Abstract
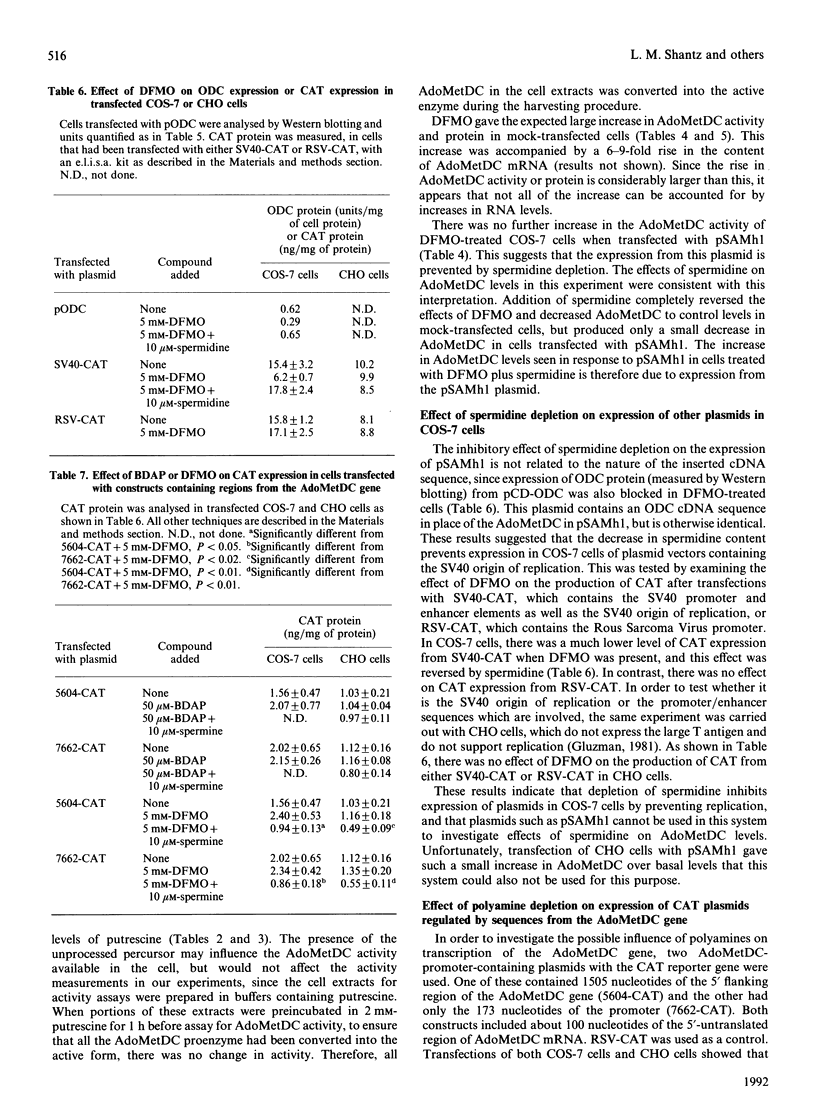
The effects of addition of exogenous spermidine and spermine and of two inhibitors of polyamine biosynthesis, alpha-difluoromethylornithine (DFMO), which decreases spermidine concentrations, and n-butyl-1,3-diaminopropane, which depletes spermine, on the expression of S-adenosylmethionine decarboxylase (AdoMetDC) activity were studied in mammalian cell lines (HT29, CHO and COS-7). AdoMetDC levels were inversely related to the polyamine content, and spermine was the more potent repressor of AdoMetDC activity, but only spermidine affected the amount of AdoMetDC mRNA. Transfection of COS-7 cells or CHO cells with plasmid constructs containing a chloramphenicol acetyltransferase (CAT) reporter gene driven by portions of the AdoMetDC promoter region indicated that CAT expression was altered by spermidine, but not by spermine, suggesting that there is a spermidine-responsive element in this promoter. Transient transfection of COS-7 cells with pSAMh1, a plasmid containing the AdoMetDC cDNA in a vector with the SV40 promoter and origin of replication, led to a large increase in AdoMetDC expression. Although treatment of COS-7 cells with n-butyl-1,3-diaminopropane greatly increased endogenous AdoMetDC activity, the spermine depletion brought about by this inhibitor did not stimulate AdoMetDC expression from pSAMh1. The pSAMh1 cDNA is missing 72 nucleotides from the 5' end of the AdoMetDC mRNA, and it is possible that translational regulation by spermine involves this region. The expression of AdoMetDC from pSAMh1 in COS-7 cells was greatly inhibited by DFMO treatment, although endogenous AdoMetDC activity was increased. The expression of other plasmids containing the SV40 origin of replication was also inhibited by DFMO in COS-7 cells, but not in CHO cells. DFMO treatment did not interfere with the expression of plasmids driven by the RSV promoter. These results suggest that low spermidine levels interfere with the replication of plasmids containing the SV40 origin of replication.
