Threatened Pugnose Shiner (Miniellus anogenus) were acclimated to low and high water temperatures crossed with low and high turbidity levels over 15 weeks. We found that fish activity and sensitivity to hypoxia increased with temperature. Additionally, acclimation to both high temperature and high turbidity resulted in lower thermal tolerance.
Keywords: fish behaviour, fish physiology, fish swimming activity, hypoxia tolerance, imperilled fish, thermal tolerance
Abstract
High turbidity and elevated water temperature are environmental stressors that can co-occur in freshwater ecosystems such as when deforestation increases solar radiation and sedimentary runoff. However, we have limited knowledge about their combined impacts on fish behaviour and physiology. We explored independent and interactive effects of sedimentary turbidity and temperature on the swimming activity and both thermal and hypoxia tolerance of the Pugnose Shiner (Miniellus anogenus, formerly Notropis anogenus), a small leuciscid fish listed as Threatened under Canada’s Species at Risk Act (SARA). Fish underwent a 15-week acclimation to two temperatures (16°C or 25°C) crossed with two turbidities (~0 NTU or 8.5 NTU). Swimming activity was measured during the first 8 weeks of acclimation. Fish in warm water were more active compared to those in cold water, but turbidity had no effect on activity. Behavioural response to hypoxia was measured after 12 weeks of acclimation, as the oxygen level at which fish used aquatic surface respiration (ASR). Fish in warm water engaged in ASR behaviour at higher oxygen thresholds, indicating less tolerance to hypoxia. Turbidity had no effect on ASR thresholds. Finally, thermal tolerance was measured as the critical thermal maximum (CTmax) after 13–15 weeks of acclimation. Acclimation to warm water increased fish CTmax and Tag (agitation temperature) but reduced the agitation window (°C difference between Tag and CTmax) and thermal safety margin (°C difference between the acclimation temperature and CTmax). Furthermore, fish in warm, turbid water had a lower CTmax and smaller thermal safety margin than fish in warm, clear water, indicating an interaction between turbidity and temperature. This reduced thermal tolerance observed in Pugnose Shiner in warm, turbid water highlights the importance of quantifying independent and interactive effects of multiple stressors when evaluating habitat suitability and conservation strategies for imperilled species.
Introduction
Freshwaters are among the most disturbed ecosystems on our planet due to multiple anthropogenic threats such as land conversion, contaminant and sediment inflow, species introductions and overharvesting (Strayer and Dudgeon, 2010; Arthington et al., 2016; Reid et al., 2019). In addition, climate change is a growing threat to freshwaters that can lead to increased average water temperature, increased frequency of high temperature extremes and changes in precipitation regimes (Heino et al., 2009; Knouft and Ficklin, 2017). Given the imperilled status of an estimated 30% of freshwater fishes (WWF, 2021) and the limited dispersal potential for many species inhabiting landlocked ecosystems (Woodward et al., 2010), there is increasing interest in understanding the independent and interactive effects of multiple stressors on fitness-related traits (Ormerod et al., 2010; Castañeda et al., 2021). Two abiotic stressors that are increasingly co-occurring in freshwater systems and may have interactive effects on fishes are high turbidity (amount of suspended organic or sedimentary particles; Kale, 2016) and elevated water temperature. In this study, we explored independent and interactive effects of these two stressors on behavioural and eco-physiological traits of the Pugnose Shiner (Miniellus anogenus, formerly Notropis anogenus), a small leuciscid fish listed as Threatened under Canada’s Species at Risk Act (SARA), for which both elevated water temperature and turbidity have been identified as potential threats (COSEWIC, 2013; Gray et al., 2014, 2016; McDonnell et al., 2021).
Habitat degradation, land use change, urban development and extreme weather events can increase sedimentary and nutrient loading in freshwater ecosystems (Kemp et al., 2011; Wurtsbaugh et al., 2019). Larger quantities of inorganic particles in the water can increase sedimentary turbidity, while nutrients such as phosphorus and nitrogen can stimulate algal blooms and increase organic turbidity (Donohue and Garcia Molinos, 2009; Wurtsbaugh et al., 2019). In turn, both sedimentary and organic turbidity can affect other abiotic factors such as water temperature or dissolved oxygen (DO) concentration. For example, deforestation of riparian habitats can increase both sedimentary runoff and solar radiation (Dudgeon et al., 2006; Souza et al., 2013). Increased sedimentary runoff can then result in larger quantities of suspended particles that absorb sunlight and warm up surrounding waters (Wotton, 1994; Kale, 2016). Warmer waters also stimulate harmful algal blooms that further spread hypoxic zones in freshwater ecosystems and increase organic turbidity (Utne-Palm, 2002; Ho and Michalak, 2020).
For fishes that typically inhabit clear-water habitats and rely on vision as a major sensory modality, increasing turbidity in freshwater ecosystems can modify their visual environment and affect multiple feeding and social behaviours (Donohue and Garcia Molinos, 2009). Suspended particles absorb and scatter light, reducing the contrast between objects and their background, which can lead to diminished visual range and poorer feeding performance in visual predators (Gardner, 1981; Utne-Palm, 2002; De Robertis et al., 2003; Wellington et al., 2010). Increased activity levels have also been observed in association with increased foraging efforts, perhaps to compensate for reduced visual range (Meager and Batty, 2007; Harvey and White, 2008). Impacts of high turbidity can scale to the community level since mating (Gray et al., 2011), territorial (Berg and Northcote, 1985) and schooling behaviours (Kelley et al., 2012; Gray et al., 2014) can be altered as individuals allocate more time and energy to forage in turbid waters, and visual signalling between conspecifics is diminished. Sedimentary turbidity can also clog or damage gills, which may reduce oxygen uptake capacities of fish and other metabolically expensive activities such as swimming (Sutherland and Meyer, 2007; Gray et al., 2014).
Elevated water temperature is increasingly recognized as a significant driver of variation in fish species and assemblages. Rising water temperatures have been associated with changes in fish distribution, behaviour and physiology (Strayer and Dudgeon, 2010; Reid et al., 2019). As ectothermic vertebrates, the body temperature of fish is regulated by their environment, which can make them highly sensitive to elevated water temperatures and extreme thermal events (Seebacher et al., 2015; Burraco et al., 2020). The thermal performance window represents the range of temperatures across which fish can maintain vital functions such as activity, growth and reproduction (Huey and Stevenson, 1979; Schulte et al., 2011). The lower and upper critical limits of this thermal performance window are referred to as critical thermal minimum (CTmin) and critical thermal maximum (CTmax), respectively. As environmental temperatures approach and eventually exceed the thermal limits of a species’ performance window, performance may decrease and death may occur if fish are unable to move to another habitat or adjust their thermal window via phenotypic plasticity and/or genetic change (Beitinger et al., 2000; Pörtner, 2010; McDonnell and Chapman, 2015). In fishes, CTmax is often quantified as the temperature at which loss of equilibrium (LOE) occurs in response to an acute increase in water temperature (Lutterschmidt and Hutchison, 1997). There is also a growing number of studies that have detected a behavioural threshold that occurs prior to CTmax and may be ecologically relevant. These studies have observed that some fish exhibit an avoidance behaviour (denoted as quick and agitated swimming) at temperatures well below their CTmax (e.g. McDonnell and Chapman, 2015; Wells et al., 2016; Turko et al., 2020; Kochhann et al., 2021). This agitation behaviour could be useful in escaping thermal stress, but it may also expose fish to predators and affect access to important resources (McDonnell and Chapman, 2015). Elevated water temperature is also associated with higher standard and maximal metabolic rates (Schulte, 2015; Neubauer and Andersen, 2019) and higher activity levels in fish (Biro et al., 2010; Bartolini et al., 2015), which may lead to compensatory responses such as an increase in foraging activity with higher predator encounter rates (Buentello et al., 2000). With the persistent threat of global warming to freshwater fish biodiversity and the various stress responses induced by elevated water temperature, there is a growing need to understand how fishes respond not only to thermal stress but also how it interacts with other stressors.
Warming waters and high turbidity are likely to have interactive effects on fishes as they both have the potential to affect aerobic metabolism. For example, elevated water temperature can increase the rate of oxygen consumption; while high turbidity can reduce oxygen uptake efficiency by clogging gills with sedimentary particles or increasing stress levels through reduced visual range. A reduction in oxygen uptake efficiency could then make it more challenging to cope with heightened metabolic demands in warming waters and also impact fish behavioural and physiological responses to other environmental disturbances such as hypoxia (Fry, 1971; McBryan et al., 2013; Claireaux and Chabot, 2016). Despite all these potential interactions between elevated water temperature and high turbidity, there are still few studies that examine the interactive effects of both stressors on freshwater fishes. We explore these potential interactions by quantifying the effects of water temperature and sedimentary turbidity on the swimming activity of Pugnose Shiner as well as their behavioural response to hypoxia and thermal tolerance.
