer must be at least 0.21 for phosphomineral release to be less than 75 % within 28 days. The graph clearly shows the time dependence of nutrients released in percent, showing phosphate release with different ratios of ethylcellulose to fertilizer (Fig. 3A), as well as the effect of fertilizer on polymer coating (Fig. 3B). As would be expected, the phospho-fertilizer that did not contain an ethylcellulose as a coating layer was dissolved completely in water in about 1 h [5].
Fig. 3.
Releasing phosphates from ethyl cellulose-coated materials having different ethyl cellulose-to-fertilizer mass ratios (A) and releasing phosphates from the uncoated materials of the NPK fertilizer (B). Reproduced from Ref. [5]. Copyright (2023) with permission from Sciendo.
Using an ethylcellulose membrane to produce a proliferating fertilizer can significantly improve crop growth and quality, due to the slow release of beneficial minerals.
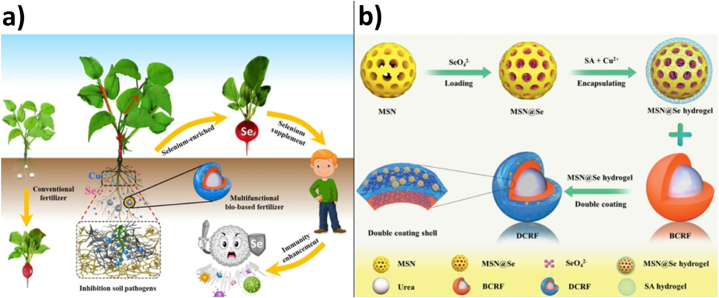
As is well-known, nitrogen is an essential microelement in plants functioning. Selenium, one of important microelements considered a leader among anticancer agents, is liable to easily washing from the plant surfaces, leaching, and deficit in soil. Like selenium, copper is a microelement playing a significant role in human life and having antifungal properties (Fig. 4А) [50].
Fig. 4.
Illustrative scheme of using DCRF in environment (A); obtaining DCRF (B) Reproduced from Ref. [50]. Copyright (2023) with permission from Elsevier.
Since the above matters are of invaluable importance for plants and people, a group of researchers prepared a biobased multipurpose two-layer controlled-release fertilizer. Urea was used as the source of nitrogen and represented the fertilizer core; biobased polyurethane was used as internal coating, and sodium alginate and copper ions formed a hydrogel as an external coating. Besides, to modify the sodium alginate hydrogel, the nanoparticles of mesoporous silicon dioxide were used, loaded with sodium selenate (Fig. 4B). This encapsulation of fertilizers made it possible to increase the duration of nitrogen and selenium release approximately 40 times to 42 days and 40 h, respectively, which made it possible to increase fruit yield by 69.9 % relative to the experiment using non-encapsulated fertilizers. This development allows controlling the release of nitrogen, selenium, and copper, which in turn increases the favorable development of crops [50].
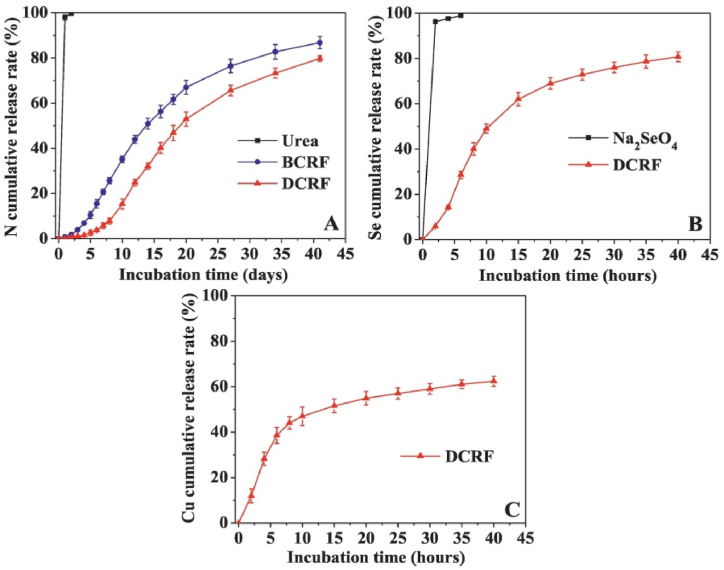
Changes in releasing N, Se, and Cu from DCRF are shown in Fig. 5. Cumulative rates of nitrogen release from urea, BCRF, and DCRF are shown in Fig. 5A. Nitrogen life time in urea and BCRF was 1 day and 30 days, respectively. Selenium and copper represented lower rate of release from the capsules and less than 80 and 60 % of these elements were released within 30 days (Fig. 5B and C). Duration of releasing nutrients from DCRF reached 42 days, while sodium alginate hydrogel slowed the nitrogen release [50].
Fig. 5.
Cumulative rate of releasing nutrients: N (A), Se (B), and Cu (C). Reproduced from Ref. [50]. Copyright (2023) with permission from Elsevier.
Fig. 6 clearly shows the effect of using DCRF as fertilizer on cherry radish seedlings (sample S2). Through controlled release, the crop was supplied with nutrients throughout the growth period, contributing to quality yields.
Fig. 6.

Digital photos of cherry radish (A) and fruit parts. Reproduced from Ref. [50]. Copyright (2023) with permission from Elsevier.
Typical raw materials of biomass, such as vegetable oil, cellulose, castor oil and starch [51], lignin and hydroxyapatite [52], graphene oxide and chitosan [33], etc., are widely used in developing biobased polymeric coatings due to their high yields and easy transformations. The authors of this paper managed to obtain a slow-release fertilizer, the polymeric coating of which was the soya oil monomer epoxidized by acrylate cured by UV radiation using Mg(OH)2 modified by stearic acid followed by immobilizing paraffin as hydrophobic fillers (Fig. 7).
Fig. 7.
Process of preparing PSF, PSF-SM, and PSF–SMP. Reproduced from Ref. [53]. Copyright (2023) with permission from Elsevier.
A systemic analysis was performed on the microstructure, thermal stability, and hydrophobic properties of the surface of the composite coatings obtained and the releasing properties of the coating fertilizers, as well as their impact upon the actual growth of maize [53].
