Abstract
The Epstein-Barr virus (EBV) glycoproteins N and M (gN and gM) are encoded by the BLRF1 and BBRF3 genes. To examine the function of the EBV gN-gM complex, recombinant virus was constructed in which the BLRF1 gene was interrupted with a neomycin resistance cassette. Recombinant virus lacked not only gN but also detectable gM. A significant proportion of the recombinant virus capsids remained associated with condensed chromatin in the nucleus of virus-producing cells, and cytoplasmic vesicles containing enveloped virus were scarce. Virus egress was impaired, and sedimentation analysis revealed that the majority of the virus that was released lacked a complete envelope. The small amount of virus that could bind to cells was also impaired in infectivity at a step following fusion. These data are consistent with the hypothesis that the predicted 78-amino-acid cytoplasmic tail of gM, which is highly charged and rich in prolines, interacts with the virion tegument. It is proposed that this interaction is important both for association of capsids with cell membrane to assemble and release enveloped particles and for dissociation of the capsid from the membrane of the newly infected cell on its way to the cell nucleus. The phenotype of EBV lacking the gN-gM complex is more striking than that of most alphaherpesviruses lacking the same complex but resembles in many respects the phenotype of pseudorabies virus lacking glycoproteins gM, gE, and gI. Since EBV does not encode homologs for gE and gI, this suggests that functions that may have some redundancy in alphaherpesviruses have been concentrated in fewer proteins in EBV.
The envelopes of herpesviruses include multiple glycoprotein species, some of which are unique to individual members of the family and others of which appear to have been conserved either in sequence, structure, or genomic organization, reflecting a common evolutionary origin. There are two multicomponent complexes of glycoproteins that fall into this latter category. The best studied of the two consists of two conserved proteins, glycoprotein H (gH) and gL that in Epstein-Barr virus (EBV) and human cytomegalovirus (HCMV) associate with an additional protein with less-obvious common ancestry. The apparently unique EBV protein is gp42 (34); in HCMV it has been designated gO (24). This is one complex that appears to have been conserved in function. Multiple studies have been done on the dependency of gH on gL for authentic processing, and the gH-gL complex has been implicated in many herpesviruses as playing a critical role in penetration (10, 15, 18, 20, 29, 36, 45). EBV is no exception in this regard. A monoclonal antibody to the third member of the complex, gp42 (34), inhibits fusion of virus with the B-cell membrane (40). It apparently does so by blocking an interaction with HLA class II which on B cells can function as a coreceptor for virus entry (33). Antibody to gH likewise inhibits entry of virus into an epithelial cell on which a distinct but as-yet-unidentified coreceptor appears to be used (41, 55).
Less information is available concerning the second conserved complex of herpesvirus glycoproteins. It consists, as far as is currently known, of only two species, gN and gM. All gN homologs are predicted to be small type 1 membrane proteins of 84 to 138 amino acids with no potential N-linked glycosylation sites. Experimentally, some, such as the 14-kDa pseudorabies virus (PRV) gN (27) and the 15-kDa EBV gN (30), have been shown to carry O-linked sugars. Others, such as the approximately 8-kDa herpes simplex virus type 1 (HSV-1) gN (1, 6), the 9-kDa bovine herpesvirus 1 (BHV-1) gN (35), and the 7-kDa varicella-zoster virus gN (50), are not glycosylated. In contrast, all gM homologs are predicted to be multiple membrane-spanning proteins of 350 to 475 amino acids, and all except one (17) that have been studied to date are glycosylated (5, 13, 27, 30, 32, 39, 44, 56). The EBV gM is expressed as several species of approximately 48, 84, and 113 kDa (30). These either represent oligomers of a single form or result from differential O-linked glycosylation; the predicted sequence of gM includes two potential N-linked glycosylation sites (3), but one is at residue 6 and thus is probably not used.
The association between gN and gM homologs was first conclusively shown for PRV (26), although previous work with BHV-1 (35) had suggested that gN was more tightly associated with the tegument than many other membrane proteins and had identified a disulfide-linked partner of 39 kDa, originally proposed to be a tegument protein but subsequently shown to be gM (56). The EBV gN has been shown to associate with gM (30) and, although there has been no formal demonstration of the HSV-1 gN homolog interacting with gM it, too, has been described as linked to the tegument by disulfide bonds (1), so its seems likely that the motif is repeated here as well. It has now perhaps been generally assumed that all gN homologs will be found in association with gM, although the nature of the association may differ from virus to virus, just as there are subtle differences in the behaviors of gH and gL. For example, there is as yet no evidence that the EBV gN-gM homologs are disulfide linked. Authentic processing of EBV gN is dependent on coexpression with gM (30), but although incorporation of PRV gN into the virion is dependent on gM, O-linked sugars are apparently added normally to PRV gN in its absence (26).
The function of the gN-gM complex is more obscure than that of gH-gL. Studies of null mutants have provided a mixed picture. The effects of eliminating either gN or gM vary somewhat between viruses, and interpretation is complicated by the effect that the loss of one of the two partners may have on the behavior of the other, an effect which in most cases has not yet been explored. Much has been made of the fact that, despite the conservation of the complex throughout the herpesvirus subfamilies, none of the proteins has been shown to be essential for replication of any virus. Indeed, in many cases only minimal effects are seen on growth of virus in vitro. Loss of the VZV gN results in a slightly smaller plaque size (50); loss of the PRV gN has a similar phenotype and produces a virus with a two- to fivefold-lower yield and delayed penetration kinetics (26). The infectivity of PRV lacking gM is reduced 10- to 50-fold and also has delayed penetration kinetics (13), although this particular phenotype may best be ascribed to the accompanying lack of gN in the virion (26). BHV-1 that lacks gN is unimpaired for growth in tissue culture (35), HSV that lacks gN is unimpaired (48), HSV that lacks gM has slightly reduced yields in tissue culture (4, 38, 39), and the glycoprotein does play a role in cell-cell fusion (10). Loss of the equine herpesvirus type 1 (EHV-1) gM produces a virus that is 100-fold less infectious, has a 2-fold reduction in plaque size, and has a slower rate of penetration (43).
Two sets of observations, however, provide complimentary explanations for the apparent paradox of conservation of nonessential proteins. The first comes from the observation that those mutant viruses that have been examined for behavior in vivo, i.e., PRV (12), HSV-1 (39), and EHV-1 (42) that have lost gM, are all significantly attenuated. The second comes from a recent analysis of a PRV mutant which lacked three glycoproteins: gM, gE, and gI (8). This virus, in contrast to virus deleted only for expression of gM, was significantly impaired in assembly. However, complementation with gM, without expression of gE or gI, restored normal maturation. Both of these sets of studies suggest that in some viruses similar, but subtly different, functions have been assigned to different glycoproteins. They appear to be redundant under the simplest conditions of growth, but their unique contributions may be revealed either if the redundancy is removed or if the environment is made more complex. We report here on the phenotype of an EBV recombinant that lacks gN. The phenotype of this virus is more striking than that of others that lack only this small component of the gN-gM complex. It suggests that functions that in other viruses may have been dispersed among a number of different proteins have in this case been more concentrated in a few.
MATERIALS AND METHODS
Cells.
(i) Akata, a Burkitt lymphoma-derived cell line that carries and can be induced to make EBV (52) (a gift of John Sixbey, St. Jude Children's Research Hospital, Memphis, Tenn.), (ii) EBV-negative Akata cells (a gift of Jeffrey Sample, St. Jude Children's Research Hospital), (iii) Raji (47), an EBV genome-positive, nonproducing human B-cell line that expresses CR2, and (iv) P3HR1Cl5 (22), an EBV-positive human B-cell line that does not express CR2 (a gift of George Miller, Yale University, New Haven, Conn.) were grown in RPMI 1640 (Sigma Chemical Co., St. Louis, Mo.) supplemented with 5% (Raji) or 10% heat-inactivated fetal bovine serum (Gibco-BRL, Grand Island, N.Y.). AGS cells (American Type Culture Collection) were grown in Ham's F-12 nutrient mixture (Gibco-BRL) supplemented with 10% heat-inactivated fetal bovine serum. Human leukocytes were obtained from heparinized adult peripheral blood by flotation on lymphocyte separation medium and depleted of T cells by a double cycle of rosetting with sheep erythrocytes as previously described (34).