Full text
PDF

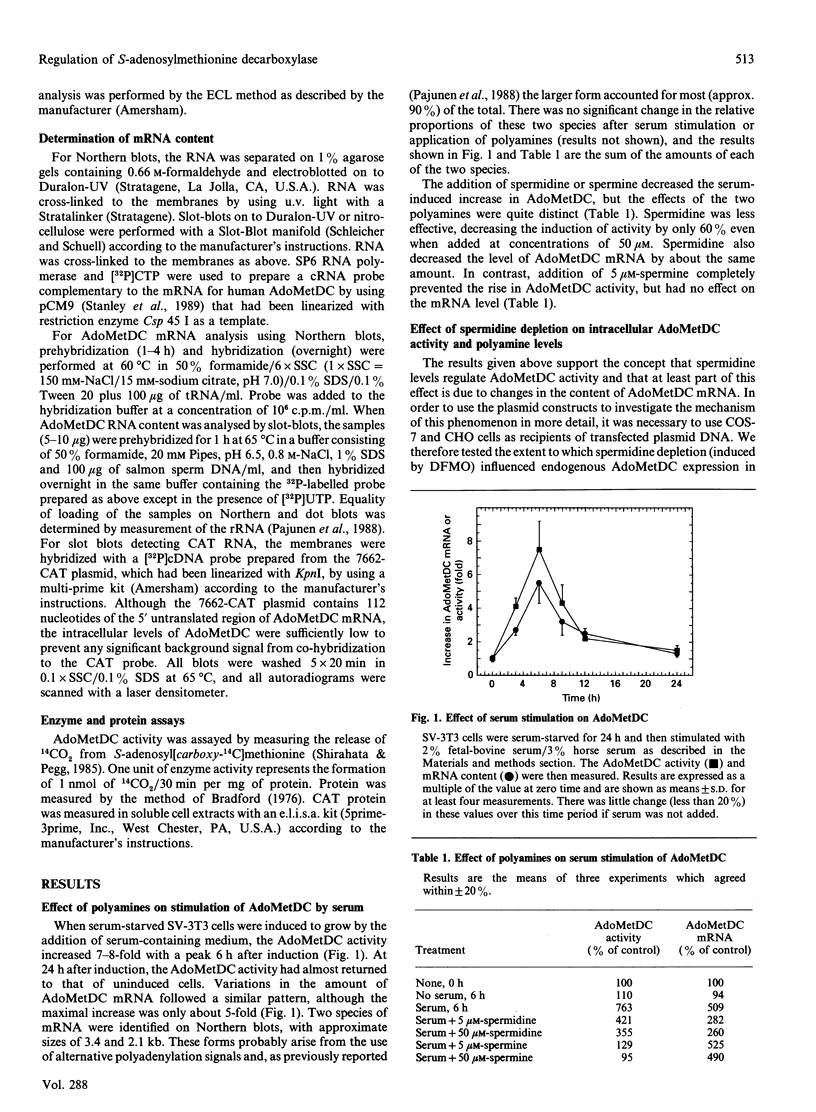
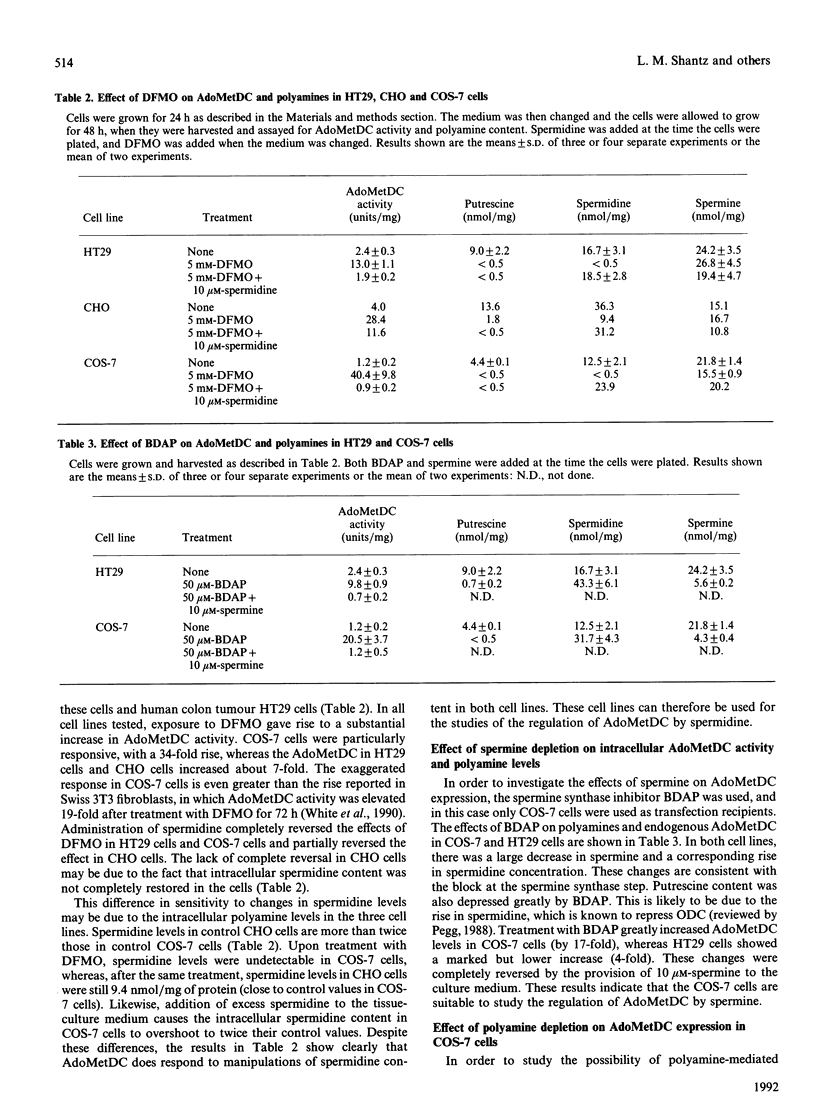
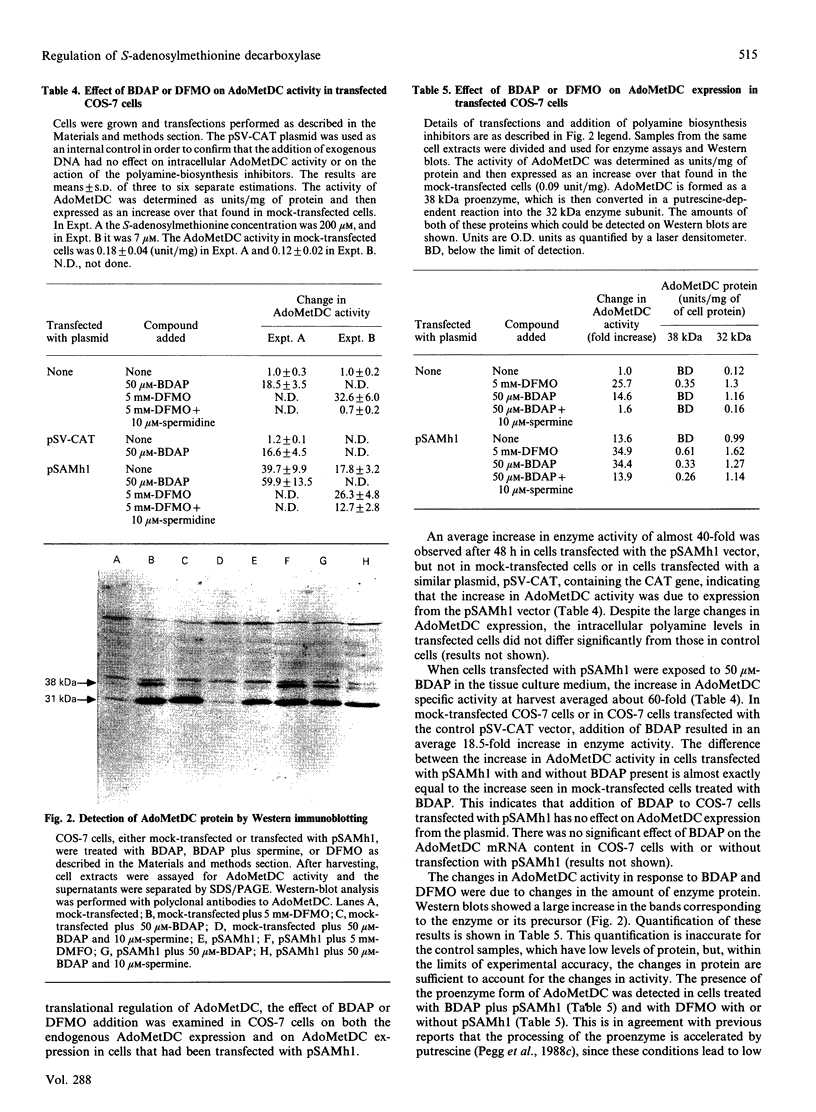


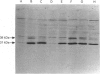
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autelli R., Stjernborg L., Khomutov A. R., Khomutov R. M., Persson L. Regulation of S-adenosylmethionine decarboxylase in L1210 leukemia cells. Studies using an irreversible inhibitor of the enzyme. Eur J Biochem. 1991 Mar 28;196(3):551–556. doi: 10.1111/j.1432-1033.1991.tb15849.x. [DOI] [PubMed] [Google Scholar]
- Baillon J. G., Kolb M., Mamont P. S. Inhibition of mammalian spermine synthase by N-alkylated-1,3-diaminopropane derivatives in vitro and in cultured rat hepatoma cells. Eur J Biochem. 1989 Jan 15;179(1):17–21. doi: 10.1111/j.1432-1033.1989.tb14515.x. [DOI] [PubMed] [Google Scholar]
- Bergeron R. J., Neims A. H., McManis J. S., Hawthorne T. R., Vinson J. R., Bortell R., Ingeno M. J. Synthetic polyamine analogues as antineoplastics. J Med Chem. 1988 Jun;31(6):1183–1190. doi: 10.1021/jm00401a019. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Holm I., Persson L., Pegg A. E., Heby O. Effects of S-adenosyl-1,8-diamino-3-thio-octane and S-methyl-5'-methylthioadenosine on polyamine synthesis in Ehrlich ascites-tumour cells. Biochem J. 1989 Jul 1;261(1):205–210. doi: 10.1042/bj2610205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne J., Alhonen L., Leinonen P. Polyamines: from molecular biology to clinical applications. Ann Med. 1991 Aug;23(3):241–259. doi: 10.3109/07853899109148056. [DOI] [PubMed] [Google Scholar]
- Kameji T., Pegg A. E. Inhibition of translation of mRNAs for ornithine decarboxylase and S-adenosylmethionine decarboxylase by polyamines. J Biol Chem. 1987 Feb 25;262(6):2427–2430. [PubMed] [Google Scholar]