The findings have shown that after UV exposure for 30–60 s poly-epoxidized soybean oil acrylate (PESOA) coating modified with stearic acid @ Mg(OH) paraffin has demonstrated a good hydrophobic ability (Fig. 8B). Great results have been reached with the coating content near 7 %. In these conditions, the start release speed was up to 1.625 %, while the duration of nitrogen release reached 63 days (Fig. 8A). This UV-curing method ensures a new and simple strategy for preparing cost-efficient biobased slow-release fertilizers [53].
Fig. 8.
(a) Performance curve of slow release: PSF-SMP 3 %, PSF-SMP 5 %, and PSF-SMP 7 %, (b) Controlled-release mechanism scheme for PSF-SMP. Reproduced from Ref. [53]. Copyright (2023) with permission from Elsevier.
Controlled-release fertilizers were developed, with a hydrophobic coating from superhydrophobic halloysite nanotubes and polyurethane based on castor oil (Fig. 9). Action duration of the slow-release nitrogen fertilizers obtained exceeded two months [54].
Fig. 9.
Scheme of obtaining a controlled-release fertilizer. Reproduced from Ref. [54]. Copyright (2023) with permission from Elsevier.
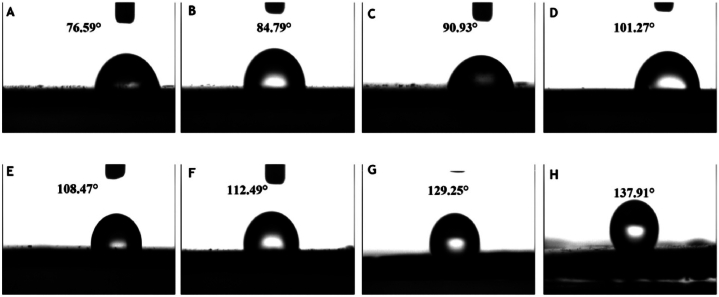
Superhydrophobic halloysite nanotubes (SHNs) were made by hydrolytic condensation of siloxane on the surface of halloysite nanotubes to increase the hydrophobic surface of the coating material equipped with bio-based castor oil. SHNs were used to modify superhydrophobic polyurethane (SHPU) for increasing the nano-sized surface roughness and reducing the surface energy. By doping halloysite nanotubes (HNTs) and polysiloxane-modified HNTs into the polyurethane coating, the researchers were able to increase the water contact angle from 84.79° to 101.27° and to 137.91°, respectively. Thus, the nanotubes blocked the micropores formed on the fertilizer coating, thereby preventing water from entering the cavity with the nutrients (Fig. 10A–H) [54].
Fig. 10.
Water contact angle of PU (A), PHU1 (B), PHU2 (C), PHU (D), SHPU1 (E), SHPU2 (F), SHPU (G), and polysiloxane-modified HNTs (H). Reproduced from Ref. [54]. Copyright (2023) with permission from Elsevier.
It was studied how polyurethane-coated urea based on the modified castor oil HNTs and SHTs upon the characteristics of nitrogen release and lifetime.
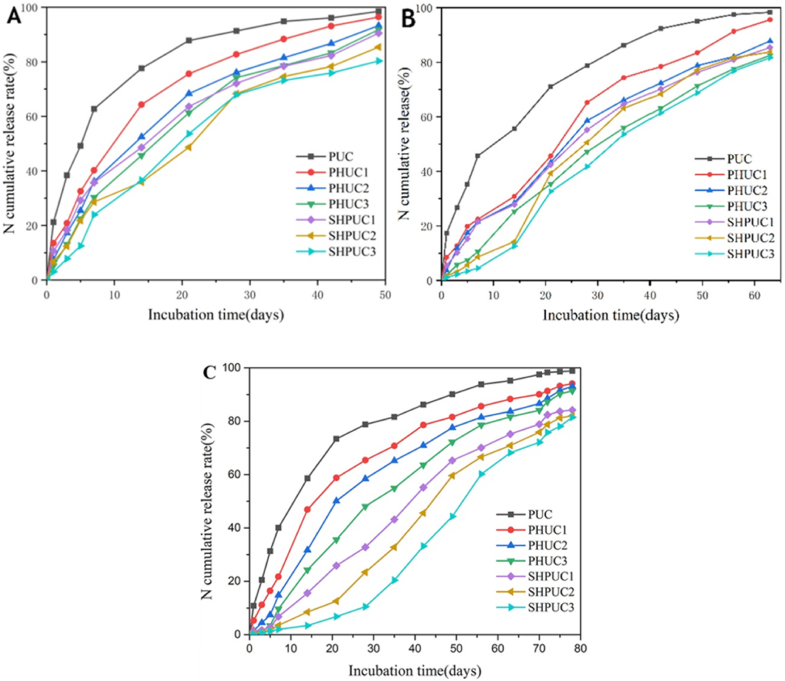
Contents of the added nanotubes considerably affected the characteristics of the PHUC and SHPUC nitrogen release (Fig. 11A–C). With increasing the contents of the HNTs and SHTs, the nitrogen release percentage decreased from 21 % down to 3 % per day and up to 49days and longevity of nitrogen release increased up to 49days for 80 % nitrogen release. As compared to PUC, PHUC and SHPUC showed a slower nitrogen release. This finding suggested that urea was contained in the HNT body and absorbed on the HNT surface, and adding HNTs could prevent from releasing nitrogen [54].
Fig. 11.
Curves of cumulative nitrogen release of PUC, PHUC, and SHPUC for (A) 3 %, (B) 5 %, and (C) 7 % of coating consumption. Reproduced from Ref. [54]. Copyright (2023) with permission from Elsevier.
This method of hydrophobically modified coating materials can become a new idea to develop highly efficient and environmentally friendly fertilizers controlled-release biobased fertilizers and sustainable development of agriculture [54].
Nano-carbon black (Nano-CB), an affordable and green nanomaterial having law surface energy, is a potential substance that provides superhydrophobic qualities to polymers without any complicated modification [55,56]. In this study, superhydrophobic nanocomposites polyurethane/black based on castor oil were developed using one-stage synthetic strategy to synthesize CRFs with the low coating contents by the three-layer coating method with a rotating drum.
Biobased polyurethane was obtained by the polyaddition of castor oil, vegetable oil containing hydroxyl groups to manufacture polyurethane directly without any modifications, and PMDI. Then polyurethane nanocomposites were obtained by including nano-carbon black into the polymer matrix (Fig. 12). It was analyzed how the nano-CB contents affect the characteristics of polyurethane nanocomposites. PU composites having different nano-CB contents were used to encapsulate the urea granules by the three-layer coating method with a rotating drum for the controlled-release fertilizers. It was investigated how the nano-CB contents affect the surface morphology and release characteristics of coated fertilizers [4].