Virus production.
EBV was obtained from Akata cells which were resuspended at a concentration of 2 × 106 per ml and induced with 100 μg of anti-human immunoglobulin G per ml for 5 days. EBV to be used for cell infection was harvested from clarified culture medium that had been passed through a 0.8-μm (pore-size) filter.
Antibodies.
Monoclonal antibody 72A1 to gp350 (23) was obtained from spent culture medium of hybridoma cells grown in RPMI 1640 supplemented with 20% heat-inactivated fetal bovine serum. Three anti-peptide antibodies were made to synthetic peptides corresponding, respectively, to residues 125 to 137 of gp25 (anti-gL) (57), to residues 44 to 69 of the predicted BLRF1 open reading frame (ORF) (anti-gN), and to residues 346 to 364 of the BBRF3 ORF (anti-gM) (30). All antibodies were purified by chromatography on protein A (Sigma) coupled to Affigel-15 (Bio-Rad, Richmond, Calif.).
Radiolabeling and immunoprecipitation.
EBV proteins were labeled biosynthetically with [3H]glucosamine (20 Ci/mmol; Amersham Corp., Arlington Heights, Ill.) for 20 h at 6 h after induction with anti-human immunoglobulin G as previously described (57). Labeled cells were solubilized in radioimmunoprecipitation buffer (50 mM Tris-HCl, pH 7.2; 0.15 M NaCl; 1% sodium deoxycholate; 0.1% sodium dodecyl sulfate [SDS]; 0.1 mM phenylmethylsulfonyl fluoride; 100 U of aprotinin per ml) and immunoprecipitated with antibody and protein A-Sepharose CL4B (Sigma). Immunoprecipitated proteins were washed, dissociated by heating to 37°C for 30 min in sample buffer containing 2-mercaptoethanol, and analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) in 9 to 18% acrylamide cross-linked with 0.28% N,N′-diallyltartardiamide, followed by fluorography.
Derivation of virus in which the BLRF1 ORF is disrupted.
Dextran-purified virus harvested from the spent medium of 8 × 109 Akata cells was digested with proteinase K, and virion DNA was purified three times by centrifugation in cesium chloride. DNA that sedimented at a density of 1.718 g/ml of cesium was digested with EcoRI. The 8.5-kb EcoRI G fragment which begins at bp 82920 of the B95-8 sequence and ends at approximately bp 91421 and includes the BLRF1 ORF (53) was cloned into the multiple cloning site of pTA.108 Strider (a gift of Joan Stader, University of Missouri-Kansas City). A 1.5-kb XmnI/HincII fragment containing the neomycin resistance (Neor) gene under control of the simian virus 40 promoter was digested from pcDNA3 (Invitrogen, San Diego, Calif.), blunt ended and cloned into the BLRF1 ORF (bp 88547 to 88852) which had been opened up at a unique SapI site 51 bp from the initiation codon and blunt ended. The 8.5-kb EcoRI G fragment, now 10 kb by virtue of the insertion of the Neor gene, was purified and used to transfect Akata cells using DEAE-dextran (2). Twenty million cells were incubated with 5 μg of DNA and 0.3 mg of dextran for 90 min at 37°C and then washed, resuspended in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (Gibco-BRL) at a concentration of 106 cells/ml, and cultured for 2 days. Cells were then plated at 104 cells per well in 96-well tissue culture plates in medium containing 500 μg of active G418 (Gibco-BRL) per ml and fed weekly with fresh drug-containing medium. Resistant clones began to emerge after approximately 3 weeks. Drug-resistant Akata cells that were shown by Southern blotting to contain wild-type episomes and episomes that had undergone homologous recombination with the Neor gene-containing fragment were induced with anti-immunoglobulin. After 5 days, 150 μl of spent culture medium, diluted 1/20, was used to infect 2.5 × 106 EBV-negative Akata cells. After two further days in culture, the cells were plated in 96-well plates at 104 cells per well in medium containing 500 μg of active G418 per ml. Resistant clones began to emerge after approximately 3 weeks.
Southern blotting.
Cells were digested overnight at 56°C with proteinase K (1 mg/ml in 100 mM NaCl, 10 mM Tris-HCl [pH 8.0], 25 mM EDTA, and 0.5% SDS), and DNA was purified by phenol-chloroform extraction and ethanol precipitation. Purified DNA was digested overnight with HindIII and separated by agarose gel electrophoresis in 0.7% agarose. Separated DNA was transferred to a nylon membrane (Magnacharge) by capillary action, cross-linked, and hybridized either with the 8.5-kb EcoRI G fragment or with the XmnI/HincII fragment of pcDNA3 containing the Neor gene. Both probes were labeled with 32P.
Slot blot assays.
The amount of EBV DNA in cells or virion particles was measured by hybridization with the BamHI W fragment of EBV DNA labeled with 32P as previously described (54) and quantified by scanning with a Molecular Dynamics Storm PhosphorImager.
Binding of [3H]thymidine-labeled virus.
Two hundred million Akata cells containing wild-type or recombinant virus were induced with goat anti-human immunoglobulin in 20 ml of medium. After 4 h, 600 μCi of [3H]thymidine (20 to 30 Ci/mmol; Amersham) was added, and the volume of medium was increased to 100 ml. After 3 days of incubation, virus was harvested from the culture supernatant by centrifugation and resuspended in 800 μl of fresh medium. EBV receptor-positive Raji and EBV receptor-negative P3HR1Cl5 cells were fixed in ice-cold 0.1% paraformaldehyde, and the ability of the radiolabeled virus to bind to the cells in the presence or absence of antibody 72A1 to gp350 was determined as previously described (40).
Binding of unlabeled virus.
Two million EBV-negative Akata cells were incubated with virus for 2 h on ice, after which cells were washed three times with medium. Cells were pelleted at 325 × g for 4 min and resuspended in 40 μl of buffer containing 90 mM Tris-borate, 2 mM EDTA, 20% Ficoll 400, and 0.01% bromophenol blue. Cells were then transferred to the wells of a Gardella gel (19) for analysis of the amount of virion DNA bound to cells. Gels were run as described earlier (25), depurinated, alkalinized, and neutralized before transfer to a Nytran membrane (Schleicher & Schuell, Inc., Keene, N.H.). EBV DNA was visualized by probing with a BamHI W fragment of EBV DNA labeled with 32P as described above.
Assays of infection of B cells.
One million EBV-negative Akata cells were incubated with virus for 2 h on ice, after which the excess virus was removed, the volume was brought to 6 ml, and the cells were incubated at 37°C. Five days later the cells were harvested and analyzed for expression of EBNA1 by Western blotting. In addition, six hundred thousand T-cell-depleted peripheral blood leukocytes were incubated for 2 h at 37°C with 240 μl of virus, plated in quintuplicate at 105 cells per well in 96-well tissue culture plates, and reincubated for 4 weeks, at which time wells were examined for the presence of transforming foci.
Polyethylene glycol-mediated infection.
Samples of 5 × 106 T-cell-depleted peripheral blood leukocytes were incubated for 1 h on ice with wild-type or recombinant virus or growth medium. Cells were washed once and gently resuspended in 1 ml of 35% polyethylene glycol 1500 (Boehringer Mannheim) or serum-free medium for 5 min. Then, 10 ml of medium was added, and the cells were centrifuged at 400 × g, resuspended in fresh growth medium, and incubated for 14 days before harvesting and Western blot analysis.
Western blotting.
Proteins were electrophoresed in polyacrylamide and then electrically transferred onto nitrocellulose membranes (0.45-μm pore size; Schleicher & Schuell) at 125 mA for 6 h. The transferred sheets were reacted overnight with blocking buffer (10 mM Tris-HCl, pH 7.2; 0.15 M NaCl; 5% skim milk; 0.05% sodium azide) containing a 1/500 dilution of EBNA1-positive human serum. They were then washed five times with wash buffer (10 mM Tris-HCl, pH 7.2; 0.15% NaCl; 0.3% Tween 20) for 10 min each time. The washed sheets were reacted with alkaline phosphatase-conjugated goat anti-human antibodies (HyClone) for 2.5 h, and the bound anti-human antibodies were detected by reacting with substrate 5-bromo-4-chloro-3-indolylphosphate (BCIP) and Nitro Blue Tetrazolium (Sigma).