The Pugnose Shiner has been listed as Threatened under Canada’s Species at Risk Act (SARA, 2019) since 2019 and under the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) since 2013. The Canadian Pugnose Shiner populations currently represent the extreme northern range of this species (Chu et al., 2005). Within Canada, a few small and isolated populations of Pugnose Shiner are found in Southern Ontario in highly vegetated streams and lakes with calm and clear water (< 2 nephelometric units (NTU); Gray et al., 2014; McCusker et al., 2014). In addition to nutrient loading and habitat degradation, turbidity has been proposed as one of the primary drivers of the decline in Canadian Pugnose Shiner (COSEWIC, 2013). Indeed, decreased swimming abilities, reduced schooling behaviour and lower hypoxia tolerance have been documented in Pugnose Shiner exposed to relatively low turbidity levels (< 10 NTU) compared to other closely related Notropis and Miniellus species (Gray et al., 2014, 2016). Climate warming may also affect the persistence of Pugnose Shiner, as this species has been observed to have a lower CTmax and smaller thermal safety margin (difference between CTmax and acclimation temperature) than other non-imperiled Notropis and Miniellus species (McDonnell et al., 2021).
To quantify the independent and interactive effects of water temperature and sedimentary turbidity on Pugnose Shiner, wild-caught fish were acclimated to clear and turbid water crossed with low and high water temperature. We measured their swimming activity in a group setting when exposed to a gradual increase in turbidity and during a long-term acclimation period. Following activity trials, we explored whether acclimation to high temperature and/or turbidity affected their response to hypoxia by quantifying agitation and aquatic surface respiration (ASR), where fish swim to the water surface and ventilate their gills in the oxygen rich layer (Chapman and Mckenzie, 2009). Measuring this behavioural response to hypoxia is ecologically relevant since ASR can increase predation risk and alter other important behaviours such as mating, competition and shoaling as fish spend more time at the water surface (Chapman and Mckenzie, 2009). After ASR trials, we finished our experiment by assessing the thermal tolerance of Pugnose Shiner with CTmax trials. An interaction between turbidity and temperature would be evident in this experiment if fish showed a different response to turbidity when acclimated to warm versus cool water. We predicted that fish acclimated to warm, turbid water would have a lower thermal tolerance than those acclimated to warm, clear water. This may reflect gill clogging from sedimentary particles and/or increased stress levels due to a reduced visual range combined with heightened metabolic demands potentially associated with an acute temperature increase during CTmax trials. We also predicted that fish acclimated to warm, turbid water would become agitated and use ASR at higher DO levels than fish acclimated to cool, clear water, again associated with reduced respiratory function in turbid water and higher metabolic demands of warm water.
Materials and Methods
Fish collection and experimental set-up
In October 2020, we live-captured Pugnose Shiner specimens from Thompson’s Bay in the upper St-Lawrence River area (between ‘44.408°N 75.908°W’ and ‘44.418°N 75.894°W’). Thompson’s Bay is one of the most important Pugnose Shiner habitats in Ontario with low turbidity levels (0.46 NTU at capture sites; Potts, 2020), high DO levels and abundant aquatic vegetation (McCusker et al., 2014). We transported live specimens to McGill University and held them for 5 months in climate-controlled chambers at 16°C ± 0.01 (mean ± SEM) under a 12 h light:dark cycle. The water temperature of 16°C represented the average water temperature for September and October recorded in previous Pugnose Shiner surveys (Fisheries and Oceans Canada (DFO), unpublished data, 2002–2016). Fish were fed a daily diet of frozen blood worm ad libitum. In March 2021, we randomly distributed 95 Pugnose Shiner among nineteen 38-L aquaria (41 × 25 × 50 cm; height × width × length) with a density of 5 fish per aquarium. Each aquarium contained a filter-less water pump (Mini Underwater Filter, Fluval) that was filled with bio-balls to allow for the growth of nitrifying bacteria while minimizing the quantity of sedimentary particles removed from the water, an oxygen diffuser and plastic vegetation for environmental enrichment. Each aquarium was also separated from other aquaria by white plastic boards to prevent visual stimuli between adjacent aquaria. We monitored water temperature, DO, pH and conductivity daily throughout the entire experiment (Handy Polaris 2, Oxyguard; pH Testr10 and EC Testr11, Thermo Fisher Scientific) and performed necessary water changes (up to 10–20 L per day) to maintain water quality during the initial holding period. Aquaria were normoxic (DO > 95% saturation), pH levels were at 8.0 ± 0.0 and water conductivity was between 400 and 700 uS.
Experimental treatments and exposure
Aquaria were randomly assigned to combinations of water temperature (16°C or 25°C) and turbidity (~0 NTU or 8.5 NTU) using a 2 × 2 crossed design. Since fish had been held at 16°C for 5 months in clear water, this experiment asked whether an increase in temperature and/or an increase in turbidity affected the Pugnose Shiner. Our elevated water temperature treatment was 2°C higher than the average water temperature recorded in July and August for sites inhabited by Pugnose Shiner in Canada (23°C; DFO, unpublished data, 2002–2016). This temperature was also selected to minimize the risk of mortality since McDonnell et al. (2021) acclimated Pugnose Shiner to water temperatures ranging from 16°C to 31°C and observed higher survivorship at temperatures between 16°C and 25°C with an increase in mortalities at 28°C and 31°C. Our turbid treatment of 8.5 NTU was selected to expose fish to turbid water while permitting visual observations of fish behaviour. This level was also close to the highest turbidity level used by Gray et al. (2014, 10 NTU) in their study on the effects of turbidity on social behaviours of Pugnose Shiner and other minnow species. Initially, each treatment combination had five replicate aquaria, except for the 16°C clear water treatment with only four replicates due to a limited number of specimens. Fish mortality per treatment was generally low, except for a few fish that did not survive fin clipping and tagging for pilot tests of a respirometry experiment. No further respirometry trials were attempted to avoid additional mortalities. All fish care, collection and experimental protocols were approved by McGill University Animal Care Committee (AUP 7951) and the following provincial and federal permits: Permit No. 20-PCAA-00030 (Fisheries and Oceans Canada), Permit No. ER-B-005-20 (Ministry of the Environment, Conservation and Parks, Ontario), License No. 1096064 (Ministry of Natural Resources and Forestry, Ontario), Permit No. 2020-07-06-2856-06-S-P (Ministère des Forêts, de la Faune et des Parcs, Quebec).
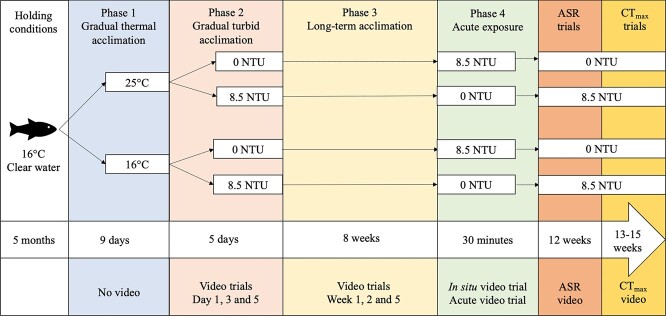
The experimental design consisted of three phases (Fig. 1). Phase 1 involved a gradual increase in water temperature (1°C/day) over 9 days for the 25°C thermal treatment using individual submersible aquarium heaters (Marina Submersible Heater, Hagen; McDonnell et al., 2021; Potts et al., 2021). During Phase 2, we gradually increased sedimentary turbidity over 5 days (1.4 NTU/day) for aquaria assigned to the 8.5 NTU turbidity treatment. Sedimentary turbidity was increased using a highly concentrated solution of natural bentonite clay powder similar to the one used in Gray et al. (2014, 2016, Healing Clay, Aztec Secret) mixed with water (30 g/L), while the ~ 0 NTU clear treatment had no clay added. We added a calculated amount of bentonite clay-water solution after any water change in turbid tanks to maintain the appropriate NTU level. We resuspended clay particles daily and measured turbidity after resuspension with a turbidity meter (2100Q Portable Turbidimeter, Hach). Phase 3 consisted of an 8-week long-term acclimation period to constant water temperatures (24.89 ± 0.01°C and 16.12 ± 0.01°C) and turbidities (0.79 ± 0.05 NTU and 8.57 ± 0.05 NTU) with daily monitoring. Following Phase 3, all fish also underwent an acute exposure to the alternative turbidity condition (30 min to either ~ 0 NTU or 8.5 NTU, results not reported here). After Phase 3 and the acute exposure, fish were acclimated for an additional 4 weeks before we measured aquatic surface respiration (ASR). Fish used in ASR trials were immediately euthanized and were not used in following critical thermal maximum (CTmax) trials to control for any residual effects of hypoxia exposure (Ackerly et al., 2018). For CTmax trials, all remaining fish were acclimated for an additional 1 to 3 weeks under the same temperature and turbidity conditions.
Figure 1.
Experimental exposure timeline used to test for the independent and interactive effects of turbidity and temperature on Pugnose Shiner. Activity was measured over 8 weeks of acclimation (Phases 2 and 3) and during an acute exposure to the alternative turbidity condition (Phase 4; results not reported), ASR thresholds were measured after 12 weeks of acclimation, and thermal tolerance was measured with CTmax trials after 13–15 weeks of acclimation.
Swimming activity trials
We recorded in situ videos using two cameras (Hero Session 4, GOPRO; Elite V50, AKASO) during Phase 2 (days 1, 3 and 5) and Phase 3 (weeks 1, 2, 5 and 8; Fig. 1). To measure fish activity, we traced a grid on the front of each aquarium to divide the viewing area into eight quadrats (12.5 × 7.5 cm, width × height). Following Gray et al. (2014), we placed a white plastic board in the aquarium to gently move the entire group of fish forward in the first half. We allowed fish to habituate for 30 min and then recorded movement over 5 min in the absence of any experimenter. Using the recorded videos, we then manually counted the number of grid quadrats visited over 5 min for each fish in the group before averaging activity data per aquarium to quantify swimming activity in a group setting. Only behavioural videos with a density of five fish were statistically analyzed due to mortalities in certain groups (tank sample size for Phase 2 and 3 reported in Table 1).