Fig. 12.
Process of preparing a fertilizer coated by superhydrophobic polyurethane. Reproduced from Ref. [4]. Copyright (2023) with permission from Elsevier.
To evaluate the effect of nano-CB layer location on the controlled nutrient yield properties of coated fertilizer, the polyurethane composite layer was coated in the innermost or outermost layer, while other layers were coated with pure polyurethane to synthesize CRFs with the coating content of 2 % [4]. The results of the kinetic analysis of urea release from the capsules demonstrate a significant increase in the wettability of the surface granules during the distribution of nano-CB on the coating surface, which leads to an acceleration of the release of fertilizer (Fig. 13A), in this connection, further work was carried out on the distribution of the modifying agent in the inner layer of polyurethane capsules.
Fig. 13.
(А) Cumulative release of nano-CB PCU nitrogen in the innermost, middle, and outermost layer. (B) Scheme of adding nano-CB at various places. (C) and (D) Cumulative nitrogen release from PCU with different nano-CB loads and coating contents, respectively. Reproduced from Ref. [4]. Copyright (2023) with permission from Elsevier.
Samples were called PU-CB-x, where PU is for polyurethane and x is for the content of nano-CB. While regulating the content of nano-CB, four types of PU-CB were obtained. With an increase in the content of nano-CB in the polymer capsule, its hydrophobicity and roughness increased. The heterogeneity of the surface contributes to the formation of air pockets, which leads to a high contact angle and low adhesion. However, with an increase in the nano-CB load to 20 %, the contact angle of the coating decreased slightly due to a decrease in surface roughness. The degree of hydrophobicity of composite encapsulated fertilizers and their roughness do not fully correlate with the dynamics of nitrogen fertilizer yield (Fig. 13B–D). The effect of increased nitrogen release kinetics with an increase in the content of nano-CB content was interpreted by partial degradation of the capsule shell during modification and a decrease in the resistance of the material to internal and external osmotic pressure, which leads to an acceleration of its destruction process.
In this study, an original concept was proposed to manufacture dual-purpose and ecofriendly SRFs with the adjustable function of releasing fertilizers and a strong adsorption ability of microelements in soil [23].
In fact, membrane materials contained biobased liquefied polyurethane and uniform, organophilic, and stratified lignin-clay nanohybrids that had ensured tortuous hydrophobic paths to reduce the release of hydrophilic nutrients (N) (Fig. 14). Meanwhile, these nanobiocomposite membranes showed a large specific surface and a unique nanolayer structure for the efficiency metal adsorption in soil, which helped ensure the restoration of the contaminated soil and the necessary microelements for the growth of plants. It is worth noting that these inexpensive and biodegradable nanocomposite membranes not only function as a CRF, increasing the efficiency of fertilizer use, but also have a beneficial effect on the soil, regulating its performance and saturation of essential micronutrients that enhance the fruitful development of crops. A close look was taken at the mechanism to regulate nutrients with these newly synthesized CRFs and the mechanism of absorbing metals with membrane materials in a soil medium.
Fig. 14.
Scheme of obtaining lignin-clay plus bio-polyurethane-coated urea. Reproduced from Ref. [23]. Copyright (2023) with permission from Elsevier.
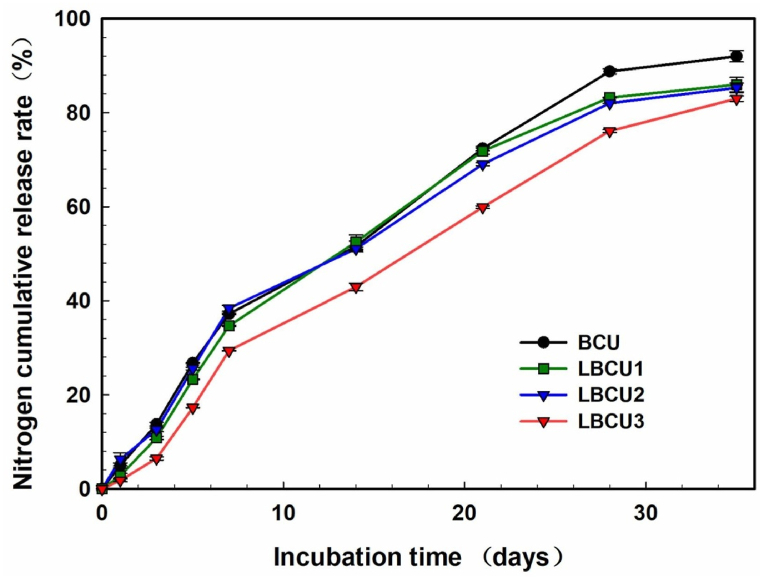
The findings show that the BCU coating has the highest water-absorption rate in all solutions, followed by LBCU1, LBCU2, and LBCU3, respectively. It is worth noting that the LBCU3 sample with an overwhelming number of nanoproducts had only about 7 % of the water-absorption rate, which suggests the hydrophobic behavior of nanocomposite coating membranes. Various salt conditions did not affect its water-absorbing ability [23]. Data on water absorption correlate well with data on the release of nitrogen fertilizers in water and soil. For LBCU3, despite the higher surface roughness, the rate of nitrogen release is lower due to higher hydrophobicity. (Fig. 15).
Fig. 15.
Release characteristics of non-modified BCU and lignin-modified LBCU. Three type of LBCU: LBCU1, LBCU2, and LBCU 3 with 10, 20, and 30 % of lignin-clay, respectively. Reproduced from Ref. [23]. Copyright (2023) with permission from Elsevier.
Fig. 16A and B shows the biomass yield of cherry radish consisting of fresh aerial and foot end. As compared to the check (CK) without any fertilizers, the yield of all variants, including urea-only fertilizer and LBCU, increased considerably on various lignin-clay nanohybrids, while the yields of fresh CRF biomass, including the upper and lower layers, were higher on addition of the lignin-clay (LBCU) nanohybrids than with U and BСU. With LBCU3, the fresh biomass yield was the highest, which can be explained through the fact that LBCU3 had the largest core urea release due to blocking the hydrophobic nanohybrid and the highest absorption of nutritive microelements in soils, which expedites the growth of plants [23].