Sedimentation analysis of virus.
A 24 to 42% continuous gradient of Nycodenz (Sigma) in 1 mM potassium phosphate containing 0.01% bacitracin was made by layering 1 ml of 42, 40, 38, 36, 34, 32, 30, 28, 26, and 24% Nycodenz in a centrifuge tube and allowing the steps to diffuse overnight at 4°C. Then, 500 μl of virus was layered on top of the gradient and centrifuged in a Beckman SW41 Ti rotor at 70,000 × g for 2 h at 4°C. Fractions (500 μl) were collected from the top. The refractive index was measured with 25 μl; 25 μl was then digested with 5 U of DNase I and analyzed by slot blot for the presence of encapsidated virus DNA. The remaining volume was dialyzed against phosphate-buffered saline, and 300 μl of each dialysate was bound to 2 × 106 paraformaldehyde-fixed EBV-negative Akata cells. Binding was measured by Gardella gel analysis as described above.
Electron microscopy.
Ten million Akata cells carrying wild-type or recombinant virus were induced with anti-immunoglobulin for 48 h, pelleted at 350 × g, and washed with cacodylate buffer (0.15 M sodium cacodylate, 2 mM calcium chloride) at 4°C. Cells were fixed overnight at 4°C in cacodylate buffer containing 4% glutaraldehyde, washed three times in buffer alone, transferred to a microcentrifuge tube, pelleted at 250 × g, and resuspended in 50 μl of cacodylate buffer containing 10% gelatin that had been prewarmed to 37°C. Cells were pelleted at 250 × g for 2 min and put on ice to harden the gelatin. Each gelatin plug was cut with a razor blade into 1-mm cubes which were rinsed twice in phosphate buffer (100 mM potassium phosphate, 10 mM magnesium chloride; pH 6.0), postfixed for 1 h with 1% osmium tetroxide, rinsed twice in buffer, rinsed twice in water, dehydrated, and embedded in Araldite for sectioning.
Complementation of mutant.
The BLRF1 ORF was cloned into the EcoRV site in the multiple cloning region of the pIRESpuro vector (Clontech Laboratories, Inc., Palo Alto, Calif.). The resulting plasmid, pIRES-gN-Puro was linearized with SspI and transfected into the gastric carcinoma cell line AGS. After 48 h the cells were trypsinized and then plated in 96-well plates in medium containing 0.2 μg of puromycin (Clontech) per ml. Drug-resistant colonies were examined for expression of gN by indirect immunofluorescence. Those in which at least 10% of the cells were positive were infected with a mixture of recombinant and wild-type viruses that had been concentrated 50-fold. After 48 h, the cells were trypsinized and plated in 96-well plates in medium containing both 0.2 μg of puromycin per ml and 500 μg of active G418 per ml. Colonies resistant to both drugs were screened by Southern blot for presence of pure recombinant virus lacking gN, for expression of gN from pIRES-gN-Puro, and for the efficiency of induction of the virus lytic cycle after treatment with 30 ng of 12-o-∼tetradecanoylphorbol-13-acetate per ml and 2.5 mM sodium butyrate.
RESULTS
Generation of recombinant EBV with a selectable marker in the BLRF1 ORF.
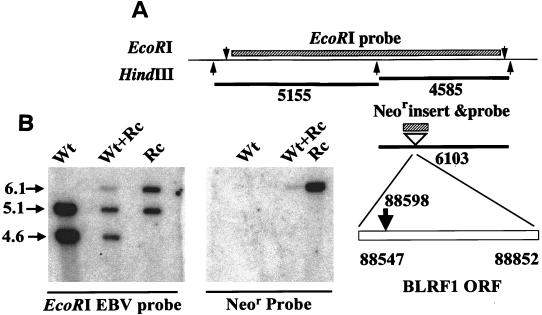
A 10-kb fragment of virus DNA that encompassed the BLRF1 ORF and contained the Neor gene 51 bp from its 5′ end was transfected into Akata cells carrying EBV episomes and cells in which recombination had occurred were obtained by selection in the presence of G418. To determine whether illegitimate or homologous recombination had occurred in each, DNA was extracted, digested with HindIII, and probed with the 8.5-kb EcoRI G fragment that corresponded to the fragment used for transfection except that it lacked the insertion of the Neor gene. This probe was predicted to visualize two fragments of 5,155 and 4,585 bp in cells harboring wild-type EBV episomes and an additional fragment of 6103 bp in cells harboring both wild-type and recombinant episomes (Fig. 1A). Three independently isolated clones obtained from separate transfections were obtained with patterns indicative of homologous recombination in one or more episomes. To derive cells that contained only recombinant episomes in the absence of wild-type episomes, cells from each were induced with anti-immunoglobulin antibody, and virus harvested from the spent culture medium was used to infect EBV-negative Akata cells. Drug-resistant clones that grew out after infection with virus from each parental clone were tested for the ability to be induced to make virus and for the presence of recombinant but not wild-type episomes. One inducible clone from each of two independently isolated parents was selected for further study. Each contained only recombinant episomes, as judged by Southern blotting and probing with the EcoRI fragment. Probing with the XmnI/HincII fragment of pcDNA3 containing the Neor gene confirmed that the resistance gene was inserted at only one site in the extracted DNA (for an example, see Fig. 1B). The phenotype of one recombinant is described in detail below.
FIG. 1.
(A) Diagram of the positions of the EcoRI and HindIII sites, numbered according to the B95-8 sequence, surrounding the EcoRI G fragment of Akata DNA targeted for homologous recombination. The boxes above indicate the position of the EcoRI G fragment used as a probe and the insertion of the Neor gene at bp 88598. Shown below are the sizes of the fragments expected from DNA from cells harboring wild-type episomes, a mixture of wild-type and recombinant episomes, or pure recombinant episomes after digestion with HindIII and probing with the EcoRI G fragment. (B) Southern blot analysis of DNA extracted from Akata cells harboring wild-type episomes (Wt), a parental clone of Akata cells harboring a mixture of wild-type and recombinant episomes (Wt+Rc), or a clone derived from the parental clone that contains only recombinant episomes (Rc). DNA was digested with HindIII, and the two identical halves of the membrane were cut apart and probed either with the EcoRI G fragment of EBV or the XmnI/HincII Neor fragment as indicated. The sizes are indicated in kilobases by arrows.
Lack of expression of gN and mature gM in cells harboring recombinant virus.
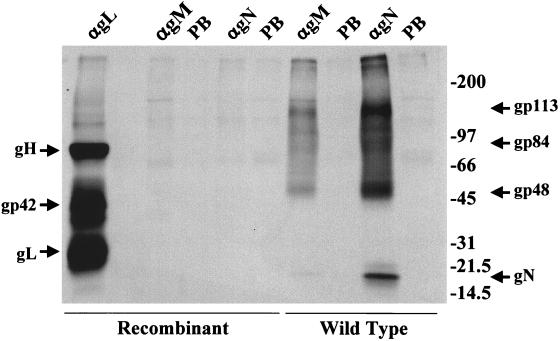
To confirm that expression of gN had been lost as a result of insertion of the Neor gene into the BLRF1 ORF, cells were induced with anti-immunoglobulin antibody, labeled with [3H]glucosamine, and immunoprecipitated with anti-gN, anti-gM, and anti-gL, which immunoprecipitates the three components of the EBV gH-gL complex: gp85, gp42, and gp25. Anti-gN immunoprecipitated the gN-gM complex from cells harboring wild-type episomes and, as previously shown (30), anti-gM immunoprecipitated gM without detectable gN (Fig. 2). As expected, the gH-gL complex, but not gN, was immunoprecipitated from cells harboring only recombinant episomes. More surprising was the finding that no detectable gM was present in these cells either, despite the fact that the sequence of the gM ORF in the recombinant virus (data not shown) was confirmed to be identical to the wild-type Akata virus sequence. The identical phenotype was seen for the second independently isolated recombinant, confirming that it was unlikely to represent a second site mutation. The failure to detect gM might reflect a very rapid turnover of gM in the absence of its partner or a failure of the protein to be glycosylated. It does, however, argue that the virus that lacks gN also lacks mature gM.