Table 1.
Biometric data and sample size for the turbidity x temperature acclimation of Pugnose Shiner (see Fig. 1 for experimental design). For Phase 2 and 3, the number of tanks with a density of 5 fish is reported for each experimental treatment. For ASR and CTmax trials, the number of individual fish used is reported for each experimental treatment. Average mass (g) ± SEM as well as average standard and total length (cm) of fish are also reported per treatment. The mass and length values were only measured for fish that underwent ASR and CTmax trials and all values were averaged together.
| Treatment | Phase 2 (# of tanks) | Phase 3 (# of tanks) | ASR (# of fish) | CTmax (# of fish) | Mass (g) | Standard length (cm) | Total length (cm) |
|---|---|---|---|---|---|---|---|
| 16°C + 0 NTU | 4 | 4 | 8 | 8 | 0.97 ± 0.05 | 4.33 ± 0.18 | 4.28 + 0.10 |
| 16°C + 8.5 NTU | 5 | 4 | 8 | 9 | 1.00 ± 0.11 | 4.09 ± 0.17 | 4.40 ± 0.20 |
| 25 °C + 0 NTU | 5 | 4 | 10 | 11 | 0.90 ± 0.04 | 4.34 ± 0.13 | 4.43 ± 0.12 |
| 25°C + 8.5 NTU | 3 | 2 | 9 | 8 | 0.97 ± 0.05 | 4.34 ± 0.16 | 4.43 ± 0.14 |
Aquatic surface respiration trials
To explore the effects of our acclimation conditions on fish response to hypoxia, we quantified the DO concentration at which fish started to become agitated (agitation threshold) and spent 90% of their time engaged in ASR behaviour (ASR90 threshold). ASR is a widespread behavioural response to hypoxia observed in many freshwater and marine fishes from both temperate and tropical ecosystems (reviewed in Chapman and McKenzie, 2009). In goldfish (Carassius auratus), ASR combined with bubble holding has been demonstrated to increase arterial blood oxygen content and therefore reduce the effects of aquatic hypoxia (Burggren, 1982). A higher ASR threshold can also be indicative of a lower tolerance to hypoxia because it is, in some species and/or populations, associated with a higher critical oxygen tension (Pcrit; oxygen level below which standard metabolic rate cannot be sustained; Chapman and McKenzie, 2009). After 12 weeks of acclimation, we conducted ASR trials on two Pugnose Shiner from each aquarium (n = 8–10 fish per treatment; Fig. 1; Table 1). Fish were not fed for 24 hours before the trial to induce a postabsorptive state. We transferred fish to individual baskets (13.5 × 15 × 12.5 cm) in a 40-L aquarium with thermal and turbidity conditions matching their acclimation conditions; and we allowed them to habituate for 2 hours. Bubble wrap covered half of the water surface to prevent oxygen diffusion, with half of the surface accessible to fish to perform ASR. We lowered DO% (percent air saturation) at a rate of 1% per min by bubbling nitrogen gas using a Witrox 1 unit, a DAQ-M instrument and the AutoResp software (Loligo Systems). DO% was lowered incrementally to levels of 100, 80, 70, 60, 50, 40, 30, 25, 20, 15, 10 and 5%. At every level, we maintained DO% constant over 5 min to allow us to record time-stamped videos, quantify agitation and ASR90 thresholds and count gill ventilations. ASR90 thresholds were measured as the DO% level at which fish spent 90% or more of their time performing ASR in the oxygen rich water surface. Agitation thresholds were measured as the DO% level at which fish started to swim with rapid bursts and occasional jumps for 30 s. Gill ventilation frequency was recorded for individual fish by averaging three ventilation counts, each of 15 s in length. For statistical analysis, gill ventilation frequency before and after the ASR90 threshold was also estimated for individual fish. Pre-ASR90 frequency was selected as the ventilation frequency recorded at the 40% DO level, a level above the ASR90 threshold of all experimental fish. Post-ASR90 frequency was selected as the ventilation frequency recorded at the 5% DO level, when all fish had crossed the ASR90 threshold. For presentation of the results, ventilation frequencies were converted to opercular beats per min (BPM). Once the three listed measurements were taken, DO% was further lowered until the next predetermined level. Trials ended at 5% DO or when fish lost equilibrium and fish were transferred to a fully aerated aquarium for recovery over 15 min. We then euthanized all fish used in ASR trials with eugenol and proceeded to weigh (g) and measure their total and standard length (cm).
Critical thermal maximum trials
After 13–15 weeks of experimental acclimation, we conducted CTmax trials using all remaining fish (n = 8–11 fish per treatment; Fig. 1; Table 1). Fish were not fed for 24 hours before the trial to induce a postabsorptive state. We transferred fish to individual baskets (13.5 × 15 × 12.5 cm) in a 40-L aquarium with temperature and turbidity conditions matching their acclimation conditions; and we allowed them to habituate for 2 hours. The experimental aquarium had three baskets and each CTmax trial was conducted with one to three fish. We raised water temperature by 0.3°C/min using a thermal control system (Witrox 1 unit and DAQ-M instrument) and monitored DO% with the AutoResp software (Loligo Systems). We measured CTmax in Pugnose Shiner as the temperature at loss of equilibrium (LOE), which is when fish became unable to maintain an upright swimming position for 30 s. We also measured the agitation temperature (Tag) of Pugnose Shiner as the temperature at which fish started to swim with rapid bursts and occasional jumps for 30 s before LOE (McDonnell et al., 2021; Potts et al., 2021). We recorded time-stamped videos of all trials to quantify CTmax and Tag temperatures. Using CTmax and Tag, two other thermal tolerance metrics were calculated. The agitation window was calculated as the difference between CTmax and Tag, as reported in Wells et al. (2016) and McDonnell et al. (2021). The thermal safety margin is generally calculated as the difference between CTmax and the maximum temperature recorded in the natural habitat of the fish (Morley et al., 2019; Turko et al., 2020). Here, we calculated a modified thermal safety margin as the difference between CTmax and the acclimation temperature (McArley et al., 2017; McDonnell et al., 2021). After CTmax trials, fish were transferred to a low water temperature aquarium for recovery over 15 min. We then euthanized, weighed (g) and measured the total and standard length (cm) of all fish used in CTmax trials.
Analysis
To analyze swimming activity of fish in a group setting, we averaged individual activity counts per aquarium and only analyzed videos with a density of five fish. Phases 2 and 3 were analyzed separately. In Phase 2, only aquaria undergoing gradual turbidity exposure were included in the analysis since we were interested in the effects of a gradual increase in turbidity on fish activity. For Phase 3, we quantified the effects of chronic turbidity exposure on fish activity. For each phase, we used the glmer.nb() function to produce general linear mixed models (GLMM; package lme4 v.1.1.30; Bates et al., 2015). Based on the guidelines in Payne et al. (2018) for data overdispersion and a dispersion parameter of 6.31 and 3.45 for the Phase 2 and 3 models, respectively; we selected a negative binomial distribution rather than a Poisson distribution. We used the default error correlations in the glmer.nb() function for random and repeated measurement components (package lme4 v.1.1.30; Bates et al., 2015). For Phase 2, we used a GLMM to test for independent and interactive effects of water temperature (16°C or 25°C) and the acclimation day, which represented our gradually increasing turbidity condition, on average fish activity (fixed effects; sig. α < 0.05). For Phase 3, we used another GLMM to test for independent and interactive effects of water temperature (16°C or 25°C), turbidity (clear or turbid) and acclimation day on average fish activity (fixed effects; sig. α < 0.05). We added tank ID as a random effect in all models. All interactions terms were kept in the models even if non-significant because we were specifically interested in testing for an interaction between turbidity and temperature (R script in Supplementary Material). Wald chi-square values (Type III) are reported for all P-values of each model using the Anova() function (package car v.3.1–0; Fox and Weisberg, 2019). All statistical analyses were done using the R Studio software version 4.2.1. (R Core Team, 2022). GLMM outputs for fish activity during Phase 2 and 3 are summarized in our Supplementary Table S1.
For CTmax and ASR trials, we used the lmer() function to produce linear mixed models (LMMs; package lme4 v.1.1.30; Bates et al., 2015). With LMMs, we tested for independent and interactive effects of water temperature and turbidity (fixed effects; sig. α < 0.05) on fish ASR90 and agitation thresholds, CTmax, Tag, agitation window and thermal safety margin. We added tank ID as a random effect in all models. Again, all interactions between temperature and turbidity were kept in the models even if non-significant because we were specifically interested in these interaction terms and their P-values, even if non-significant, can be informative (R script in Supplementary Material). For gill ventilation data, we used a GLMM to test for the effects of water temperature and turbidity (fixed effects; sig. α < 0.05) on gill ventilation frequency with tank ID as a random effect (R script in Supplementary Material). We also included individual fish as a repeated measure effect in this model since we compared pre-ASR90 and post-ASR90 gill ventilation frequency of the same fish to test if gill ventilation rates declined once fish crossed the ASR90 threshold. We selected a Poisson distribution for this GLMM since gill frequency was represented by discrete count data with a dispersion parameter of 0.41, resulting in relatively conservative P-values (Payne et al., 2018). Again, we used the default error correlations in the lmer() and glmer() functions for random and repeated measure effects (package lme4 v.1.1.30; Bates et al., 2015). Fish mass and standard length were initially included as covariates, but removed from all models as they had no significant effect (α > 0.05). We performed multiple comparison post-hoc tests (Holm-Bonferroni method) among the different thermal and turbidity treatment combinations for all significant interactions (sig. α < 0.05; package emmeans v.1.8.1–1; Lenth, 2022). Wald chi-square values (Type III) are reported for all P-values of each model using the Anova() function (package car v.3.1–0; Fox and Weisberg, 2019). All LMM outputs for our ASR and CTmax trials and the GLMM output for our gill ventilation data are reported in our Supplementary Tables S2 and S3.