Fig. 16.
In the photo: Oilseed radish (Raphanus sativus L. var. radculus pers) (A) and (B). Reproduced from Ref. [23]. Copyright (2023) with permission from Elsevier.
It is expected that this affordable, biodegradable, multifunctional nanocomposite lignin-based membrane will solve the issues related to the low utilization coefficients of fertilizers and to contaminating the delayed runoff with fertilizers and metals, which ensures a technical and theoretical support for the industrial application of coated CRFs [23].
In the experiment chitosan nanoparticles (CSNPs) showed ability to catch crude lipase and alkane hydroxylase enzymes from bacteria Alcanivorax borkumensis by ionotropic gelation. Enzymes in immobilized form prolonged their time of living, retaining up to 70 % of the initial activity after 5 days.
It is worth noting that the study used two solvents, namely double-distilled water (DDW) and phosphate buffer sodium (PBS), simulating the pH that can correspond to the real environmental conditions. DDW gave the best result in both samples; the alkane hydroxylase-saturated CSNPs (Fig. 17A) did not start releasing until 24 h later, and most (74.3 %) were released after 17 days (∼400 h), while the lipase-loaded CSNPs (Fig. 17B) had a complete controllable yield of 79.3 % after the same 17 days [57].
Fig. 17.
Release profile of alkane hydroxylase (A) and lipase (B) from CSNPs loaded with enzymes in two different solutions: Double-distilled water and phosphate-buffered saline. Reproduced from Ref. [57]. Copyright (2023) with permission from Elsevier.
Some researchers tried various binders to regulate the releases of macro- and microelements [7,58]. Bentonite clay, gypsum, gelatin, polyol, sulfur, starch, Zn nano particles, ZnO were previously coated using neem oil, paraffin wax, palm oil, and polyol as a binder of an urea fertilizer [7,[58], [59], [60]]. ZnO NP surface coating on urea was studied recently, using vegetable oil. Notwithstanding that some researchers used bentonite as a binder, no one used is as a source of nutrients [61,62]. In this study, nano bentonite was used as the source of Zn. The focus was on developing a macro- and microelement fertilizer from zinc-coated slow-release urea. For this purpose, ZnO NPs (21–41 nm) and zinc-enriched nano bentonite (6–50 nm) were applied to urea using stearic acid, paraffin oil, and paraffin wax as a binder [63].
The findings have shown that urea coated with nano bentonite (ZU1 and ZU3) released considerably less Zn as compared to urea coated with ZnO NPs (ZU2 and ZU4) (Fig. 18A). This difference in the Zn release is probably related to the low amounts of coated zinc (1 %) in form of zinc-enriched nano bentonite, as compared to the ZnO NPs (2 %). The highest release of Zn from ZU1 and ZU2 was recorded after 15 days, while zinc release from ZU3 and ZU4 continued even on the 30th day (Fig. 18A). This may be associated with a higher stability of paraffin oil and paraffin wax using in coating ZU3 and ZU4 [63].
Fig. 18.
Characteristics of releasing Zn from Zn-rich nanobentonite coated with urea, and ZnO NPs coated by urea (A); nature of nitrogen releasing from zinc-rich nanobentonite, and urea coated with ZnO NPs (B). Reproduced from Ref. [63]. Copyright (2023) with permission from Elsevier.
Nitrogen release analysis has proven the slower N release from coated urea granules, as compared to uncoated urea (Fig. 18B). It was noted that urea granule coatings in ZU3 and ZU4 considerably decreases the N release, as compared to ZU1 and ZU2 [63].
To better visualize the above literature data, there is a table presented that contains the most important characteristics of encapsulated fertilizers (Table 1).
Table 1.
– Characteristics of encapsulated fertilizers.
| Fertilizer | Coating (shell) | Active coating content in % | Release time in days | Release % | Release rate in % | Source | |
|---|---|---|---|---|---|---|---|
| Ammonium nitrate | poly (β-hydroxy butyrate) | 50 | 76 | 20 % | [14] | ||
| NKP | Ethyl cellulose | 0.16:0.29 | 1 | ∼15 % | [5] | ||
| Urea | Bio-based polyurethane coating with the alginate hydrogel mesh | 42 | nitrogen | ∼72 | [50] | ||
| 40 | selenium | ∼81 | |||||
| 2 | copper | ∼65 | |||||
| Urea | Mg(OH) modified with stearic acid followed by immobilizing with paraffin as hydrophobic fillers | 3 | 63 | 100 | [53] | ||
| 5 | 63 | 98 | |||||
| 7 | 63 | 80 | 1.625 | ||||
| Urea | Superhydrophobic halloysite nanotubes and castor-oil-based polyurethane | 7 % | 78 | 100 % | [54] | ||
| Urea | Polyurethane composites based on castor oil and nanocarbon black | 10 | 30 | 83 | 0.32 | [4] | |
| 15 | 25 | 85 | 2.03 | ||||
| 20 | 18 | 95 | 2.26 | ||||
| Urea | Bio-polyurethane-coated lignin-clay | 10 | 35 | 82.5 | 4.7 | [23] | |
| 20 | 35 | 82 | 4.1 | ||||
| 30 | 35 | 81 | 3.6 | ||||
| Alkane hydroxylase | Chitosan nanoparticles | 8 | 80.3 | [57] | |||
| Lipase | 8 | 88.1 | |||||
| Urea | Zinc-rich bentonite + vegetable oil | 1 | 10 | 90 | [63] | ||
| ZnO NPs + vegetable oil | 2 | 5 | 102 | ||||
| Zinc-rich bentonite + Stearic acid + Ca (OH)2+ Paraffin oil + Paraffin wax | 1 | 15 | 88 | ||||
| ZnO NPs + stearic acid + Ca(OH)2+ Paraffin oil + paraffin wax | 2 | 15 | 78 | ||||
Thus, literature data suggest a variety of possibilities to create pluripotential controlled-release fertilizers of various compositions.
3. Conclusions and prospectives
Concluding this review, it would be worth to note the most important prospects for the development of “smart” fertilizers in agriculture, to highlight their main advantages, as well as to indicate the disadvantages.
3.1. A number of pros of SRFs and CRFs
-
1.
With the metered technology of nutrient inputs of fertilizers with slow and controlled release, you can avoid high concentrations of ions (or elements) in the soil, which can “poison” the crop.
