Abstract
Purpose
To describe the use of an amniotic membrane graft (AMG) with fibrin sealant to address an overfiltering trabeculectomy flap encountered intraoperatively.
Observations
A 35-year-old female with severe primary open angle glaucoma underwent trabeculectomy with mitomycin C due to uncontrolled intraocular pressure (IOP). Intraoperatively, the elastic nature of the scleral flap led to overfiltration, causing persistent anterior chamber shallowing despite numerous sutures. To decrease but not completely shut down aqueous outflow through the trabeculectomy flap, we utilized AMG and fibrin sealant to stabilize the flap. Postoperatively, the patient had a formed anterior chamber, elevated bleb and significantly reduced IOP, without the need for additional glaucoma medications.
Conclusions and importance
Amniotic membrane grafts (AMG) with fibrin sealant may help regulate aqueous flow efflux, maintain anterior chamber stability, and mitigate the risk of postoperative hypotony in trabeculectomy surgery. AMG was chosen in this setting given its anti-inflammatory, anti-fibrotic properties, as well as its optically clear nature to allow for post-operative visualization of the flap. AMG allows for early postoperative stabilization of the scleral flap without complete obstruction, and may be useful in patients at risk of early postoperative hypotony.
Keywords: Amniotic membrane graft, Overfiltering bleb, Trabeculectomy, Hypotony, Scleral flap, Fibrin glue
Highlights
-
•
Amniotic membrane graft with fibrin sealant can regulate aqueous efflux through an overfiltering trabeculectomy flap.
-
•
Amniotic membrane graft can mitigate risk for early postoperative hypotony without causing long term flap obstruction.
-
•
Optically clear amniotic membrane graft allows for visualization during post-operative procedures.
1. Introduction
Glaucoma, a leading cause of irreversible blindness worldwide, is characterized by progressive optic nerve damage often associated with elevated intraocular pressure (IOP).1 IOP is the major risk factor for glaucoma progression, and IOP reduction is currently the primary objective of medical and surgical treatments for glaucoma.2 First introduced by John Cairns in 1968, trabeculectomy remains the gold standard surgical intervention for reducing IOP in patients with advanced glaucoma.3,4 Especially in patients requiring a low IOP goal or in low-resource healthcare settings, trabeculectomy is preferred given the ability to achieve a durable, low IOP without implants.
Trabeculectomy surgery involves dissecting through the conjunctiva and Tenon's capsule to the sclera, and subsequently creating a fistula to the anterior chamber that is regulated by a partial thickness scleral flap that allows for controlled efflux of aqueous humor.3,5 Intraoperatively, trabeculectomy can rarely be complicated by persistent over-filtration, particularly in high myopes or young patients with more elastic sclera. In instances where additional scleral flap sutures are inadequate to control aqueous flow and maintain anterior chamber formation, surgical options remain limited. Methods to address postoperative hypotony and leaks have been well documented,6, 7, 8 but intraoperative strategies are less well described. In this report, we describe the use of an amniotic membrane graft (AMG) to address an overfiltering trabeculectomy flap intraoperatively and avoid the need to permanently shut down flow using a scleral or corneal patch graft. The AMG with fibrin sealant provided support to the scleral flap and reduced overfiltration in the early postoperative period, without causing long-term obstruction of the flap.
2. Case report
A 35-year-old African American female was referred by an outside provider for consideration for glaucoma surgical intervention. She had severe juvenile open angle glaucoma (JOAG) in the left eye with an IOP in the low 40s on four classes of topical glaucoma medications and oral acetazolamide. Her fellow eye was labeled as a glaucoma suspect without any notable visual field loss. Her presenting visual acuity (VA) was 20/40, with a notable afferent pupillary defect. She was myopic with a refraction of −1.25 D in the left eye. Her Humphrey visual field 24-2 showed a dense superior and inferior arcuate defect, split fixation, with a mean deviation of −18.37. Her OCT nerve fiber layer showed clear thinning in the left eye, but the scans were also confounded by myopia. She had no prior ocular surgeries. Due to her persistently elevated IOP on maximally tolerated glaucoma medications, the patient was scheduled for trabeculectomy with mitomycin C of the left eye.