Results
Swimming activity trials
Overall, acclimation to warm water resulted in an increase in swimming activity of Pugnose Shiner (Figs 2 and 3). During Phase 2, fish in warm and cool water were exposed to increasing turbidity over a 5-day period. Pugnose Shiner were more active in warm water (25°C) than in cool water (16°C; χ2 = 4.22 P = 0.04), but there was no evidence of activity changing as days progressed and turbidity increased (χ2 = 1.09, P = 0.30; Fig. 2). During Phase 3, fish acclimated to warm water were more active than fish acclimated to cool water throughout the 8-week period of long-term acclimation (χ2 = 11.45, P = 0.0007; Fig. 3). However, there was no significant effect of turbidity (χ2 = 1.00, P = 0.32) or acclimation day (χ2 = 0.0005, P = 0.98), nor was there a significant interaction between temperature and turbidity (χ2 = 0.02, P = 0.89).
Figure 2.
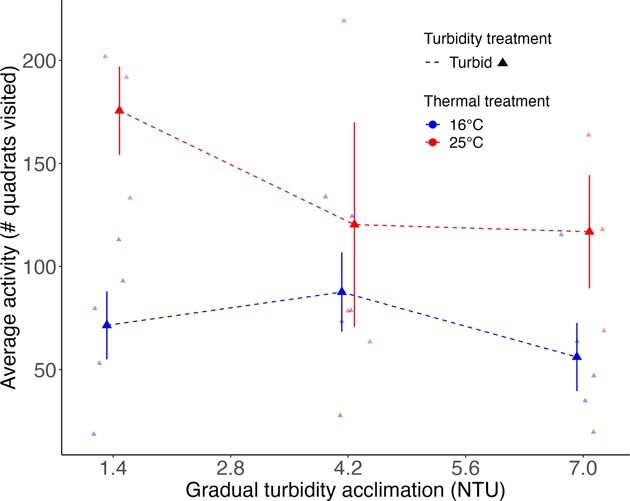
Swimming activity of Pugnose Shiner in a group setting under gradual turbidity increase over 5 days (Phase 2). Thermal treatment is indicated by colour (blue line at the bottom of the figure for 16°C and red line at the top of the figure for 25°C). Only aquaria undergoing gradual turbidity acclimation were analyzed (dashed lines and triangle points). Activity was measured as the total number of quadrats visited over 5 min per fish, and individual activity counts were averaged per aquarium. Larger points on the graph represent mean ± SEM while smaller points represent the raw data. Error bars and means in the figure are calculated using the raw data and are not corrected for the variance due to the aquarium random effect.
Figure 3.
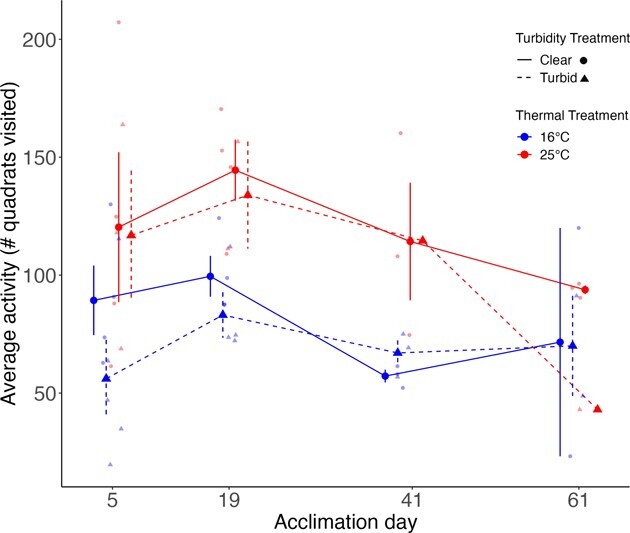
Swimming activity of Pugnose Shiner in a group setting during Phase 3 (8-week acclimation). Thermal treatment is indicated by colour (blue lines at the bottom of the figure for 16°C and red lines at the top of the figure for 25°C), and turbidity treatment is indicated by line and shape type (solid and circles for ~ 0 NTU (clear water) and dashed and triangles for 8.5 NTU (turbid water)). Larger points on the graph represent mean ± SEM while smaller points represent the raw data. Absence of error bars for the swimming activity recorded on days 41 and 61 for the turbid, warm treatment indicates that only one aquarium had a density of 5 fish at the end of Phase 3 due to fish mortality. Error bars and means in the figure are calculated using the raw data and are not corrected for the variance due to the aquarium random effect.
Aquatic surface respiration trials
Pugnose Shiner acclimated to warm water for 12 weeks exhibited higher ASR90 thresholds (mean ASR90 threshold = 19.85 ± 1.08% DO; mean ± SEM) than fish acclimated to cool water (mean ASR90 threshold = 12.23 ± 0.82% DO; χ2 = 19.87, P < 0.0001; Fig. 4A). Similarly, the DO% level at which fish became agitated in response to progressive hypoxia was higher in Pugnose Shiner acclimated to warm water (mean agitation threshold = 11.13 ± 1.25% DO) than in fish acclimated to cool water (mean agitation threshold = 6.33 ± 0.54% DO; χ2 = 8.04, P = 0.0045; Fig. 4B). We did not detect an effect of turbidity on ASR90 thresholds (χ2 = 0.0005, P = 0.98) or a significant interaction between temperature and turbidity (χ2 = 1.52, P = 0.22). Similarly, we did not detect an effect of turbidity on agitation thresholds (χ2 = 0.003, P = 0.96) or a significant interaction between temperature and turbidity (χ2 = 1.05, P = 0.31). Finally, Pugnose Shiner acclimated to warm water exhibited a higher opercular BPM than fish acclimated to cool water both pre-ASR90 and post-ASR90 thresholds (χ2 = 15.47, P < 0.0001; Fig. 5), while acclimation to turbidity had no effect (χ2 = 1.22, P = 0.27), nor was there a significant interaction between temperature and turbidity (χ2 = 1.60, P = 0.21).
Figure 4.
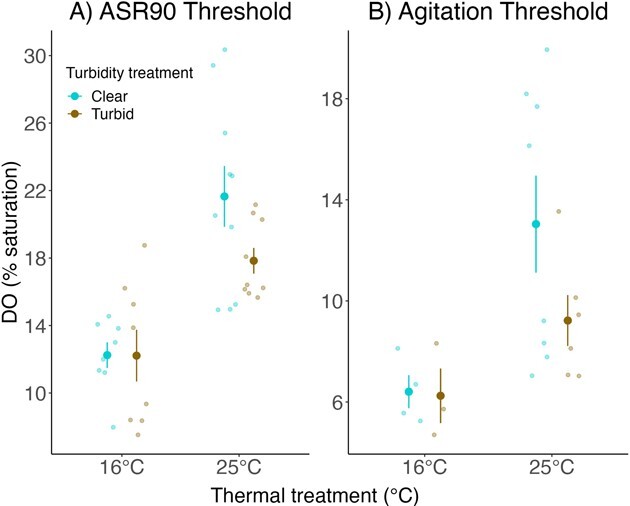
ASR90 thresholds (A) and agitation thresholds (B) of Pugnose Shiner after a 12-week acclimation period. Water temperature during the acclimation period is indicated on the x-axis and turbidity is indicated by colour (blue for clear water (~ 0 NTU) and brown for turbid water (8.5 NTU)). Larger points on the graph represent mean ± SEM while smaller points represent the raw data. Error bars and means in the figure are calculated using the raw data and are not corrected for the variance due to the aquarium random effect.
Figure 5.
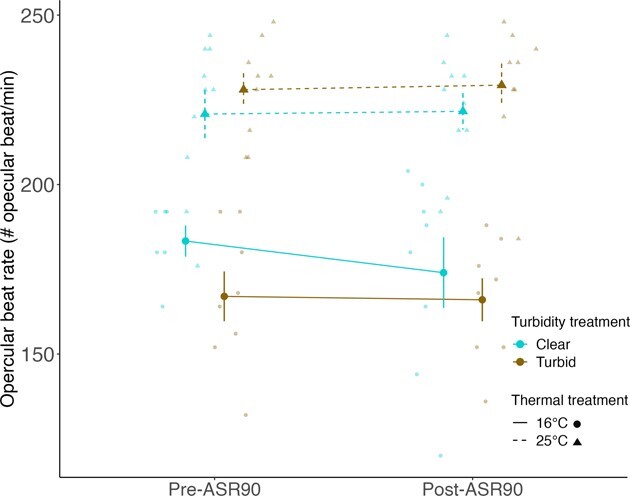
Opercular BPM (beat/min) of Pugnose Shiner pre-ASR90 and post-ASR90 thresholds. Pre-ASR90 BPM is the rate recorded at 40% DO, a level above the ASR90 threshold of all experimental fish and post-ASR90 BPM is the rate recorded at 5% DO, when all fish had crossed the ASR90 threshold. Water temperature is indicated by line and shape type (solid and circles for 16°C and dashed and triangles for 25°C) and turbidity is indicated by colour (blue for clear water (~ 0 NTU) and brown for turbid water (8.5 NTU)). Larger points on the graph represent mean ± SEM while smaller points represent the raw data. Error bars and means in the figure are calculated using the raw data and are not corrected for the variance due to the aquarium random effect.