-
2.
Due to the action of SRFs and CRFs, it is possible to carry out planned plant fertilization less often, thereby reducing the amount of arable work, saving time and energy.
-
3.
Due to the slow release of nutrients, plants get sufficient and timely nutrition, and therefore a better development of the aboveground as well as root biomass can be observed [14].
-
4.
SRFs and CRFs can be used both outdoors and in greenhouses. In closed greenhouses they also provide plants with all the necessary useful substances in a single application.
-
5.
There is a significant reduction in the loss of nutrients in the form in which they are needed by the plant. That is, due to the slow release, there is no modification of a useful element in the soil (nitrate needed by plants does not have time to change into ammonia).
-
6.
Due to the fact that the nutrients remain in the required modification, no dangerous gases containing nitrogen (e.g. ammonia or laughing gas) are released. Thus, SRFs and CRFs keep the environment clean [64].
-
7.
Considering the environmental aspect, it is worth noting that the action of controlled-release fertilizers prevents the natural problems that could occur with conventional fertilizers, namely water pollution, biomaterial burning, eutrophication, greenhouse effect, energy costs [24].
3.2. Cons of SRFs and CRFs
-
1.
The release rate of some forms of the presented fertilizers due to the nature of the coating material or the variety of the nutrient itself may not always be effective. In one case the useful element may be released too quickly, in another very slowly or not completely.
-
2.
As for fertilizers containing sulfur in the coating material, the improperly designed shape of the membrane, can lead to turf damage or poor yields, since the sulfur film is often very fragile, causing pores and cracks, and can also be destroyed by exposure to soil bacteria [30].
-
3.
When developing a controlled-release fertilizer, it is worth considering how the nutrient and the protective coating correlate (can work in tandem), since the two components may have acidic properties, which can ultimately lower the soil pH several times and have a detrimental effect on the crop.
-
4.
It is worth paying more attention to the elaboration of the prolongation layer of fertilizer, as most often it is synthesized from polymers that may not decompose well or not decompose at all, thus creating an environmental problem. Preference should be given to a coating based on biological materials or biowaste.
-
5.
SRFs and CRFs are a fairly new solution in the agricultural industry, which requires regular and careful elaboration, which in turn leads to increased cost of encapsulated fertilizers [24].
-
6.
The release rate of nutrients from such fertilizers is sharply dependent on the ambient temperature, moisture, in some cases soil acidity. This is why at the beginning and at the end of the season, in the spring and autumn, when the temperature in the fields are low, the release of nutrients can occur slower [65]. As a result of which the nutrition for crops may be insufficient. In contrast, at elevated temperatures or high humidity, the release of nutrients is accelerated, which can cause a root burn [66].
Up to date, high cost is the main limiting factor for the development of coated fertilizers in agriculture, which is the reason the reason for infrequent use worldwide (the market share of CRFs is less than 1 %). However, CRFs can be used in high value-added areas of agriculture to enhance soil properties and increase soil productivity. This trend may be especially pronounced in that countries where agricultural products are expensive. For example, in Europe controlled release fertilizers account for a much larger share of the market. It is mentioned that eight to ten percent out of the total mass of fertilizers used [66]. Another illustrative example, almost 70 % of coated fertilizers are used in rice cultivation is registered in Japan. In general, this share will grow due to advancements in fabrication methods, application strategies, and improvement of their properties. As a result, CRFs will provide a revolutionary breakthrough in agriculture in the nearest future.
Prolonged-acting fertilizers carry enormous potential for use in agriculture. They have a number of advantages over traditional fertilizers, the use of which most often leads to environmental problems, poor yields, inefficient energy and labor costs. With the proper development of slow release/controlled release fertilizers, labor in the agribusiness can be greatly alleviated and what is more important, a quality of the crop can be significantly raised, which in the end will reduce the world hunger on the planet to a minimum.
CRediT authorship contribution statement
Alexey P. Dovzhenko: Visualization, Validation, Methodology. Olga A. Yapryntseva: Resources, Investigation, Data curation. Kirill O. Sinyashin: Software, Methodology, Formal analysis. Tinatin Doolotkeldieva: Writing – review & editing, Validation, Formal analysis, Conceptualization. Rustem R. Zairov: Writing – review & editing, Writing – original draft, Visualization, Resources, Project administration.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Aleksander Butlerov reports equipment, drugs, or supplies, statistical analysis, travel, and writing assistance were provided by Institute of Chemistry, Kazan (Volga region) Federal University. Rustem R. Zairov has patent pending to patent will be filed later. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was funded by a grant from the Ministry of Science and Higher Education of the Russian Federation for large scientific projects in priority areas of scientific and technological development (075-15-2024-646).