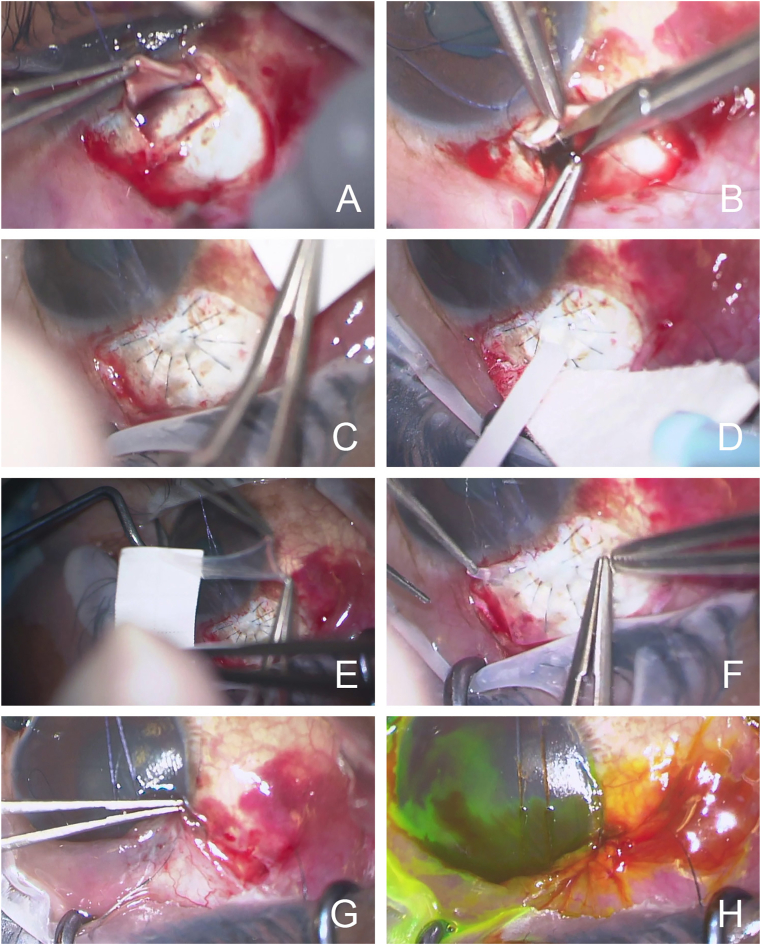
Intraoperatively, the dissection through the conjunctiva and Tenon's capsule was performed in a fornix-based fashion. Upon creation of the partial thickness scleral flap, the scleral was noted to be particularly elastic (Fig. 1A). The scleral flap was approximately 3.0 mm by 4.5 mm in size and rectangular in shape. A Kelly punch was used to create the sclerostomy, and a surgical iridectomy was created (Fig. 1B). Despite the placement of multiple 10-0 nylon sutures to create better apposition of the flap edges, the scleral flap continued to over-filter with persistent anterior chamber shallowing (Fig. 1C). Eventually, a total of ten sutures were placed. To avoid permanent shut down of aqueous flow using a scleral patch graft, the decision was made to buttress the scleral flap with an AMG and fibrin sealant. The two components of the fibrin sealant were combined on top of the scleral flap - “protein solution” (human fibrinogen + aprotinin) and “thrombin solution” (human thrombin in CaCl2) (Fig. 1D). The AMG was sized to cover the scleral window and the adjacent sutures, approximately 4.5 mm by 6.0 mm in a rectangular shape. The AMG was peeled carefully from the nitrocellulose support paper and placed mesenchymal side down, epithelium side up (Fig. 1E and F). Notably the anterior chamber stopped shallowing and remained formed after this step. Care was taken to free the edges of the AMG from the edges of the conjunctiva and the conjunctiva was closed (Fig. 1G). A small amount of viscoelastic (Healon Pro) was left in the anterior chamber. The conjunctiva was reapproximated to the limbus using a 10-0 nylon in a combined double-winged suture and running purse string suture fashion. The closure was noted to be Seidel negative at the conclusion of the case (Fig. 1H).
Fig. 1.
Intraoperative images of the use of an amniotic membrane graft (AMG) to bolster an overfiltering trabeculectomy flap. A) A partial thickness scleral flap was created. B) A Kelly punch was used to create a sclerostomy followed by a surgical iridectomy. C) Despite multiple 10-0 nylon sutures, the anterior chamber remain shallow with persistent aqueous efflux through the scleral flap. D) The two components of the fibrin sealant were combined on top of the scleral flap (fibrinogen and thrombin). E) The AMG was peeled carefully from the nitrocellulose support paper F) The AMG was placed over the scleral flap (mesenchymal side down, epithelium side up). G) The conjunctiva was closed over the AMG. H) At the end of the case, the closure was Seidel negative.