Critical thermal maximum trials
During CTmax trials, Pugnose Shiner acclimated to warm water became agitated and lost equilibrium at higher temperatures than fish acclimated to cool water (χ2 = 357.72, P < 0.0001 for CTmax and χ2 = 277.01, P < 0.0001 for Tag). Additionally, there was a significant interaction between temperature and turbidity for CTmax (χ2 = 5.86, P = 0.016; Fig. 6A). CTmax was lower in fish acclimated to warm, turbid water (36.42 ± 0.28°C) than in fish acclimated to warm, clear water (37.16± 0.06°C; P < 0.0001; post-hoc). However, when fish were acclimated to cool water, there was no difference in CTmax between clear or turbid treatments (31.63 ± 0.21°C; P > 0.05; post-hoc). Agitation temperature (Tag) was much higher in fish acclimated to warm water (35.96 ± 0.11°C) than in fish acclimated to cool water (29.05 ± 0.29°C; χ2 = 277.01, P < 0.0001; Fig. 6B). There was no evidence that acclimation to turbidity affected Tag (χ2 = 3.36, P = 0.07), and there was no significant interaction between turbidity and temperature (χ2 = 2.21, P = 0.14).
Figure 6.
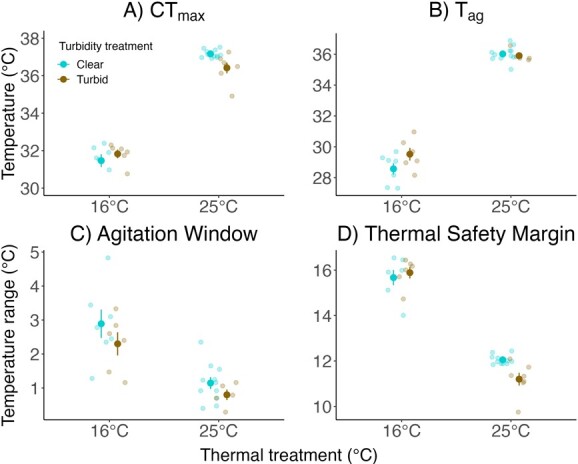
CTmax (A), Tag (B), agitation window (C) and thermal safety margin (D) of Pugnose Shiner after a 13 to 15-week acclimation period. Water temperature used during the acclimation period is indicated on the x-axis, and turbidity is indicated by colour (blue for clear water (~ 0 NTU) and brown for turbid water (8.5 NTU)). Larger points on the graph represent mean ± SEM while smaller points represent the raw data. Error bars and means in the figure are calculated using the raw data and are not corrected for the variance due to the aquarium random effect.
Pugnose Shiner in warm water had smaller agitation windows than fish in cool water (2.62 ± 0.27°C at 16°C and 1.04 ± 0.13°C at 25°C; Fig. 6C; χ2 = 22.08, P < 0.0001) with no significant effect of turbidity (χ2 = 1.92, P = 0.17) and no significant interaction between temperature and turbidity (χ2 = 0.17, P = 0.69). The thermal safety margin was much smaller in fish acclimated to warm water than in fish acclimated to cold water (χ2 = 142.26, P < 0.0001; Fig. 6D). There was no significant independent effect of turbidity (χ2 = 0.39, P = 0.54), but there was a significant interaction between turbidity and water temperature (χ2 = 5.42, P = 0.02). For fish acclimated to warm water, the thermal safety margin declined from 12.05 ± 0.06°C at ~ 0 NTU to 11.2 ± 0.28°C at 8.5 NTU (P < 0.0001; post-hoc), while fish acclimated to cold water showed an average thermal safety margin of 15.78 ± 0.21°C.
Discussion
Swimming activity, aquatic surface respiration (ASR) and critical thermal maximum (CTmax) trials revealed multiple effects of elevated water temperature and turbidity on Pugnose Shiner behaviour and physiology. Acclimation to warm water (25°C) increased fish activity and thermal tolerance (CTmax and Tag). However, fish acclimated to warm water also showed an earlier response to a gradual decrease in DO levels as well as a smaller agitation window and thermal safety margin than fish acclimated to cool water. Furthermore, fish acclimated to warm, turbid water exhibited a lower CTmax and had smaller thermal safety margin than fish acclimated to warm, clear water. We discuss the observed patterns in relation to the multiple stressor literature on the effects of turbidity, hypoxia and thermal stress, as well as the conservation implications of our results for the imperilled Pugnose Shiner.
Swimming activity trials
Pugnose Shiner acclimated to warm water were more active than fish acclimated to cool water, while acclimation to turbidity had no effect on fish activity. Increased activity was an immediate response to high water temperature that did not vary significantly over time. This is consistent with previous studies where activity increased when fish were reared and/or acclimated to warmer temperatures (e.g. Biro et al., 2010; Bartolini et al., 2015; Colchen et al., 2017; McDonnell et al., 2019). Since higher temperatures have been associated with higher metabolic rates, fish in warmer water may show higher activity levels because of an increased metabolism (Bartolini et al., 2015; Neubauer and Andersen, 2019). Fish in warm water were also more active throughout the long-term acclimation period and acclimation time did not significantly affect fish activity level. This might indicate that fish were unable to lower their standard metabolic rate through thermal compensation over the acclimation period. It is to be noted that our repeated measurements of activity over a long-term period may reflect fish acclimation to the experimental arena based on the overall decreasing trends shown in our Figs 2 and 3. However, this decrease in activity may have been missed due to the limited sample size in our study. Activity generally represents a large proportion of fish energy budgets, meaning that higher activity levels could reduce other energy intensive functions (Rennie et al., 2005). Therefore, wild populations of Pugnose Shiner under continuous thermal stress may exhibit reduced growth and reproduction over time if activity levels remain heightened.
Turbidity had no independent or interactive effect on Pugnose Shiner activity. The absence of any turbidity effect could reflect the relatively low turbidity level used in our experiment (8.5 NTU), which was necessary to allow us to visually record fish behaviour. Gray et al. (2014) reported reduced school cohesion of Pugnose Shiner acclimated to relatively low turbidity levels (< 10 NTU), which suggests an impact of even low levels of turbidity on schooling behaviour. However, this response may not necessarily be captured in average swimming activity of fish in a group setting. It would be ecologically relevant to explore the effects of higher turbidity levels or acute turbidity exposure on the activity of Pugnose Shiner to mimic the effects of rainy season runoff or flooding events (Gray et al., 2011).
Aquatic surface respiration trials
Pugnose Shiner acclimated to warm water became agitated and used ASR at higher DO levels than fish in cool water, suggesting less tolerance to hypoxia. This effect of temperature on hypoxia tolerance, where hypoxia tolerance has been measured as critical oxygen tension (Pcrit; oxygen level below which standard metabolic rate cannot be sustained), ASR thresholds and/or LOE, has been documented in multiple fish species (e.g. Remen et al., 2013; He et al., 2015; Jung et al., 2020; McArley et al., 2020). The potential effects of elevated water temperature on hypoxia tolerance have also been explored in the context of the oxygen- and capacity-limited thermal tolerance (OCLTT) framework (Pörtner and Farrell, 2008; Pörtner, 2010). According to the OCLTT, fish maximal metabolic rate increases with temperature until the oxygen demands exceed the capacity of the cardiovacular system to provide oxygen to tissues (Pörtner, 2010). As a result, fish aerobic scope (difference between the maximal and standard metabolic rate) and performance may decrease (Pörtner, 2010). Consistent with the OCLTT is the idea that fish upper thermal limits should be highly sensitive to aquatic hypoxia since this stressor directly limits the amount of oxygen available for aerobic metabolism (Holeton, 1980; Pörtner, 2010; McBryan et al., 2013). Similarly, fish hypoxia tolerance may be reduced under high water temperature conditions that increase oxygen demands (McBryan et al., 2013). In Pugnose Shiner, previous studies have shown reduced thermal tolerance of juveniles and adults acclimated or acutely exposed to reduced oxygen levels (McDonnell et al., 2021; Potts et al., 2021). In our study, we explored the effects of chronic exposure to elevated water temperature on ASR behaviour. The increase in the ASR90 and agitation threshold in fish acclimated to high water temperature supports a thermal dependency of this response to hypoxia. Together with earlier studies on the Pugnose Shiner, there is increasing evidence for links between hypoxia and thermal tolerance in this imperilled fish. Future studies on the effects of elevated water temperature on Pugnose Shiner should integrate Pcrit as a more direct evaluation of physiological responses to hypoxia under different thermal and turbid environments since it may yield informative and potentially different results compared to a behavioural response such as ASR.