References
- 1.Akay G. Sustainable ammonia and advanced symbiotic fertilizer production using catalytic multi-reaction-zone reactors with nonthermal plasma and simultaneous reactive separation. ACS Sustain. Chem. Eng. 2017;5:11588–11606. doi: 10.1021/acssuschemeng.7b02962. [DOI] [Google Scholar]
- 2.Chen X., Cui Z., Fan M., Vitousek P., Zhao M., Ma W., Wang Z., Zhang W., Yan X., Yang J., Deng X., Gao Q., Zhang Q., Guo S., Ren J., Li S., Ye Y., Wang Z., Huang J., Tang Q., Sun Y., Peng X., Zhang J., He M., Zhu Y., Xue J., Wang G., Wu L., An N., Wu L., Ma L., Zhang W., Zhang F. Producingmore grain with lower environmental costs. Nature. 2014;514:486–489. doi: 10.1038/nature13609. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S., Yang Y., Gao B., Wan Y., Li Y.C., Zhao C. Bio-based interpenetrating network polymer composites from locust sawdust as coating material for environmentally friendly controlled-release urea fertilizers. J. Agric. Food Chem. 2016;64:5692–5700. doi: 10.1021/acs.jafc.6b01688. [DOI] [PubMed] [Google Scholar]
- 4.Liang D., Wang Y., Shi H., Luo Z., Quirino R.L., Lu Q., Zhang C. Controllable release fertilizer with low coating content enabled by superhydrophobic castor oil-based polyurethane nanocomposites prepared through a one-step synthetic strategy. Ind. Crops Prod. 2022;189 doi: 10.1016/j.indcrop.2022.115803. [DOI] [Google Scholar]
- 5.Lubkowski K., Smorowska A., Sawicka M., Wróblewska E., Dzienisz A., Kowalska M., Sadłowski M. Ethylcellulose as a coating material in controlled-release fertilizers. Pol. J. Chem. Technol. 2019;21:52–58. doi: 10.2478/pjct-2019-0010. [DOI] [Google Scholar]
- 6.Trenkel . 2013. Slow and Controlled-Release and Stabilized Fertilizers. Paris. [Google Scholar]
- 7.Azeem B., Kushaari K., Man Z.B., Basit A., Thanh T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Contr. Release. 2014;181:11–21. doi: 10.1016/j.jconrel.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Lawrencia D., Wong S.K., Low D.Y.S., Goh B.H., Goh J.K., Ruktanonchai U.R., Soottitantawat A., Lee L.H., Tang S.Y. Controlled release fertilizers: a review on coating materials and mechanism of release. Plants. 2021;10:1–26. doi: 10.3390/plants10020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gil-Ortiz R., Naranjo M.Á., Ruiz-Navarro A., Atares S., García C., Zotarelli L., Bautista A.S., Vicente O. Enhanced agronomic efficiency using a new controlled-released, polymeric-coated nitrogen fertilizer in rice. Plants. 2020;9:1–17. doi: 10.3390/plants9091183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Liu M., Ni B., Xie L. κ-Carrageenan-sodium alginate beads and superabsorbent coated nitrogen fertilizer with slow-release, water-retention, and anticompaction properties. Ind. Eng. Chem. Res. 2012;51:1413–1422. doi: 10.1021/ie2020526. [DOI] [Google Scholar]
- 11.Cole J.C., Smith M.W., Penn C.J., Cheary B.S., Conaghan K.J. Nitrogen, phosphorus, calcium, and magnesium applied individually or as a slow release or controlled release fertilizer increase growth and yield and affect macronutrient and micronutrient concentration and content of field-grown tomato plants. Sci. Hortic. 2016;211:420–430. doi: 10.1016/j.scienta.2016.09.028. [DOI] [Google Scholar]
- 12.Cong Z., Yazhen S., Changwen D., Jianmin Z., Huoyan W., Xiaoqin C. Evaluation of waterborne coating for controlled-release fertilizer using Wurster fluidized bed. Ind. Eng. Chem. Res. 2010;49:9644–9647. doi: 10.1021/ie101239m. [DOI] [Google Scholar]
- 13.Shaviv A., Mikkelsen R.L. Controlled-release fertilizers to increase efficiency of nutrient use and minimize environmental degradation - a review. Fert. Res. 1993;35:1–12. doi: 10.1007/BF00750215. [DOI] [Google Scholar]
- 14.Kontárová S., Přikryl R., Škarpa P., Kriška T., Antošovský J., Gregušková Z., Figalla S., Jašek V., Sedlmajer M., Menčík P., Mikolajová M. Slow-release nitrogen fertilizers with biodegradable poly(3-hydroxybutyrate) coating: their effect on the growth of maize and the dynamics of N release in soil. Polymers. 2022;14 doi: 10.3390/polym14204323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S., Shen T., Yang Y., Li Y.C., Wan Y., Zhang M., Tang Y., Allen S.C. Controlled-release urea reduced nitrogen leaching and improved nitrogen use efficiency and yield of direct-seeded rice. J. Environ. Manag. 2018;220:191–197. doi: 10.1016/j.jenvman.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Kottegoda N., Sandaruwan C., Priyadarshana G., Siriwardhana A., Rathnayake U.A., Berugoda Arachchige D.M., Kumarasinghe A.R., Dahanayake D., Karunaratne V., Amaratunga G.A.J. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano. 2017;11:1214–1221. doi: 10.1021/acsnano.6b07781. [DOI] [PubMed] [Google Scholar]
- 17.Xiao X., Yu L., Xie F., Bao X., Liu H., Ji Z., Chen L. One-step method to prepare starch-based superabsorbent polymer for slow release of fertilizer. Chem. Eng. J. 2017;309:607–616. doi: 10.1016/j.cej.2016.10.101. [DOI] [Google Scholar]
- 18.Da Cruz D.F., Bortoletto-Santos R., Guimarães G.G.F., Polito W.L., Ribeiro C. Role of polymeric coating on the phosphate availability as a fertilizer: insight from phosphate release by Castor polyurethane coatings. J. Agric. Food Chem. 2017;65:5890–5895. doi: 10.1021/acs.jafc.7b01686. [DOI] [PubMed] [Google Scholar]
- 19.Chen J., Lü S., Zhang Z., Zhao X., Li X., Ning P., Liu M. Environmentally friendly fertilizers: a review of materials used and their effects on the environment. Sci. Total Environ. 2018;613–614:829–839. doi: 10.1016/j.scitotenv.2017.09.186. [DOI] [PubMed] [Google Scholar]