FIG. 2.
Electrophoretic analysis in 9 to 18% polyacrylamide of proteins immunoprecipitated from Akata cells harboring wild-type or recombinant episomes. The cells were induced with anti-immunoglobulin antibody and labeled with [3H]glucosamine. Proteins were immunoprecipitated by anti-gL (αgL), which immunoprecipitates the EBV gH-gL complex of gH (85 kDa), gL (25 kDa), and gp42, by anti-gN (αgN), which immunoprecipitates the 15-kDa gN and the three 113-, 84-, and 48-kDa species of gM, by anti-gM (αM), and by preimmune antibody (PB). The sizes are indicated on the right in kilodaltons.
Recombinant virus exits cells less efficiently than does wild-type virus.
To examine whether recombinant virus could exit cells normally, a slot blot assay was used to assess the total amount of virus-specific DNA associated with induced cells and the DNase-resistant, encapsidated DNA that could be pelleted from spent culture medium after it had been filtered through a 0.8-μm-pore-size filter to remove the cells. A comparison of the ratios of the two for recombinant and wild-type viruses showed that, although they varied from induction to induction, cells harboring recombinant virus consistently released less encapsidated virion DNA than did those harboring wild-type virus (Table 1). This contrasts with three other recombinant viruses in which no such consistent reduction was seen (7, 41, 54).
TABLE 1.
Comparison of the relative amounts of virus released from cells making wild-type or recombinant virus
| Expt no. | Extracellular virion DNA as a percentage of intracellular virus DNA at 48 h postinfection
|
|
|---|---|---|
| Wild-type virus | Recombinant virus | |
| 1 | 9.2 | 2.9 |
| 2 | 14.3 | 8.3 |
| 3 | 12.5 | 6.3 |
| 4 | 10.4 | 5.2 |
| Avg | 11.6 | 5.7 |
Recombinant virus associates with electron-dense material in the nucleus.
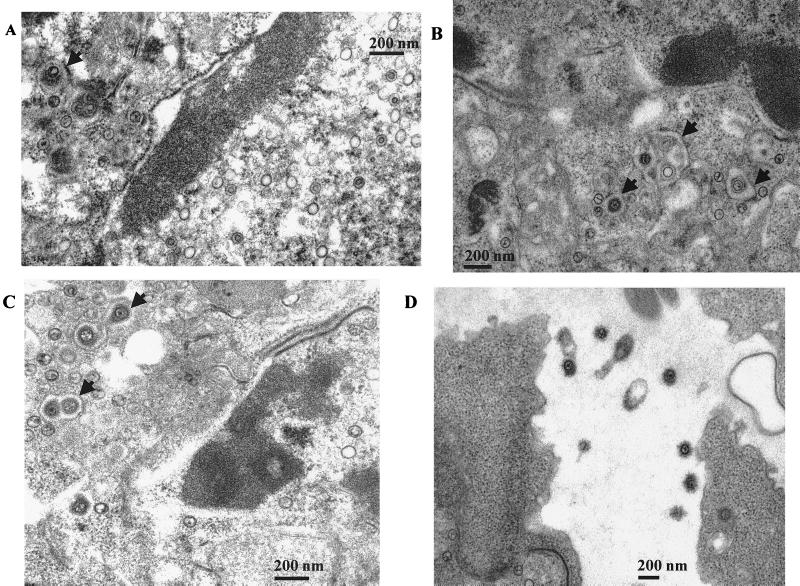
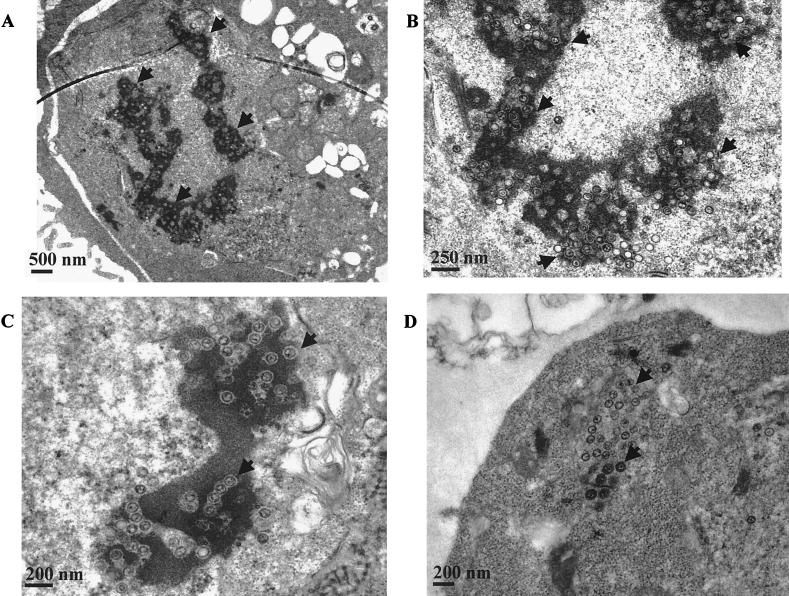
The impairment in egress of recombinant virus stimulated an analysis of virus-producing cells by electron microscopy. Cells making wild-type virus contained the expected condensed and marginated chromatin and capsids in various stages of maturation in the nucleus (Fig. 3). Virus particles could also be seen in vesicles in the cytoplasm, and enveloped virus was visible outside the cell. In contrast, in cells making virus that lacks gN there was a marked accumulation of capsids within the condensed chromatin itself (Fig. 4). Although clearly not all particles were within chromatin, a majority were. Subjectively few viruses were seen in vesicles in the cytoplasm, and the number of cells with extracellular virus was at too low a frequency to detect.
FIG. 3.
Electron micrographs of induced Akata cells producing wild-type virus showing virus in the nucleus (A), in the cytoplasm (B and C), and extracellular virus (D). The arrows indicate enveloped virus and enveloped virus in vesicles in the cytoplasm.
FIG. 4.
Electron micrographs of induced Akata cells producing recombinant virus lacking gN showing virus associated with chromatin in the nucleus (A to C) or in the cytoplasm (D). The arrows in panels A to C indicate virus in the condensed chromatin in the nucleus. The arrows in panel D indicate nonenveloped virus in the cytoplasm.
Recombinant virus binds to B cells with the same specificity as the wild-type virus, but less encapsidated DNA attaches to receptor-positive cells.
To determine whether the recombinant virus that did egress from the cells was able to bind to receptor-positive cells with the same specificity as wild-type virus, virus was labeled intrinsically with [3H]thymidine, and its ability to bind to receptor-negative P3HR1Cl5 cells or receptor-positive Raji cells in the presence or absence of antibody to gp350 was evaluated. The amount of acid-precipitable radioactivity bound to the cells could be reduced by antibody to an amount similar to that associated with receptor-negative cells, indicating that its specificity of binding had not been altered. The binding of [3H]thymidine-labeled recombinant or wild-type virus to receptor-positive and -negative cells was as follows. The acid-precipitable radioactivity (in counts per minute) bound to Raji cells (i.e., receptor-positive cells) was 1,488 cpm in the absence of antibody and 460 cpm in the presence of antibody 72A1. Radioactivity bound to P3HR1Cl5 cells (i.e., receptor-negative cells) was 362 cpm. This is consistent with the behavior of wild-type virus and other recombinant viruses in which gp350 is not disrupted (7, 40, 54). However, the amount of encapsidated virion DNA that bound to receptor-positive cells was significantly reduced for recombinant virus as judged by Gardella gel analysis. The amount of recombinant and wild-type virion DNA in virus stocks was equilibrated, and equal amounts of each were incubated with EBV-negative Akata cells on ice. Excess virus was removed by washing, and the cells were lysed and digested in the wells of a Gardella gel. Linear virion DNA was run into the gel, Southern blotted, and probed. This protocol allowed analysis of far more cells than could be looked at on a cell blot. The signal could be quantitated by scanning, and scans of exposures within the linear range showed that for different virus preparations the amount of recombinant viral DNA that bound to cells was 10- to 30-fold lower than with wild type. A representative experiment is shown in Fig. 5 in which the identical phenotype is shown by the second independently isolated recombinant virus.