Pugnose Shiner acclimated to warm water also exhibited a higher gill ventilation frequency (or opercular beats per min; BPM) than fish acclimated to cool water, potentially reflecting the increased rate of oxygen consumption associated with higher metabolic rates. Furthermore, fish in warm water maintained higher rates of gill ventilation throughout the ASR trials and engagement in ASR behaviour did not reduce gill ventilation rates, suggesting a relatively low efficiency of ASR in this species. In some fish species, the onset of ASR can reduce gill ventilation rates, likely due to an increase in oxygen uptake (Chapman and Liem, 1995). Pugnose Shiner exposed to thermal stress may then have engaged in ASR at higher oxygen concentrations and with higher gill ventilation frequencies to fufill greater metabolic demands and delay performance reduction (Holeton, 1980; McBryan et al., 2013).
Turbidity showed no significant effect on Pugnose Shiner ASR behaviour. In addition, gill ventilation frequency was similar between fish acclimated to clear or turbid water. An earlier study on Pugnose Shiner reported a higher Pcrit in fish acclimated to low levels of turbidity, which is consistent with the idea that turbidity may clog fish gills and reduce oxygen uptake efficiency (Gray et al., 2016). Gills were not examined post-ASR trials, but Gray et al. (2016) reported a buildup of grey mucus on gill lamellae after acclimating Pugnose Shiner to 7 NTU for 3 to 6 months. Thus, Pugnose Shiner acclimated to 8.5 NTU for 3 months in our study could have also experienced a mucus buildup on their gills. Pugnose Shiner also had access to the water surface during ASR trials, whereas Pcrit is measured in a closed system. Therefore, it is possible that access to the water surface compensated for any respiratory impairment due to high turbidity. Again, future studies measuring a physiological response to hypoxia such as Pcrit or LOE could show an interactive or independent effect of a low turbidity level such as the one used in this experiment.
Critical thermal maximum trials
Pugnose Shiner acclimated to warm water exhibited a higher CTmax and Tag than fish acclimated to cool water, a pattern that has been previously observed in this species (McDonnell et al., 2021; Potts et al., 2021) and in several other fish species (reviewed in Beitinger et al., 2000; Comte and Olden, 2017; Morley et al., 2019). Despite evidence for thermal acclimation capacities, Pugnose Shiner acclimated to warm water had a smaller thermal safety margin than fish acclimated to cool water. This is consistent with previous studies on multiple freshwater fish species including Pugnose Shiner (Campos et al., 2021; McDonnell et al., 2021; Potts et al., 2021). Therefore, wild populations of Pugnose Shiner in warm waters might have smaller thermal buffers against environmental warming compared to populations found in cooler waters. Fish in warm water also had smaller agitation windows, which can allow them to delay agitation and maintain normal behaviours for longer in warming waters. However, fish would potentially have less time to escape extreme thermal events in their environment before losing equilibrium. In addition, there is evidence that Pugnose Shiner may be more sensitive to thermal stress than other closely related species. McDonnell et al. (2021) quantified the CTmax of Pugnose Shiner across several acclimation temperatures. Using literature-derived data on other congeners, they found that Pugnose Shiner exhibited a lower CTmax and smaller thermal safety margins than non-imperilled congeners under comparable acclimation conditions.
Acclimation to turbidity decreased CTmax and the thermal safety margin of Pugnose Shiner acclimated to warm water. This reduced thermal tolerance could be due to a higher stress level in fish exposed to sedimentary turbidity that limited visual range and altered social behaviours and/or clogging of the gills that diminished oxygen uptake efficiency while oxygen demands increased with temperature. The turbidity level used in our study was well above the mean turbidity level (0.46 NTU) measured in Thompson’s Bay (site of specimen collection; Potts, 2020), but lower than the maximum observed at the same site (12.5 NTU; Potts, 2020). Although there was only a small decrease in CTmax of fish acclimated to warm, turbid waters, our data supports several reports over the years that Pugnose Shiner is sensitive to turbidity (Edwards et al., 2012), and that effects can be observed at low levels of sedimentary loading, an observation also supported by the studies of Gray et al. (2014, 2016).
Limitations
The absence of turbidity effect in behavioural metrics such as swimming activity, ASR thresholds and agitation temperatures may be due to variability among individuals in association with different personalities or other phenotypic traits. These behavioural metrics may then be underpowered compared to a physiological threshold such as CTmax, for which we observed an interactive effect between temperature and turbidity. Furthermore, a limited sample size had to be used throughout the experiment to minimize the impact of our study on the natural population of Thompson’s Bay, which may have affected the power of our statistical analysis of the behavioural parameters measured. It is also possible that exposing fish to greater turbidity levels that are still ecologically relevant or using another turbidity medium other than bentonite clay could show different results. Nonetheless, it remains concerning that we observed an interactive effect of turbidity on fish thermal tolerance at a relatively low level, one that is already under the maximum turbidity value recorded in some natural Pugnose Shiner habitats. Therefore, the potential effects of sedimentary turbidity on Pugnose Shiner should not be disregarded without further studies on other behavioural and physiological metrics that play important roles in the survival and health of this species.
Conclusion
The mechanisms underlying the effects of multiple stressors on fishes remain difficult to understand as freshwater ecosystems continue to undergo severe anthropogenic disturbances and climate variability. Isolated populations of imperilled fish with limited range expansion capacities will have to either adapt in situ to these disturbances or continue to decline. To mitigate population decline, species repatriation has been suggested as a potential conservation action for multiple imperilled fish species, including Pugnose Shiner (Edwards et al., 2012; Lamothe et al., 2019). This strategy has been applied with success in Chaumont Bay (Lake Ontario; New York), where thousands of pond-reared Pugnose Shiner have been released to restore the isolated population in the area (Foster et al., 2021). However, knowledge gaps still surround the biology and ecology of Pugnose Shiner that might diminish the efficiency of such conservation strategy over the long-term. COSEWIC (2013) has listed turbidity and sediment loading as one of the main threats for this species in addition to habitat loss and degradation. Our study shows that relatively low levels of sedimentary turbidity decreased the thermal tolerance of this species under warm water conditions. Future conservation strategies should then combine actions to reduce sediment loading in critical habitats for Pugnose Shiner with pond-rearing techniques to improve the long-term success of repatriation efforts and increase the protection of extant populations. Furthermore, other threats listed by COSEWIC (2013) such as loss of aquatic vegetation, nutrient loading and algal blooms, range shifts of predators and invasive species may interact with sedimentary turbidity and result in severe impacts on Pugnose Shiner behaviour and physiology. Therefore, our study highlights the importance of exploring potential interactions between stressors when evaluating threats to imperilled species and potential recovery strategies.
Supplementary Material
Acknowledgements
We thank Jessica Reemeyer and Rebecca Pahulje for their help with fish collection and fish care at McGill University. We also thank David Hunt for his support and statistical analysis guidance.
Contributor Information
Liana Fortin-Hamel, Department of Biology, McGill University, 1205 avenue du Docteur-Penfield, Montreal, Quebec, Canada, H3A 1B1.
Lauren J Chapman, Department of Biology, McGill University, 1205 avenue du Docteur-Penfield, Montreal, Quebec, Canada, H3A 1B1.
Author contributions
L.F.H. designed and carried out all laboratory experiments to collect scientific data, analyzed all datasets, and wrote the manuscript. L.J.C. contributed biological expertise, conceptual and data analysis guidance, and manuscript editing.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
This work was supported by the Department of Fisheries and Oceans Canada Nature Fund for Aquatic Species at Risk [G253657 to L.J.C.]; a National Sciences and Engineering Research Council of Canada Discovery Grant [RGPIN/04315–2020 to L.J.C.], the Distinguished James McGill Professorship Research Funds [to L.J.C.]; a Canada Graduate Scholarship—Master’s program from the Natural Sciences and Engineering Research Council of Canada [555939 to L.F.H] and a B1X scholarship from the Fonds de Recherche du Québec—Nature et Technologies [289559 to L.F.H].