- 20.Bolan N., Kunhikrishnan A., Thangarajan R., Kumpiene J., Park J., Makino T., Kirkham M.B., Scheckel K. Remediation of heavy metal(loid)s contaminated soils - to mobilize or to immobilize? J. Hazard Mater. 2014;266:141–166. doi: 10.1016/j.jhazmat.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Khalid S., Shahid M., Niazi N.K., Murtaza B., Bibi I., Dumat C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochemical Explor. 2017;182:247–268. doi: 10.1016/j.gexplo.2016.11.021. [DOI] [Google Scholar]
- 22.Yao Z., Li J., Xie H., Yu C. Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ. Sci. 2012;16:722–729. doi: 10.1016/j.proenv.2012.10.099. [DOI] [Google Scholar]
- 23.Zhang S., Yang M., Meng S., Yang Y., Li Y.C., Tong Z. Biowaste-derived, nanohybrid-reinforced double-function slow-release fertilizer with metal-adsorptive function. Chem. Eng. J. 2022;450 doi: 10.1016/j.cej.2022.138084. [DOI] [Google Scholar]
- 24.Trenkel M.E. 1997. Controlled-Release and Stabilized Fertilizers in Agriculture. [Google Scholar]
- 25.Dubey A., Mailapalli D.R. Zeolite coated urea fertilizer using different binders: fabrication, material properties and nitrogen release studies. Environ. Technol. Innov. 2019;16 doi: 10.1016/j.eti.2019.100452. [DOI] [Google Scholar]
- 26.Hara Y. Suppressive effect of sulfate on establishment of rice seedlings in submerged soil may be due to sulfide generation around the seeds, plant prod. Sci. 2013;16 doi: 10.1626/pps.16.50. [DOI] [Google Scholar]
- 27.Degryse F., Ajiboye B., Baird R., da Silva R.C., McLaughlin M.J. Oxidation of elemental sulfur in granular fertilizers depends on the soil-exposed surface area. Soil Sci. Soc. Am. J. 2016;80 doi: 10.2136/sssaj2015.06.0237. [DOI] [Google Scholar]
- 28.Ranadev P., Ashwin R., Joseph Bagyaraj D., Shinde A.H. Sulfur oxidizing bacteria in agro ecosystem and its role in plant productivity—a review. J. Appl. Microbiol. 2023;134 doi: 10.1093/jambio/lxad161. [DOI] [PubMed] [Google Scholar]
- 29.Rousk J., Bååth E., Brookes P.C., Lauber C.L., Lozupone C., Caporaso J.G., Knight R., Fierer N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010;4 doi: 10.1038/ismej.2010.58. [DOI] [PubMed] [Google Scholar]
- 30.Sim D.H.H., Tan I.A.W., Lim L.L.P., Hameed B.H. Encapsulated biochar-based sustained release fertilizer for precision agriculture: a review. J. Clean. Prod. 2021;303 doi: 10.1016/j.jclepro.2021.127018. [DOI] [Google Scholar]
- 31.Liu Y.H., Wang T.J., Qin L., Jin Y. Urea particle coating for controlled release by using DCPD modified sulfur. Powder Technol. 2008;183:88–93. doi: 10.1016/j.powtec.2007.11.022. [DOI] [Google Scholar]
- 32.Mehmood A., Khan Niazi M.B., Hussain A., Beig B., Jahan Z., Zafar N., Zia M. Slow-release urea fertilizer from sulfur, gypsum, and starch-coated formulations. J. Plant Nutr. 2019;42:1218–1229. doi: 10.1080/01904167.2019.1609502. [DOI] [Google Scholar]
- 33.Li Q., Wu S., Ru T., Wang L., Xing G., Wang J. Synthesis and performance of polyurethane coated urea as slow/controlled release fertilizer. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2012;27:126–129. doi: 10.1007/s11595-012-0421-7. [DOI] [Google Scholar]
- 34.Jarosiewicz A., Tomaszewska M. Controlled-release NPK fertilizer encapsulated by polymeric membranes. J. Agric. Food Chem. 2003;51:413–417. doi: 10.1021/jf020800o. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M., Yang J. Preparation and characterization of multifunctional slow release fertilizer coated with cellulose derivatives. Int. J. Polym. Mater. Polym. Biomater. 2021;70:774–781. doi: 10.1080/00914037.2020.1765352. [DOI] [Google Scholar]
- 36.Zvomuya F., Rosen C.J., Russelle M.P., Gupta S.C. Nitrate leaching and nitrogen recovery following application of polyolefin-coated urea to potato. J. Environ. Qual. 2003;32:480. doi: 10.2134/jeq2003.0480. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar A., Biswas D.R., Datta S.C., Roy T., Moharana P.C., Biswas S.S., Ghosh A. Polymer coated novel controlled release rock phosphate formulations for improving phosphorus use efficiency by wheat in an Inceptisol. Soil Tillage Res. 2018;180:48–62. doi: 10.1016/j.still.2018.02.009. [DOI] [Google Scholar]
- 38.Puoci F., Iemma F., Spizzirri U.G., Cirillo G., Curcio M., Picci N. Polymer in agriculture: a review. Am. J. Agric. Biol. Sci. 2008;3 doi: 10.3844/ajabssp.2008.299.314. [DOI] [Google Scholar]
- 39.Pena B., Gumi T. State of the art of polysulfone microcapsules. Curr. Org. Chem. 2013;17 doi: 10.2174/138527213805289105. [DOI] [Google Scholar]
- 40.Zhao R., Li Y., Li X., Li Y., Sun B., Chao S., Wang C. Facile hydrothermal synthesis of branched polyethylenimine grafted electrospun polyacrylonitrile fiber membrane as a highly efficient and reusable bilirubin adsorbent in hemoperfusion. J. Colloid Interface Sci. 2018;514 doi: 10.1016/j.jcis.2017.12.059. [DOI] [PubMed] [Google Scholar]
- 41.Abd El-Aziz M.E., Salama D.M., Morsi S.M.M., Youssef A.M., El-Sakhawy M. Development of polymer composites and encapsulation technology for slow-release fertilizers. Rev. Chem. Eng. 2022;38 doi: 10.1515/revce-2020-0044. [DOI] [Google Scholar]
- 42.Li T., Lü S., Wang Z., Huang M., Yan J., Liu M. Lignin-based nanoparticles for recovery and separation of phosphate and reused as renewable magnetic fertilizers. Sci. Total Environ. 2021;765 doi: 10.1016/j.scitotenv.2020.142745. [DOI] [PubMed] [Google Scholar]
- 43.Chi Y., Zhang G., Xiang Y., Cai D., Wu Z. Fabrication of reusable temperature-controlled-released fertilizer using a palygorskite-based magnetic nanocomposite. Appl. Clay Sci. 2018;161 doi: 10.1016/j.clay.2018.04.024. [DOI] [Google Scholar]
- 44.Katsumi N., Kusube T., Nagao S., Okochi H. Accumulation of microcapsules derived from coated fertilizer in paddy fields. Chemosphere. 2021;267 doi: 10.1016/j.chemosphere.2020.129185. [DOI] [PubMed] [Google Scholar]