- Kolb M., Danzin C., Barth J., Claverie N. Synthesis and biochemical properties of chemically stable product analogues of the reaction catalyzed by S-adenosyl-L-methionine decarboxylase. J Med Chem. 1982 May;25(5):550–556. doi: 10.1021/jm00347a014. [DOI] [PubMed] [Google Scholar]
- Kramer D. L., Khomutov R. M., Bukin Y. V., Khomutov A. R., Porter C. W. Cellular characterization of a new irreversible inhibitor of S-adenosylmethionine decarboxylase and its use in determining the relative abilities of individual polyamines to sustain growth and viability of L1210 cells. Biochem J. 1989 Apr 15;259(2):325–331. doi: 10.1042/bj2590325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Madhubala R., Secrist J. A., 3rd, Pegg A. E. Effect of inhibitors of S-adenosylmethionine decarboxylase on the contents of ornithine decarboxylase and S-adenosylmethionine decarboxylase in L1210 cells. Biochem J. 1988 Aug 15;254(1):45–50. doi: 10.1042/bj2540045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. M. Polyamines. Essays Biochem. 1987;23:82–115. [PubMed] [Google Scholar]
- Pajunen A., Crozat A., Jänne O. A., Ihalainen R., Laitinen P. H., Stanley B., Madhubala R., Pegg A. E. Structure and regulation of mammalian S-adenosylmethionine decarboxylase. J Biol Chem. 1988 Nov 15;263(32):17040–17049. [PubMed] [Google Scholar]
- Palvimo J. J., Eisenberg L. M., Jänne O. A. Protein-DNA interactions in the cAMP responsive promoter region of the murine ornithine decarboxylase gene. Nucleic Acids Res. 1991 Jul 25;19(14):3921–3927. doi: 10.1093/nar/19.14.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Jones D. B., Secrist J. A., 3rd Effect of inhibitors of S-adenosylmethionine decarboxylase on polyamine content and growth of L1210 cells. Biochemistry. 1988 Mar 8;27(5):1408–1415. doi: 10.1021/bi00405a003. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Kameji T., Shirahata A., Stanley B., Madhubala R., Pajunen A. Regulation of mammalian S-adenosylmethionine decarboxylase. Adv Enzyme Regul. 1988;27:43–55. doi: 10.1016/0065-2571(88)90008-8. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988 Feb 15;48(4):759–774. [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. S-adenosylmethionine decarboxylase: a brief review. Cell Biochem Funct. 1984 Jan;2(1):11–15. doi: 10.1002/cbf.290020105. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Wechter R., Pajunen A. Increase in S-adenosylmethionine decarboxylase in SV-3T3 cells treated with S-methyl-5'-methylthioadenosine. Biochem J. 1987 May 15;244(1):49–54. doi: 10.1042/bj2440049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Wechter R., Poulin R., Woster P. M., Coward J. K. Effect of S-adenosyl-1,12-diamino-3-thio-9-azadodecane, a multisubstrate adduct inhibitor of spermine synthase, on polyamine metabolism in mammalian cells. Biochemistry. 1989 Oct 17;28(21):8446–8453. doi: 10.1021/bi00447a026. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Wiest L., Pajunen A. Detection of proenzyme form of S-adenosylmethionine decarboxylase in extracts from rat prostate. Biochem Biophys Res Commun. 1988 Jan 29;150(2):788–793. doi: 10.1016/0006-291x(88)90460-3. [DOI] [PubMed] [Google Scholar]
- Persson L., Khomutov A. R., Khomutov R. M. Feedback regulation of S-adenosylmethionine decarboxylase synthesis. Biochem J. 1989 Feb 1;257(3):929–931. doi: 10.1042/bj2570929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson L., Stjernborg L., Holm I., Heby O. Polyamine-mediated control of mammalian S-adenosyl-L-methionine decarboxylase expression: effects on the content and translational efficiency of the mRNA. Biochem Biophys Res Commun. 1989 May 15;160(3):1196–1202. doi: 10.1016/s0006-291x(89)80130-5. [DOI] [PubMed] [Google Scholar]