Data availability
Supplementary material, datasets and R script codes underlying this article are publicly available from Figshare at https://doi.org/10.6084/m9.figshare.25180445
References
- Ackerly KL, Krahe R, Sanford CP, Chapman LJ (2018) Effects of hypoxia on swimming and sensing in a weakly electric fish. J Exp Biol 221: jeb172130. 10.1242/jeb.172130. [DOI] [PubMed] [Google Scholar]
- Arthington AH, Dulvy NK, Gladstone W, Winfield IJ (2016) Fish conservation in freshwater and marine realms: status, threats and management. Aquat Conserv 26: 838–857. 10.1002/aqc.2712. [DOI] [Google Scholar]
- Bartolini T, Butail S, Porfiri M (2015) Temperature influences sociality and activity of freshwater fish. Environ Biol Fishes 98: 825–832. 10.1007/s10641-014-0318-8. [DOI] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48. 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Beitinger TL, Bennett WA, McCauley RW (2000) Temperature tolerances of north American freshwater fishes exposed to dynamic changes in temperature. Environ Biol Fishes 58: 237–275. 10.1023/A:1007676325825. [DOI] [Google Scholar]
- Berg L, Northcote TG (1985) Changes in territorial, gill-flaring, and feeding behavior in juvenile coho salmon (Oncorhynchus kisutch) following short-term pulses of suspended sediment. Can J Fish Aquat Sci 42: 1410–1417. 10.1139/f85-176. [DOI] [Google Scholar]
- Biro PA, Beckmann C, Stamps JA (2010) Small within-day increases in temperature affects boldness and alters personality in coral reef fish. Proc R Soc B 277: 71–77. 10.1098/rspb.2009.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buentello JA, Delbert MG, Neill WH (2000) Effects of water temperature and dissolved oxygen on daily feed consumption, feed utilization and growth of channel catfish (Ictalurus punctatus). Aquac 182: 339–352. 10.1016/S0044-8486(99)00274-4. [DOI] [Google Scholar]
- Burggren WW (1982) “Air gulping” improves blood oxygen transport during aquatic hypoxia in the goldfish, Carassius auratus. Physiol Zool 55: 327–334. 10.1086/physzool.55.4.30155860. [DOI] [Google Scholar]
- Burraco P, Orizaola G, Monaghan P, Metcalfe NB (2020) Climate change and ageing in ectotherms. Glob Chang Biol 26: 5371–5381. 10.1111/gcb.15305. [DOI] [PubMed] [Google Scholar]
- Campos DF, Amanajás RD, Almeida-Val VMF, Val AL (2021) Climate vulnerability of south American freshwater fish : thermal tolerance and acclimation. J Exp Zool A Ecol Integr Physiol 335: 723–734. 10.1002/jez.2452. [DOI] [PubMed] [Google Scholar]
- Castañeda RA, Ackerman JD, Chapman LJ, Cooke SJ, Cuddington K, Dextrase AJ, Jackson DA, Koops MA, Krkošek M, Loftus KKet al. (2021) Approaches and research needs for advancing the protection and recovery of imperilled freshwater fishes and mussels in Canada. Can J Fish Aquat Sci 78: 1356–1370. 10.1139/cjfas-2020-0374. [DOI] [Google Scholar]
- Chapman LJ, Liem KF (1995) Papyrus swamps and the respiratory ecology of Barbus neumayeri. In Luczkovich JJ, Motta PJ, Norton SF, Liem KF, eds, Ecomorphology of Fishes. Springer, Dordrecht, pp. 183–197 [Google Scholar]
- Chapman LJ, Mckenzie DJ (2009) Behavioral responses and ecological consequences. In Richards JG, Farrell AP, Brauner CJ, eds, Hypoxia. Elsevier, San Diego, pp. 25–77 [Google Scholar]
- Chu C, Mandrak NE, Minns CK (2005) Potential impacts of climate change on the distributions of several common and rare freshwater fishes in Canada : climate change and fish distributions in Canada. Divers Distrib 11: 299–310. 10.1111/j.1366-9516.2005.00153.x. [DOI] [Google Scholar]
- Claireaux G, Chabot D (2016) Responses by fishes to environmental hypoxia : integration through Fry’s concept of aerobic metabolic scope. J Fish Biol 88: 232–251. 10.1111/jfb.12833. [DOI] [PubMed] [Google Scholar]
- Colchen T, Teletchea F, Fontaine P, Pasquet A (2017) Temperature modifies activity, inter-individual relationships and group structure in a fish. Curr Zool 63: 175–183. 10.1093/cz/zow048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comte L, Olden JD (2017) Evolutionary and environmental determinants of freshwater fish thermal tolerance and plasticity. Glob Chang Biol 23: 728–736. 10.1111/gcb.13427. [DOI] [PubMed] [Google Scholar]
- COSEWIC (2013) Cosewic assessment and status report on the Pugnose shiner Notropis anogenus in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa, 1–32. https://www.canada.ca/en/environment-climate-change/services/species-risk-public-registry/cosewic-assessments-status-reports/pugnose-shiner-2013.html. [Google Scholar]
- De Robertis A, Ryer CH, Veloza A, Brodeur RD (2003) Differential effects of turbidity on prey consumption of piscivorous and planktivorous fish. Can J Fish Aquat Sci 60: 1517–1526. 10.1139/f03-123. [DOI] [Google Scholar]
- Donohue I, Garcia Molinos J (2009) Impacts of sediment loads on the ecology of lakes. Biol Rev 84: 517–531. 10.1111/j.1469-185X.2009.00081.x. [DOI] [PubMed] [Google Scholar]
- Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, Lévêque C, Naiman RJ, Prieur-Richard AH, Soto D, Stiassny MLJet al. (2006) Freshwater biodiversity : importance, threats, status and conservation challenges. Biol Rev 81: 163–182. 10.1017/S1464793105006950. [DOI] [PubMed] [Google Scholar]
- Edwards AL, Matchett SP, Doherty A, Staton SK (2012) Recovery strategy for the Pugnose Shiner (Notropis anogenus) in Canada (proposed). Fisheries and Oceans Canada, Ottawa, pp. 1–72. https://sararegistry.gc.ca/document/doc1555p/ind_e.cfm. [Google Scholar]
- Foster JR, Lehman BC, Carlson DM, Ratchford JM, Robbins B, Soukup MW (2021) Empirical support for a strategy for the restoration of Pugnose Shiner (Notropis anogenus). Restor Ecol 29: el3431. 10.1111/rec.13431. [DOI] [Google Scholar]
- Fox J, Weisberg S (2019) An R companion to applied regression. Sage, California [Google Scholar]
- Fry FEJ (1971) The effect of environmental factors on the physiology of fish. In Hoar WS, Randall DJ, eds, Environmental Relations and Behavior. Elsevier, New York, pp. 1–98 [Google Scholar]
- Gardner MB (1981) Effects of turbidity on feeding rates and selectivity of bluegills. Trans Am Fish Soc 110: 446–450. . [DOI] [Google Scholar]
- Gray SM, Bieber FME, McDonnell LH, Chapman LJ, Mandrak NE (2014) Experimental evidence for species-specific response to turbidity in imperilled fishes: species-specific response to turbidity. Aquat Conserv: Mar Freshw 24: 546–560. 10.1002/aqc.2436. [DOI] [Google Scholar]
- Gray SM, McDonnell LH, Mandrak N, Chapman LJ (2016) Species-specific effects of turbidity on the physiology of imperiled blackline shiners Notropis spp. in the Laurentian Great Lakes. Endanger Species Res 31: 271–277. 10.3354/esr00774. [DOI] [Google Scholar]
- Gray SM, Sabbah S, Hawryshyn CW (2011) Experimentally increased turbidity causes behavioural shifts in Lake Malawi cichlids: turbidity shifts behaviour in cichlids. Ecol Freshw Fish 20: 529–536. 10.1111/j.1600-0633.2011.00501.x. [DOI] [Google Scholar]
- Harvey BC, White JL (2008) Use of benthic prey by salmonids under turbid conditions in a laboratory stream. Trans Am Fish 137: 1756–1763. 10.1577/T08-039.1. [DOI] [Google Scholar]
- He W, Cao ZD, Fu SJ (2015) Effect of temperature on hypoxia tolerance and its underlying biochemical mechanism in two juvenile cyprinids exhibiting distinct hypoxia sensitivities. Comp Biochem Physiol A Mol Integr Physiol 187: 232–241. 10.1016/j.cbpa.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Heino J, Virkkala R, Toivonen H (2009) Climate change and freshwater biodiversity : detected patterns, future trends and adaptations in northern regions. Biol Rev Camb Philos Soc 84: 39–54. 10.1111/j.1469-185X.2008.00060.x. [DOI] [PubMed] [Google Scholar]
- Ho JC, Michalak AM (2020) Exploring temperature and precipitation impacts on harmful algal blooms across continental U.S. lakes. Limnol Oceanogr 65: 992–1009. 10.1002/lno.11365. [DOI] [Google Scholar]
- Holeton GF (1980) Oxygen as an environmental factor of fishes. In: Ali MA, ed. Environmental Physiology of Fishes. Springer, Boston, pp. 7–32, 10.1007/978-1-4899-3659-2_2. [DOI] [Google Scholar]
- Huey RB, Stevenson RD (1979) Integrating thermal physiology and ecology of ectotherms : a discussion of approaches. Am Zool 19: 357–366. 10.1093/icb/19.1.357. [DOI] [Google Scholar]
- Jung EH, Brix KV, Richards JG, Val AL, Brauner CJ (2020) Reduced hypoxia tolerance and survival at elevated temperatures may limit the ability of Amazonian fishes to survive in a warming world. Sci Total Environ 748: 141349. 10.1016/j.scitotenv.2020.141349. [DOI] [PubMed] [Google Scholar]
- Kale VS (2016) Consequence of temperature, pH, turbidity and dissolved oxygen water quality parameters. Int Adv Res J Sci Eng Technol 3: 186–190. 10.17148/IARJSET.2016.3834. [DOI] [Google Scholar]
- Kelley J, Phillips B, Cummins G, Shand J (2012) Changes in the visual environment affect colour signal brightness and shoaling behaviour in a freshwater fish. Anim Behav 83: 783–791. 10.1016/j.anbehav.2011.12.028. [DOI] [Google Scholar]
- Kemp P, Sear D, Collins A, Naden P, Jones I (2011) The impacts of fine sediment on riverine fish. Hydrol Process 25: 1800–1821. 10.1002/hyp.7940. [DOI] [Google Scholar]
- Knouft J, Ficklin D (2017) The potential impacts of climate change on biodiversity in flowing freshwater systems. Annu Rev Ecol Evol Syst 48: 111–133. 10.1146/annurev-ecolsys-110316-022803. [DOI] [Google Scholar]
- Kochhann D, Sarmento CG, Oliveira JC, Queiroz HL, Val AL, Chapman LJ (2021) Take time to look at the fish : behavioral response to acute thermal challenge in two Amazonian cichlids. J Exp Zool A Ecol Integr Physiol 335: 735–744. 10.1002/jez.2541. [DOI] [PubMed] [Google Scholar]
- Lamothe KA, Drake DAR, Pitcher TE, Broome JE, Dextrase AJ, Gillespie A, Mandrak NE, Poesch MS, Reid SM, Vachon N (2019) Reintroduction of fishes in Canada: a review of research progress for SARA-listed species. Environ Rev 27: 575–599. 10.1139/er-2019-0010. [DOI] [Google Scholar]
- Lenth RV (2022) emmeans: Estimated Marginal Means, aka Least-Squares Means. https://CRAN.R-project.org/package=emmeans (date last accessed 20 May 2023).