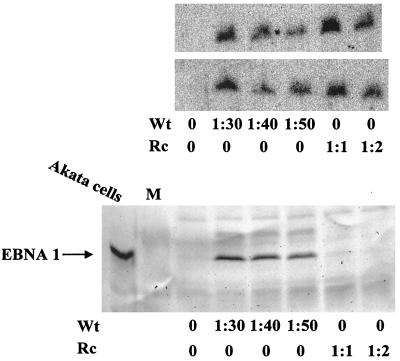
FIG. 5.
Southern blot of Gardella gel analysis of the amount of virion DNA that bound to EBV-negative Akata cells. The amounts of virion DNA in wild-type (Wt) or one of two independently isolated recombinant viruses (Rc1 and Rc2) that were added were equilibrated. Scanning of exposures of Southern blots within the linear range indicated that at least 10-fold more of the wild type than the recombinant virion DNA bound to cells.
That the specificity of virus binding was unaltered suggested either that the recombinant virus preparations contained a large number of empty capsids that competed for binding or that a majority of the virus was damaged in some way. To test the first possibility, stocks of recombinant and wild-type viruses that contained the same amounts of virion DNA were added singly or as mixtures to EBV-negative Akata cells, and the amounts of DNA that bound were again measured by Gardella gel analysis. Scanning of blots at exposures within the linear range indicated that the amount of virion DNA bound in the mixtures was the same as would have been expected from simple addition of each virus (Fig. 6). Thus, although less recombinant virus bound to cells there was no evidence that this was because the recombinant stocks contained empty virions that could compete for binding.
FIG. 6.
Southern blot of Gardella gel analyses of the amounts of wild-type (Wt) and recombinant viruses (Rc) that bound to EBV-negative Akata cells alone or in combination. The starting concentration of each virus stock was equilibrated for virus DNA content. Scanning of the Southern blot at exposures within the linear range indicated that the amount of virion DNA bound in the mixtures of the different viruses was in each case what would have been expected from a simple addition of each virus. The two panels represent duplicate analyses.
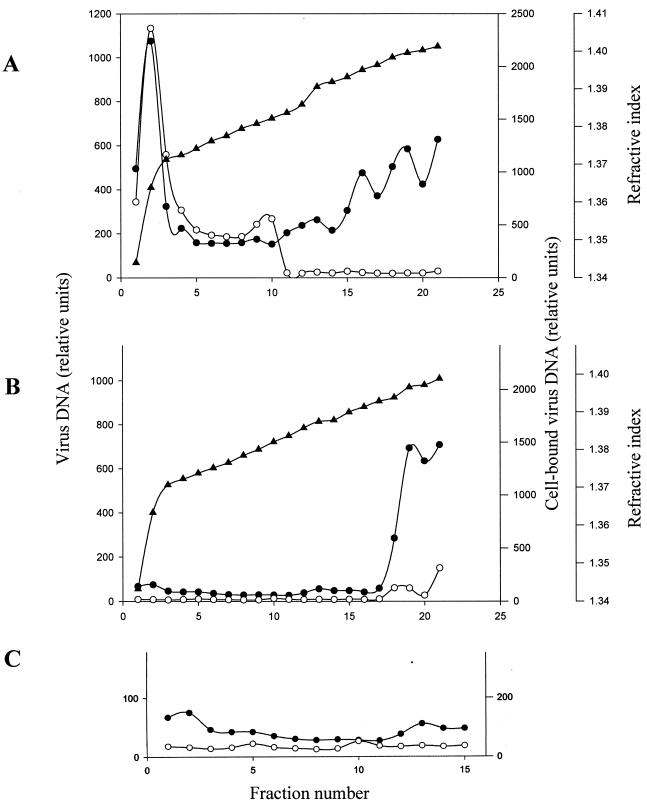
To test the second possibility, i.e., that virus was damaged in some way, equal amounts of recombinant and wild-type viruses, as judged by virion DNA content, were sedimented through a 24 to 42% Nycodenz gradient. Fractions were collected, and aliquots of each were analyzed by slot blot to measure viral DNA content and by Gardella gel analysis to measure their ability to bind to EBV-negative Akata cells. The majority of wild-type virus measured both in terms of DNA content and in terms of ability to bind to cells was found in the first 10 fractions at the top of the gradient (Fig. 7A) at a density consistent with that previously reported (16). In contrast, the majority of recombinant virus, measured by the same criteria, was present in fractions 18 through 21 (Fig. 7B), a finding consistent with previous observations of de-enveloped or partially damaged enveloped virions sedimenting at this higher density (51). Also, a much smaller proportion of the recombinant virus was able to bind to cells, although expansion of the scale of Fig. 7B (Fig. 7C) demonstrates the presence of a small amount of virus sedimenting in fractions 5 and 10 that was able to bind to cells. Both observations were consistent with the release of only a very small amount of completely enveloped recombinant virus. The viability of cells producing recombinant virus was consistently about 50% less than those making wild-type virus at 72 h after induction, even though similar numbers of cells were induced. This suggested that improperly enveloped particles might be being released from the recombinant cells as a result of cell death and lysis.
FIG. 7.
Sedimentation profiles in 24 to 42% Nycodenz of wild-type virus (A) and recombinant virus (B). The scale of the first 15 fractions of panel B are enlarged in panel C. Samples of each fraction were measured by slot blot analysis for the relative amounts of virion DNA and by Gardella gel analysis for the relative amounts of virion DNA that could bind to EBV-negative Akata cells. Equal amounts of virion DNA were loaded for each virus. Variables: ▴, refractive index; ●, virion DNA; ○, virus binding.
Recombinant virus is reduced in infectivity for B cells, but this loss cannot be solely attributed to reduced binding.
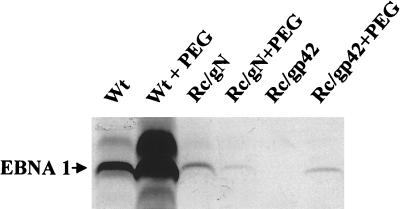
The ability of two independently isolated recombinant viruses to transform resting B cells was reduced equally by 1,000-fold (Table 2), although the binding of virus had been reduced by a factor of <100-fold (Fig. 5). This suggested that recombinant virus was also defective in a step postbinding. To confirm this, the amounts of wild-type and recombinant viruses added to EBV-negative Akata cells were adjusted so that the infectivity of equal amounts of bound virus could be compared. Even when equal or slightly more recombinant virion DNA than wild type was bound to cells, infectivity of the recombinant remained significantly reduced (Fig. 8). To determine whether this represented a block in virus cell fusion, wild-type virus, a recombinant virus that lacks gp42 and is known to be deficient in fusion, and the gN-minus virus were bound to normal B cells. Cells and bound virus were then treated with the exogenous fusogen polyethylene glycol. The infectivity of virus lacking gp42 was restored at a low level, and polyethylene glycol treatment even increased the infectivity of wild-type virus in the experiment shown. Polyethylene glycol can also rescue a virus that is lacking gH (41). In repeated attempts, however, it was never possible to increase the infectivity of the small amount of virus lacking gN that bound to cells (Fig. 9).
TABLE 2.
Comparison of the ability of wild-type and two independently isolated recombinant viruses to transform T-cell-depleted peripheral blood leukocytes
| Virus dilution | No. of transformed wells/total no. of wells infected with:
|
||
|---|---|---|---|
| Recombinant virus 1 | Recombinant virus 2 | Wild-type virus | |
| 1/1 | 2/5 | 1/5 | 5/5 |
| 1/2 | 1/5 | 1/5 | 5/5 |
| 1/4 | 0/5 | 0/5 | 5/5 |
| 1/8 | 0/5 | 0/5 | 5/5 |
| 1/16 | 0/5 | 0/5 | 5/5 |
| 1/32 | 0/5 | 0/5 | 5/5 |
| 1/64 | 0/5 | 0/5 | 5/5 |
| 1/128 | 0/5 | 0/5 | 5/5 |
| 1/256 | NDa | ND | 3/5 |
| 1/512 | ND | ND | 2/5 |
| 1/1,024 | ND | ND | 2/5 |
| 1/2,048 | ND | ND | 2/5 |
| 1/4,096 | ND | ND | 0/5 |
| 1/8,192 | ND | ND | 0/5 |
| None | 0/8 | ||
ND, not done.