- Porter C. W., Bergeron R. J. Enzyme regulation as an approach to interference with polyamine biosynthesis--an alternative to enzyme inhibition. Adv Enzyme Regul. 1988;27:57–79. doi: 10.1016/0065-2571(88)90009-x. [DOI] [PubMed] [Google Scholar]
- Porter C. W., Pegg A. E., Ganis B., Madhabala R., Bergeron R. J. Combined regulation of ornithine and S-adenosylmethionine decarboxylases by spermine and the spermine analogue N1 N12-bis(ethyl)spermine. Biochem J. 1990 May 15;268(1):207–212. doi: 10.1042/bj2680207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkka A., Ihalainen R., Aatsinki J., Pajunen A. Structure and organization of the gene encoding rat S-adenosylmethionine decarboxylase. FEBS Lett. 1991 Oct 21;291(2):289–295. doi: 10.1016/0014-5793(91)81304-q. [DOI] [PubMed] [Google Scholar]
- Shirahata A., Pegg A. E. Increased content of mRNA for a precursor of S-adenosylmethionine decarboxylase in rat prostate after treatment with 2-difluoromethylornithine. J Biol Chem. 1986 Oct 15;261(29):13833–13837. [PubMed] [Google Scholar]
- Shirahata A., Pegg A. E. Regulation of S-adenosylmethionine decarboxylase activity in rat liver and prostate. J Biol Chem. 1985 Aug 15;260(17):9583–9588. [PubMed] [Google Scholar]
- Stanley B. A., Pegg A. E., Holm I. Site of pyruvate formation and processing of mammalian S-adenosylmethionine decarboxylase proenzyme. J Biol Chem. 1989 Dec 15;264(35):21073–21079. [PubMed] [Google Scholar]
- White M. W., Degnin C., Hill J., Morris D. R. Specific regulation by endogenous polyamines of translational initiation of S-adenosylmethionine decarboxylase mRNA in Swiss 3T3 fibroblasts. Biochem J. 1990 Jun 15;268(3):657–660. doi: 10.1042/bj2680657. [DOI] [PMC free article] [PubMed] [Google Scholar]