- Lutterschmidt WI, Hutchison VH (1997) The critical thermal maximum: history and critique. Can J Zoo 75: 1561–1574. 10.1139/z97-783. [DOI] [Google Scholar]
- McArley TJ, Hickey AJR, Herbert NA (2017) Chronic warm exposure impairs growth performance and reduces thermal safety margins in the common triplefin fish (Forsterygion lapillum). J Exp Biol 220: 3527–3535. 10.1242/jeb.162099. [DOI] [PubMed] [Google Scholar]
- McArley TJ, Hickey AJR, Herbert NA (2020) Acute high temperature exposure impairs hypoxia tolerance in an intertidal fish. PloS One 15: e0231091. 10.1371/journal.pone.0231091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBryan TL, Anttila K, Healy TM, Schulte PM (2013) Responses to temperature and hypoxia as interacting stressors in fish : implications for adaptation to environmental change. Integra Comp Biol 53: 648–659. 10.1093/icb/ict066. [DOI] [PubMed] [Google Scholar]
- McCusker MR, Mandrak NE, Doka S, Gertzen EL, Wieren JF, McKenna JE, Carlson DM, Lovejoy NR (2014) Estimating the distribution of the imperiled pugnose shiner (Notropis anogenus) in the St. Lawrence River using a habitat model. J Great Lakes Res 40: 980–988. 10.1016/j.jglr.2014.09.014. [DOI] [Google Scholar]
- McDonnell LH, Chapman LJ (2015) At the edge of the thermal window: effects of elevated temperature on the resting metabolism, hypoxia tolerance and upper critical thermal limit of a widespread African cichlid. Conserv Physiol 3: cov050. 10.1093/conphys/cov050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell LH, Mandrak NE, Kaur S, Chapman LJ (2021) Effects of acclimation to elevated water temperature and hypoxia on thermal tolerance of the threatened pugnose shiner (Notropis anogenus). Can J Fish Aquat Sci 78: 1257–1267. 10.1139/cjfas-2020-0362. [DOI] [Google Scholar]
- McDonnell LH, Reemeyer JE, Chapman LJ (2019) Independent and interactive effects of long-term exposure to hypoxia and elevated water temperature on behavior and thermal tolerance of an equatorial cichlid. Physiol Biochem Zool 92: 253–265. 10.1086/702712. [DOI] [PubMed] [Google Scholar]
- Meager JJ, Batty RS (2007) Effects of turbidity on the spontaneous and prey-searching activity of juvenile Atlantic cod (Gadus morhua). Philos Trans R Soc Lond B Biol Sci 362: 2123–2130. 10.1098/rstb.2007.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley SA, Peck LS, Sunday JM, Heiser S, Bates AE (2019) Physiological acclimation and persistence of ectothermic species under extreme heat events. Glob Ecol Biogeogr 28: 1018–1037. 10.1111/geb.12911. [DOI] [Google Scholar]
- Neubauer P, Andersen KH (2019) Thermal performance of fish is explained by an interplay between physiology, behaviour and ecology. Conserv Physiol 7: coz025. 10.1093/conphys/coz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod SJ, Dobson M, Hildrew AG, Townsend CR (2010) Multiple stressors in freshwater ecosystems. Freshw Biol 55: 1–4. 10.1111/j.1365-2427.2009.02395.x. [DOI] [Google Scholar]
- Payne EH, Gebregziabher M, Hardin JW, Ramakrishnan V, Egede LE (2018) An empirical approach to determine a threshold for assessing overdispersion in Poisson and negative binomial models for count data. Commun Stat Simul Comput 47: 1722–1738. 10.1080/03610918.2017.1323223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pörtner HO (2010) Oxygen- and capacity-limitation of thermal tolerance : a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213: 881–893. 10.1242/jeb.037523. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322: 690–692. 10.1126/science.1163156. [DOI] [PubMed] [Google Scholar]
- Potts LB (2020) Hot and already bothered : Exploring effects of warming waters on an imperiled freshwater fish, pugnose shiner (Notropis anogenus) (Master’s thesis). McGill University, Montreal, Quebec [Google Scholar]
- Potts LB, Mandrak NE, Chapman LJ (2021) Coping with climate change : phenotypic plasticity in an imperilled freshwater fish in response to elevated water temperature. Aquat Conserv 31: 2726–2736. 10.1002/aqc.3620. [DOI] [Google Scholar]
- R Core Team (2021) R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- Reid AJ, Carlson AK, Creed IF, Eliason EJ, Gell PA, Johnson PTJ, Kidd KA, MacCormack TJ, Olden JD, Ormerod SJet al. (2019) Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev 94: 849–873. 10.1111/brv.12480. [DOI] [PubMed] [Google Scholar]
- Remen M, Oppedal F, Imsland AK, Olsen RE, Torgersen T (2013) Hypoxia tolerance thresholds for post-smolt Atlantic salmon : dependency of temperature and hypoxia acclimation. Aquaculture 416-417: 41–47. 10.1016/j.aquaculture.2013.08.024. [DOI] [Google Scholar]
- Rennie MD, Collins NC, Shuter BJ, Rajotte JW, Couture P (2005) A comparison of methods for estimating activity costs of wild fish populations : more active fish observed to grow slower. Can J Fish Aquat Sci 62: 767–780. 10.1139/f05-052. [DOI] [Google Scholar]
- SARA (2019) Pugnose shiner (Notropis anogenus). https://species-registry.canada.ca/index-en.html#/species/108-280. (date last accessed 10 May 2023).
- Schulte PM (2015) The effects of temperature on aerobic metabolism : towards a mechanistic understanding of the responses of ectotherms to a changing environment. J Exp Biol 218: 1856–1866. 10.1242/jeb.118851. [DOI] [PubMed] [Google Scholar]
- Schulte PM, Healy TM, Fangue NA (2011) Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr Comp Biol 51: 691–702. 10.1093/icb/icr097. [DOI] [PubMed] [Google Scholar]
- Seebacher F, White CR, Franklin CE (2015) Physiological plasticity increases resilience of ectothermic animals to climate change. Nat Clim Chang 5: 61–66. 10.1038/nclimate2457. [DOI] [Google Scholar]
- Souza ALT, Fonseca DG, Libório RA, Tanaka MO (2013) Influence of riparian vegetation and forest structure on the water quality of rural low-order streams in SE Brazil. For Ecol Manage 298: 12–18. 10.1016/j.foreco.2013.02.022. [DOI] [Google Scholar]
- Strayer DL, Dudgeon D (2010) Freshwater biodiversity conservation : recent progress and future challenges. J North Am Benthol Soc 29: 344–358. 10.1899/08-171.1. [DOI] [Google Scholar]
- Sutherland AB, Meyer JL (2007) Effects of increased suspended sediment on growth rate and gill condition of two southern Appalachian minnows. Environ Biol Fishes 80: 389–403. 10.1007/s10641-006-9139-8. [DOI] [Google Scholar]
- Turko AJ, Nolan CB, Balshine S, Scott GR, Pitcher TE (2020) Thermal tolerance depends on season, age and body condition in imperilled redside dace Clinostomus elongatus. Conserv Physiol 8: coaa062. 10.1093/conphys/coaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utne-Palm AC (2002) Visual feeding of fish in a turbid environment: physical and behavioural aspects. Mar Freshw Behav Physiol 35: 111–128. 10.1080/10236240290025644. [DOI] [Google Scholar]
- Wellington CG, Mayer CM, Bossenbroek JM, Stroh NA (2010) Effects of turbidity and prey density on the foraging success of age 0 year yellow perch Perca flavescens. J Fish Biol 76: 1729–1741. 10.1111/j.1095-8649.2010.02612.x. [DOI] [PubMed] [Google Scholar]
- Wells ZRR, McDonnell LH, Chapman LJ, Fraser DJ (2016) Limited variability in upper thermal tolerance among pure and hybrid populations of a cold-water fish. Conserv Physiol 4: cow063. 10.1093/conphys/cow063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward G, Perkins DM, Brown LE (2010) Climate change and freshwater ecosystems : impacts across multiple levels of organization. Philos Trans R Soc Lond B Biol Sci 365: 2093–2106. 10.1098/rstb.2010.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotton RS (1994) The classification of particulate and dissolved matter. In Wotton RS, ed, The biology of particles in aquatic systems. CRC Press, Boca Raton, pp. 1–6 [Google Scholar]
- Wurtsbaugh WA, Paerl HW, Dodds WK (2019) Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. WIREs Water 6: e1373. 10.1002/wat2.1373. [DOI] [Google Scholar]
- WWF . 2021The world’s forgotten fishes. WWF International, Switzerland. https://wwf.panda.org/discover/our_focus/freshwater_practice/the_world_s_forgoforg_fishes/. (date last accessed 20 July 2023). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplementary material, datasets and R script codes underlying this article are publicly available from Figshare at https://doi.org/10.6084/m9.figshare.25180445