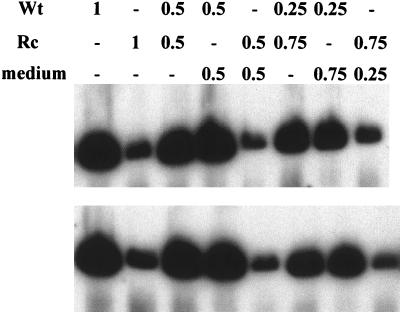
FIG. 8.
Comparison of the infectivity of wild-type (Wt) and recombinant (Rc) viruses after attachment of equal amounts of virion DNA. (Upper panel) Southern blot of Gardella gel analysis, in duplicate, of amounts of virion DNA bound to EBV-negative Akata cells after addition of different dilutions of wild-type and recombinant viruses. (Lower panel) Infectivity of the same dilutions of virus measured by Western blot analysis for the EBV latent protein EBNA1.
FIG. 9.
Western blot analysis for EBNA1 expression in T-cell-depleted human mononuclear cells infected in the absence or presence of polyethylene glycol (PEG) with wild-type Akata virus (Wt), recombinant virus lacking gN (Rc/gN), or recombinant virus lacking gp42 (Rc/gp42).
Complementation of recombinant virus by expression of gN in trans.
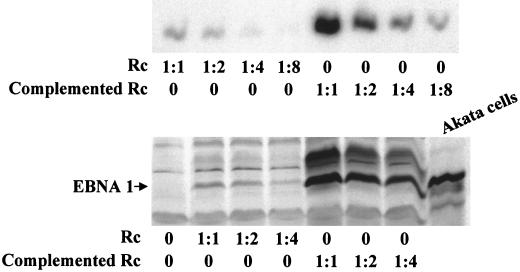
Repeated attempts to establish an Akata cell line that constitutively expressed gN from an integrated plasmid were unsuccessful. To confirm that the phenotype of the virus that lacked gN could be attributed to interruption of the BLRF1 ORF, recombinant virus was rescued either into the gastric carcinoma cell line AGS or into an AGS-derived cell line in which approximately 10% of cells expressed gN from the integrated plasmid pIRES-gN-Puro. Cells that were resistant to treatment with both puromycin and G418 were screened for the ability to be induced to make virus. Virus was obtained from a clone in which approximately 15% of cells could be induced to enter the lytic cycle. Virus from the parental AGS line or from the clone that expressed gN were adjusted for DNA content and examined for the ability to bind to and infect EBV-negative Akata cells. Both binding and infectivity of the partially complemented virus were increased (Fig. 10).
FIG. 10.
Binding and infectivity of equal amounts of recombinant viruses that had been produced from AGS cells (Rc) or from AGS expressing gN from an integrated plasmid (Complemented Rc). (Upper panel) Southern blot of Gardella gel analysis of amounts of dilutions of virion DNA bound to EBV-negative Akata cells. (Lower panel) Infectivity of dilutions of virus measured by Western blot analysis for the EBV latent protein EBNA1.
DISCUSSION
Although glycoproteins gN and gM are highly conserved among the herpesviruses, they have generally been considered to be nonessential proteins whose loss has a relatively small effect on replication in vitro. The results presented here demonstrate a very different phenotype for EBV, in which loss of gN sequences also leads to loss of the mature form of gM. EBV lacking the gN-gM complex is defective in several steps in the normal replication cycle (Fig. 11). Wild-type virus is thought to bud through the inner nuclear membrane and either follow the default exocytic pathway to the cell surface or perhaps undergo a second step of de-envelopment and re-envelopment (21). Enveloped particles that are released attach to a new cell and fuse with the cell membrane, and the capsid moves to the nucleus. Replication of the recombinant virus differed from this model in several respects. First, a significant proportion of the recombinant virus remained associated with condensed chromatin in the nucleus of the virus-producing cell. Although some enveloped virus could be detected in the cytoplasm, the vesicles containing enveloped particles that could be readily seen in cells producing wild-type virus were not found. Second, enveloped virus was not released in sufficient quantity to be visualized at the cell surface. This was despite the fact that only twofold differences were seen in the amount of encapsidated virion DNA released from cells. It was, however, consistent with the sedimentation analysis that indicated that much of the virus that was released lacked a complete envelope and also with the failure of much of the released virus to bind to cells. The rapid decrease in the viability of cells producing recombinant virus suggested that these incompletely enveloped particles might be released by premature cell lysis. Finally, the small amount of enveloped virus that could bind to cells was defective in a step following fusion or penetration that, at least speculatively, might result from impaired movement of virus from the cell surface to the nucleus to establish a new infection.
FIG. 11.
Comparison of replication of wild-type virus and virus lacking the gN-gM complex. (A) Wild-type virus. Wild-type virus buds through the inner nuclear membrane and either follows the default exocytic pathway to the cell surface or undergoes a second step of de-envelopment and re-envelopment. Enveloped particles bind to a new cell and fuse with the cell membrane, and the capsid moves away from the membrane to the nucleus. (B) Virus lacking the gN-gM complex. Many gN-null capsids associate with condensed chromatin. A few appear as enveloped particles in vesicles, and a significant amount of the virus is released without an intact envelope. Virus that remains able to bind to new cells is impaired in infectivity at a step following fusion, perhaps involving movement of capsids away from the cell membrane to the nucleus.
The most obvious potential common denominator in this apparently diverse series of defects is the interaction of a partially or completely tegumented capsid with its envelope. The association of capsids with condensed chromatin is consistent with a failure to associate correctly with the inner nuclear membrane. This in turn would lead to diminished yields of vesicles containing enveloped virus in the cytoplasm. If a de-envelopment and re-envelopment step occurs after exit from the nucleus, the effect might be compounded to produce a significantly reduced yield of infectious extracellular virus. Movement of the capsid away from the membrane of a newly infected cell with which virus has fused requires a dissociation of capsid and envelope. This, intuitively, must involve an alteration in behavior of some of the same proteins as are involved in association and assembly.
gN is a very small protein with a predicted cytoplasmic tail of only 9 amino acids (3). In contrast, gM, which is predicted to be a protein that spans the membrane multiple times, has a potential cytoplasmic tail of 78 amino acids which is highly charged and rich in prolines. The potential for an interaction between tegument proteins would thus appear to be greater for EBV gM than for gN. In this respect the recent findings concerning the role of PRV gM in virion maturation may be pertinent, if not exactly parallel. Although deletion of PRV gM alone had only a relatively small impact on replication (13), the loss of gM, gE, and gI resulted in virus that was impaired in morphogenesis at a stage before envelopment (8). The contribution of gM was suggested to be in directing tegumented capsids to the budding site for secondary envelopment; the loss of the cytoplasmic tail of gE, which contributes to the phenotype, was thought to play a subordinate role in the presence of gM (9). Loss of the PRV gN produced a virus that was impaired in penetration but had no effect on morphogenesis (26). EBV contains no gE or gI homologs and replicates primarily in lymphocytes that do not grow contiguously. The functions that are to some extent shared between gE and gM in PRV may then be entirely carried out by gM in EBV.
The most important caveat to this interpretation is, of course, that the recombinant under study here is not genetically a gM-null virus. In fact, the failure to detect gM was a surprise in that previous expression of gM from a T7 promoter in the absence of gN had produced a protein that was indistinguishable from the wild-type form made in virus-producing cells (30). Since gM is produced at levels that, at least with our present reagents, we can only detect with a glucosamine label, this could mean either that the protein is not fully processed or that it is turned over at a very rapid rate. However, since the levels of expression of gM under control of a T7 promoter are likely to be much higher than from its endogenous viral promoter, we currently favor the latter hypothesis which suggests that the loss of gN, at least in the context of additional virus proteins with which gM might interact, leads to its destabilization. Derivation of a recombinant virus in which the BBRF3 ORF that encodes gM has been disrupted is clearly a priority, as is a search for potential interactive proteins.