- 45.Hüffer T., Metzelder F., Sigmund G., Slawek S., Schmidt T.C., Hofmann T. Polyethylene microplastics influence the transport of organic contaminants in soil. Sci. Total Environ. 2019;657 doi: 10.1016/j.scitotenv.2018.12.047. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Y., Liu X., Wang J. Ecotoxicological effects of microplastics and cadmium on the earthworm Eisenia foetida. J. Hazard Mater. 2020;392 doi: 10.1016/j.jhazmat.2020.122273. [DOI] [PubMed] [Google Scholar]
- 47.Klinpituksa P., Kosaiyakanon P. Superabsorbent polymer based on sodium carboxymethyl cellulose grafted polyacrylic acid by inverse suspension polymerization. Int. J. Polym. Sci. 2017;2017 doi: 10.1155/2017/3476921. [DOI] [Google Scholar]
- 48.Cheng D., Liu Y., Yang G., Zhang A. Water- and fertilizer-integrated hydrogel derived from the polymerization of acrylic acid and urea as a slow-release N fertilizer and water retention in agriculture. J. Agric. Food Chem. 2018;66:5762–5769. doi: 10.1021/acs.jafc.8b00872. [DOI] [PubMed] [Google Scholar]
- 49.Khalil M.I., Schmidhalter U., Gutser R., Heuwinkel H. Comparative efficacy of urea fertilization via supergranules versus prills on nitrogen distribution, yield response and nitrogen use efficiency of spring wheat. J. Plant Nutr. 2011;34 doi: 10.1080/01904167.2011.544349. [DOI] [Google Scholar]
- 50.Ma X., Zhang S., Yang Y., Tong Z., Shen T., Yu Z., Xie J., Yao Y., Gao B., Li Y.C., Helal M.I.D. Development of multifunctional copper alginate and bio-polyurethane bilayer coated fertilizer: controlled-release, selenium supply and antifungal. Int. J. Biol. Macromol. 2023;224:256–265. doi: 10.1016/j.ijbiomac.2022.10.121. [DOI] [PubMed] [Google Scholar]
- 51.Liao Y., Cao B., Liu L., Wu X., Guo S., Mi C., Li K., Wang M. Structure and properties of bio-based polyurethane coatings for controlled-release fertilizer. J. Appl. Polym. Sci. 2021;138:1–7. doi: 10.1002/app.50179. [DOI] [Google Scholar]
- 52.Elhassani C.E., Essamlali Y., Aqlil M., Nzenguet A.M., Ganetri I., Zahouily M. Urea-impregnated HAP encapsulated by lignocellulosic biomass-extruded composites: a novel slow-release fertilizer. Environ. Technol. Innov. 2019;15 doi: 10.1016/j.eti.2019.100403. [DOI] [Google Scholar]
- 53.Shi H., Liang D., Deng H., Xie F., Chen Z., Chen Y., Lu Q., Liu X., Zhang C. Bio-based superhydrophobic polymer coatings for slow-release fertilizers via a UV-curing encapsulation method. Ind. Crops Prod. 2022;188 doi: 10.1016/j.indcrop.2022.115580. [DOI] [Google Scholar]
- 54.Wang C., Song S., Yang Z., Liu Y., He Z., Zhou C., Du L., Sun D., Li P. Hydrophobic modification of castor oil-based polyurethane coated fertilizer to improve the controlled release of nutrient with polysiloxane and halloysite. Prog. Org. Coatings. 2022;165 doi: 10.1016/j.porgcoat.2022.106756. [DOI] [Google Scholar]
- 55.Hu X., Tang C., He Z., Shao H., Xu K., Mei J., Lau W.M. Highly stretchable superhydrophobic composite coating based on self-adaptive deformation of hierarchical structures. Small. 2017;13:1–10. doi: 10.1002/smll.201602353. [DOI] [PubMed] [Google Scholar]
- 56.Wu S., Du Y., Alsaid Y., Wu D., Hua M., Yan Y., Yao B., Ma Y., Zhu X., He X. Superhydrophobic photothermal icephobic surfaces based on candle soot. Proc. Natl. Acad. Sci. U. S. A. 2020;117 doi: 10.1073/pnas.2001972117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kadri T., Cuprys A., Rouissi T., Brar S.K., Daghrir R., Lauzon J.M. Nanoencapsulation and release study of enzymes from Alkanivorax borkumensis in chitosan-tripolyphosphate formulation. Biochem. Eng. J. 2018;137:1–10. doi: 10.1016/j.bej.2018.05.013. [DOI] [Google Scholar]
- 58.Irfan M., Khan Niazi M.B., Hussain A., Farooq W., Zia M.H. Synthesis and characterization of zinc-coated urea fertilizer. J. Plant Nutr. 2018;41:1625–1635. doi: 10.1080/01904167.2018.1454957. [DOI] [Google Scholar]
- 59.Junejo N., Khanif M.Y., Dharejo K.A., Abdu A., Abdul-Hamid H. A field evaluation of coated urea with biodegradable materials and selected urease inhibitors. Afr. J. Biotechnol. 2011;10:19729–19736. doi: 10.5897/AJB11.2415. [DOI] [Google Scholar]
- 60.Vashishtha M., Dongara P., Singh D. Improvement in properties of urea by phosphogypsum coating. Int. J. ChemTech Res. 2010;2:36–44. [Google Scholar]
- 61.Dimkpa C.O., Andrews J., Fugice J., Singh U., Bindraban P.S., Elmer W.H., Gardea-Torresdey J.L., White J.C. Facile coating of urea with low-dose ZnO nanoparticles promotes wheat performance and enhances Zn uptake under drought stress. Front. Plant Sci. 2020;11:1–12. doi: 10.3389/fpls.2020.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dimkpa C.O., Fugice J., Singh U., Lewis T.D. Development of fertilizers for enhanced nitrogen use efficiency – trends and perspectives. Sci. Total Environ. 2020;731 doi: 10.1016/j.scitotenv.2020.139113. [DOI] [PubMed] [Google Scholar]
- 63.Umar W., Czinkota I., Gulyás M., Aziz T., Hameed M.K. Development and characterization of slow release N and Zn fertilizer by coating urea with Zn fortified nano-bentonite and ZnO NPs using various binders. Environ. Technol. Innov. 2022;26 doi: 10.1016/j.eti.2021.102250. [DOI] [Google Scholar]
- 64.Mikkelsen R.L., Williams H.M., Behel A.D. Nitrogen leaching and plant uptake from controlled-release fertilizers. Fert. Res. 1994;37:43–50. doi: 10.1007/BF00750672. [DOI] [Google Scholar]
- 65.Shahena S., Rajan M., Chandran V., Mathew L. Conventional methods of fertilizer release. Control. Release Fertil. Sustain. Agric. 2021 doi: 10.1016/b978-0-12-819555-0.00001-7. [DOI] [Google Scholar]
- 66.Naik M.R., Kumar B.K., Manasa K. Polymer coated fertilizers as advance technique in nutrient management, AN ASIAN J. Soil Sci. 2017;12 doi: 10.15740/has/ajss/12.1/228-232. [DOI] [Google Scholar]