Loss of the gN-gM complex, although it reduces virus infectivity considerably, is not lethal. This implies that there is cooperativity or redundancy between the complex and other EBV membrane proteins. gB may be one such protein, since a recombinant virus lacking gB has been shown to assemble neither nucleocapsids nor enveloped particles (31). EBV also expresses a glycoprotein encoded by the BFRF1 gene, which is a homolog of HSV UL34 (14). HSV mutants that fail to express the UL34 protein make DNA containing capsids but little enveloped virus (49). At least three other EBV glycoproteins, (i) gp78, the product of the BILF2 ORF (37), (ii) gp60, the product of the BILF1 ORF (W. J. Kenyon and L. M. Hutt-Fletcher, unpublished data), and (iii) the unknown product of the BMRF2 ORF (46) are all potential players since none has yet been ascribed a function. Derivation of EBV mutants by homologous recombination and selection is extremely labor-intensive and time-consuming. The availability of newer technologies (11), which may make the process much less cumbersome, will hopefully facilitate identification of all the molecules involved in the critical processes of assembly and disassembly of the virion.
ACKNOWLEDGMENTS
This research was supported by Public Health Service grant AI20662 from the National Institute of Allergy and Infectious Diseases.
We thank Brian Hatch of the Electron Microscopy Core of the School of Biological Sciences, University of Missouri-Kansas City, for expert technical assistance.
REFERENCES
- 1.Adams R, Cunningham C, Davison M D, MacLean C A, Davison A J. Characterization of the protein encoded by the gene UL49A of herpes simplex virus type 1. J Gen Virol. 1998;78:813–823. doi: 10.1099/0022-1317-79-4-813. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Siedman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1997. [Google Scholar]
- 3.Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Seguin C, Tuffnell P S, Barrell B G. DNA sequence and expression of the B95–8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 4.Baines J D, Roizman B. The open reading frames UL3, UL4, UL10, and UL16 are dispensible for the replication of herpes simplex virus 1 in cell culture. J Virol. 1991;65:938–944. doi: 10.1128/jvi.65.2.938-944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baines J D, Roizman B. The UL10 gene of herpes simplex virus 1 encodes a novel viral glycoprotein, gM, which is present in the virion and in the plasma membrane of infected cells. J Virol. 1993;67:1441–1452. doi: 10.1128/jvi.67.3.1441-1452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker D E, Roizman B. The unique sequence of herpes simplex virus 1 L component contains an additional translated open reading frame designated UL49.5. J Virol. 1992;66:562–566. doi: 10.1128/jvi.66.1.562-566.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borza C, Hutt-Fletcher L M. Epstein-Barr virus recombinant lacking expression of glycoprotein gp150 infects B cells normally but is enhanced for infection of the epithelial line SVKCR2. J Virol. 1998;72:7577–7582. doi: 10.1128/jvi.72.9.7577-7582.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brack A R, Dijkstra J M, Granzow H, Klupp B G, Mettenleiter T C. Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus. J Virol. 1999;73:5364–5372. doi: 10.1128/jvi.73.7.5364-5372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brack A R, Klupp B G, Granzow H, Tirabassi R, Enquist L W, Mettenleiter T C. Role of the cytoplasmic tail of pseudorabies virus glycoprotein E in virion formation. J Virol. 2000;74:4004–4016. doi: 10.1128/jvi.74.9.4004-4016.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis-Poynter N, Bell S, Minson T, Browne H. Analysis of the contributions of herpes simplex virus type 1 membrane proteins to the induction of cell-cell fusion. J Virol. 1994;68:7586–7590. doi: 10.1128/jvi.68.11.7586-7590.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delecluse H J, Hilsendegen T, Pich D, Zeidler R, Hammerschmidt W. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc Natl Acad Sci USA. 1998;95:8245–8250. doi: 10.1073/pnas.95.14.8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dijkstra J M, Gerdts V, Klupp B G, Mettenleiter T C. Deletion of glycoprotein gM of pseudorabies virus results in attenuation for the natural host. J Gen Virol. 1997;78:2147–2151. doi: 10.1099/0022-1317-78-9-2147. [DOI] [PubMed] [Google Scholar]
- 13.Dijkstra J M, Visser N, Mettenleiter T C, Klupp B G. Identification and characterization of pseudorabies virus glycoprotein gM as a nonessential virion component. J Virol. 1996;70:5684–5688. doi: 10.1128/jvi.70.8.5684-5688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farina A, Santarello R, Gonnella R, Bei R, Muraro R, Cardinali G, Uccini S, Ragona G, Frati L, Faggioni A, Angeloni A. The BFRF1 gene of Epstein-Barr virus encodes a novel protein. J Virol. 2000;74:3235–3244. doi: 10.1128/jvi.74.7.3235-3244.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forghani B, Ni L, Grose C. Neutralization epitope of the varicella-zoster virus gH-gL glycoprotein complex. Virology. 1994;199:458–462. doi: 10.1006/viro.1994.1145. [DOI] [PubMed] [Google Scholar]
- 16.Fowler E, Raab-Traub N, Hester S. Purification of biologically active Epstein-Barr virus by affinity chromatography and non-ionic density gradient centrifugation. J Virol Methods. 1985;11:59–74. doi: 10.1016/0166-0934(85)90125-9. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs W, Mettenleiter T C. DNA sequence of the UL6 to UL20 genes of infectious laryngotracheitis virus and characterization of the UL10 gene product as a non-glycosylated and nonessential virion protein. J Gen Virol. 1999;80:2173–2182. doi: 10.1099/0022-1317-80-8-2173. [DOI] [PubMed] [Google Scholar]
- 18.Fuller A O, Santos R E, Spear P G. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989;63:3435–3443. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardella T, Medveczky P, Sairenji T, Mulder C. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J Virol. 1984;50:248–254. doi: 10.1128/jvi.50.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gompels U A, Minson A. The properties and sequence of glycoprotein H of herpes simplex type 1. Virology. 1986;153:230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- 21.Gong M, Kieff E. Intracellular trafficking of two major Epstein-Barr virus glycoproteins, gp350/220 and gp110. J Virol. 1990;64:1507–1516. doi: 10.1128/jvi.64.4.1507-1516.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heston L, Rabson M, Brown N, Miller G. New Epstein-Barr virus variants from cellular subclones of P3J-HR-1 Burkitt lymphoma. Nature. 1982;295:160–163. doi: 10.1038/295160a0. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman G J, Lazarowitz S G, Hayward S D. Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antigen. Proc Natl Acad Sci USA. 1980;77:2979–2983. doi: 10.1073/pnas.77.5.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber M T, Compton T. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J Virol. 1998;72:8191–8197. doi: 10.1128/jvi.72.10.8191-8197.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurley E A, Thorley-Lawson D A. B cell activation and the establishment of Epstein-Barr virus latency. J Exp Med. 1988;168:2059–2075. doi: 10.1084/jem.168.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jöns A, Dijkstra J M, Mettenleiter T C. Glycoproteins M and N of pseudorabies virus form a disulfide-linked complex. J Virol. 1998;72:550–557. doi: 10.1128/jvi.72.1.550-557.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jöns A, Granzlow H, Kuchling R, Mettenleiter T C. The UL49.5 gene of pseudorabies virus codes for an O-glycosylated structural protein of the virus envelope. J Virol. 1996;70:1237–1241. doi: 10.1128/jvi.70.2.1237-1241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kari B, Li W, Cooper R, Goertz R, Radeke B. The human cytomegalovirus UL100 gene encodes the gC-II glycoproteins recognized by group 2 monoclonal antibodies. J Gen Virol. 1994;75:3081–3086. doi: 10.1099/0022-1317-75-11-3081. [DOI] [PubMed] [Google Scholar]
- 29.Keller P M, Davison A J, Lowe R S, Riemen M W, Ellis R W. Identification and sequence of the gene encoding gpIII, a major glycoprotein of varicella-zoster virus. Virology. 1987;157:526–533. doi: 10.1016/0042-6822(87)90295-9. [DOI] [PubMed] [Google Scholar]
- 30.Lake C M, Molesworth S J, Hutt-Fletcher L M. The Epstein-Barr virus (EBV) gN homolog BLRF1 encodes a 15 kilodalton glycoprotein that cannot be authentically processed unless it is co-expressed with the EBV gM homolog BBRF3. J Virol. 1998;72:5559–5564. doi: 10.1128/jvi.72.7.5559-5564.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S K, Longnecker R. The Epstein-Barr virus glycoprotein 110 carboxy-terminal tail domain is essential for lytic virus replication. J Virol. 1997;71:4092–4097. doi: 10.1128/jvi.71.5.4092-4097.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehner R, Meyer H, Mach M. Identification and characterization of a human cytomegalovirus gene coding for a membrane protein that is conserved among human herpesviruses. J Virol. 1989;63:3792–3800. doi: 10.1128/jvi.63.9.3792-3800.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q X, Spriggs M K, Kovats S, Turk S M, Comeau M R, Nepom B, Hutt-Fletcher L M. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J Virol. 1997;71:4657–4662. doi: 10.1128/jvi.71.6.4657-4662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q X, Turk S M, Hutt-Fletcher L M. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J Virol. 1995;69:3987–3994. doi: 10.1128/jvi.69.7.3987-3994.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang X, Chow B, Raggo C, Babiuk L A. Bovine herpesvirus 1 UL49.5 homolog gene encodes a novel viral envelope protein that forms a disulfide-linked complex with a second virion structural protein. J Virol. 1996;70:1448–1454. doi: 10.1128/jvi.70.3.1448-1454.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu D X, Gompels U A, Foa-Tomasi L, Campadelli-Fiumi G. Human herpesvirus 6 glycoprotein H and L homologues are components of the gp100 complex and the gH external domain is the target for neutralizing monoclonal antibodies. Virology. 1993;197:12–22. doi: 10.1006/viro.1993.1562. [DOI] [PubMed] [Google Scholar]
- 37.Mackett M, Conway M J, Arrand J R, Haddad R S, Hutt-Fletcher L M. Characterization and expression of a glycoprotein encoded by the Epstein-Barr virus BamHI 1 fragment. J Virol. 1990;64:2545–2552. doi: 10.1128/jvi.64.6.2545-2552.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacLean C A, Efstathiou S, Elliott M L, Jamieson F E, McGeoch D J. Investigation of herpes simplex virus type 1 genes encoding multiply inserted membrane proteins. J Gen Virol. 1991;72:897–906. doi: 10.1099/0022-1317-72-4-897. [DOI] [PubMed] [Google Scholar]
- 39.MacLean C A, Robertson L M, Jamieson F E. Characterization of the UL10 gene product of herpes simplex virus type 1 and investigations of its role in vivo. J Gen Virol. 1993;74:975–983. doi: 10.1099/0022-1317-74-6-975. [DOI] [PubMed] [Google Scholar]
- 40.Miller N, Hutt-Fletcher L M. A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J Virol. 1988;62:2366–2372. doi: 10.1128/jvi.62.7.2366-2372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molesworth S J, Lake C M, Borza C M, Turk S M, Hutt-Fletcher L M. Epstein-Barr virus gH is essential for penetration of B cell but also plays a role in attachment of virus to epithelial cells. J Virol. 2000;74:6324–6332. doi: 10.1128/jvi.74.14.6324-6332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neubauer A, Beer M, Brandmuller C, R. K O, Osterrieder N. Equine herpesvirus 1 mutants devoid of glycoprotein B or M are apathogenic for mice but induce protection against challenge infection. Virology. 1997;239:36–45. doi: 10.1006/viro.1997.8857. [DOI] [PubMed] [Google Scholar]
- 43.Osterreider N, Neubauer A, Brandmuller C, Braun B, Kaadden O-R, Baines J. The equine herpesvirus type 1 glycoprotein gp21/22a, the herpes simplex virus type 1 gM homolog, is involved in virus penetration and cell-to cell spread of virions. J Virol. 1996;70:4110–4115. doi: 10.1128/jvi.70.6.4110-4115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osterrieder N, Neubauer A, Fakler B, Brandmuller C, Kaaden O R, Baines J D. Synthesis and processing of the equine herpesvirus 1 glycoprotein M. Virology. 1997;232:230–239. doi: 10.1006/viro.1997.8561. [DOI] [PubMed] [Google Scholar]
- 45.Peeters B, Dewind N, Broer R, Gielkins A, Moormann R. Glycoprotein H of pseudorabies virus is essential for entry and cell-to-cell spread of the virus. J Virol. 1992;66:3888–3892. doi: 10.1128/jvi.66.6.3888-3892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penaranda M E, Lagenaur L A, Pierek L T, Berline J W, MacPhail L A, Greenspan D, Greenspan J, Palefsky J M. Expression of Epstein-Barr virus BMRF-2 and BDLF-3 genes in hairy leukoplakia. J Gen Virol. 1997;78:3361–3370. doi: 10.1099/0022-1317-78-12-3361. [DOI] [PubMed] [Google Scholar]
- 47.Pulvertaft R J V. Cytology of Burkitt's tumor (African lymphoma) Lancet. 1964;i:238–240. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- 48.Pyles R B, Sawtell N M, Thompson R L. Herpes simplex virus type 1 dUTPase mutants are attenuated for neurovirulence, neuroinvasiveness, and reactivation from latency. J Virol. 1992;66:6706–6713. doi: 10.1128/jvi.66.11.6706-6713.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roller R J, Zhou Y, Schnetzer R, Ferguson J, DeSalvo D. Herpes simplex virus type 1 UL34 gene product is required for viral envelopment. J Virol. 2000;74:117–129. doi: 10.1128/jvi.74.1.117-129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ross J, Williams M, Cohen J I. Disruption of the varicella-zoster virus dUTPase and the adjacent ORF9A gene results in impaired growth and reduced syncytia formation in vitro. Virology. 1997;234:186–195. doi: 10.1006/viro.1997.8652. [DOI] [PubMed] [Google Scholar]
- 51.Sathananthan B, Rodahl E, Flatmark T, Langeland N, Haarr L. Purification of herpes simplex virus type 1 by density gradient centrifugation and estimation of the sedimentation coefficient of the virion. APMIS. 1997;105:238–246. doi: 10.1111/j.1699-0463.1997.tb00564.x. [DOI] [PubMed] [Google Scholar]
- 52.Takada K. Cross-linking of cell surface immunoglobulin induces Epstein-Barr virus in Burkitt lymphoma lines. Int J Cancer. 1984;33:27–32. doi: 10.1002/ijc.2910330106. [DOI] [PubMed] [Google Scholar]
- 53.Takada K, Horinouchi K, Ono Y, Aya T, Osato T, Takahashi M, Hayasaka S. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes. 1991;5:147–156. doi: 10.1007/BF00571929. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Hutt-Fletcher L M. Epstein-Barr virus lacking glycoprotein gp42 can bind to B cells but is not able to infect. J Virol. 1998;72:158–163. doi: 10.1128/jvi.72.1.158-163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, Kenyon W J, Li Q X, Mullberg J, Hutt-Fletcher L M. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J Virol. 1998;72:5552–5558. doi: 10.1128/jvi.72.7.5552-5558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu S X, Zhu X P, Letchworth G J. Bovine herpesvirus 1 glycoprotein M forms a disulfide-linked heterodimer with the UL49.5 protein. J Virol. 1998;72:3029–3036. doi: 10.1128/jvi.72.4.3029-3036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yaswen L R, Stephens E B, Davenport L C, Hutt-Fletcher L M. Epstein-Barr virus glycoprotein gp85 associates with the BKRF2 gene product and is incompletely processed as a recombinant protein. Virology. 1993;195:387–396. doi: 10.1006/viro.1993.1388. [DOI] [PubMed] [Google Scholar]