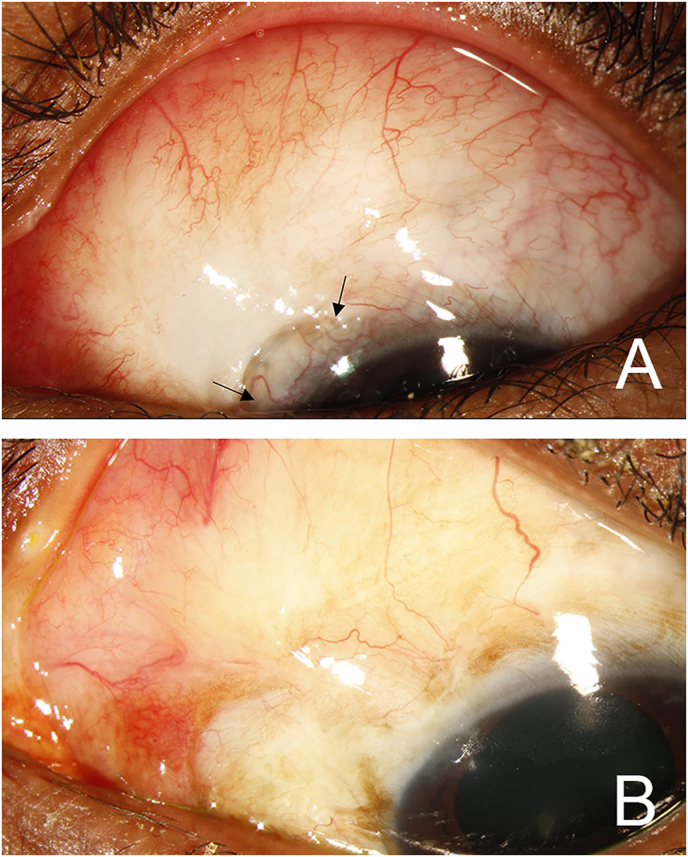
On postoperative day one, the patient's VA was 20/150, IOP was 3 mmHg with a Seidel negative, raised bleb. She had a formed anterior chamber and no choroidal effusions. She was started on prednisolone acetate four times per day and atropine two times per day. All her IOP-lowering medications were stopped. OCT of the macula did not show significant macular folds or choroidal undulations during the first postoperative week. Throughout the first postoperative month, the patient maintained a formed anterior chamber despite a slow bleb leak at the nasal limbus appearing at post-operative week one which resolved following the application of a bandage contact lens. At postoperative month one, VA was 20/70 and IOP was 4 mmHg with a formed anterior chamber (Fig. 2A). At postoperative month two, VA was 20/60, IOP was 10 mmHg with a deep anterior chamber. She was maintained on prednisolone acetate four times per day and remained off IOP-lowering medications. She was then lost to follow up for four months. At postoperative month six, VA was 20/70, and IOP was 14 mmHg. She was then lost again to follow up with unclear medication usage in the interim. At postoperative month ten, the patient's VA was 20/60 and IOP was 14 mmHg with an elevated bleb (Fig. 2B).
Fig. 2.
External slit lamp image of the bleb at postoperative month one (A) and postoperative month ten (B). (A) At postoperative month one, the patient had an elevated bleb, formed anterior chamber and Seidel negative conjunctival closure. Flap sutures (arrows) were visible under the conjunctiva. (B) At postoperative month ten, the bleb was diffusely elevated.
3. Discussion
Trabeculectomy, a cornerstone surgical technique for managing glaucoma, provides significant and durable reduction in intraocular pressure (IOP).3 Despite the benefits of trabeculectomy, an over-filtering trabeculectomy flap can be challenging to manage, and poses a risk for vision loss due to hypotony-associated complications such as choroidal effusions or maculopathy. Our patient's young age and associated elastic sclera contributed to difficulties in controlling aqueous outflow through the ostomy and achieving a stable anterior chamber intraoperatively.9 Our case report demonstrates the successful use of AMG and fibrin sealant to bolster the scleral flap to decrease aqueous flow efflux and stabilize the anterior chamber without long term obstruction of the flap.
AMGs were first utilized for skin transplantation in 1910 by John David, and are used in a variety of ophthalmic procedures ranging from pterygium excision to ocular surface reconstruction.10,11 It offers a unique biological scaffold that promotes epithelialization, modulates inflammatory responses, and reduces scarring.12 The use of AMG in trabeculectomy has been documented for a wide variety of intentions. AMG has been described in reinforcing late-onset bleb leaks and conjunctival buttonholes with varying success.13, 14, 15 Other groups have utilized AMG during the initial trabeculectomy surgery for its antifibrotic properties in attempt to decrease scarring, also with varied results.16, 17, 18, 19, 20, 21 In this case, we expand the use of AMG in the initial intraoperative setting to situations where persistent overfiltration is encountered. In young myopes, a larger scleral flap with good apposition of flap edges can reduce risk of overfiltration. In cases of persistent overfiltration, surgeons often consider the use of a scleral or corneal patch graft over the scleral flap, which can result in a permanent reduction of flow and need for further intervention to address IOP. Alternatively, the eye can be filled with a heavier viscoelastic to encourage chamber maintenance with a less intensive post-operative steroid course to promote more rapid healing, but this risks significant post-operative hypotony with need for further intervention.
Our decision to use AMG in our patient was aimed at avoiding both permanent shut-down of the scleral flap whilst mitigating the significant risk of post-operative hypotony associated with over-filtration. The AMG provided structural support and physical tamponade to aqueous flow, resulting in stabilization of the anterior chamber. Additionally, the AMG resorbs in approximately 7–14 days, allowing sufficient time for early fibrosis to stabilize the flap without causing permanent obstruction.22 Lastly, the AMG is optically clear, allowing for a clear view for postoperative needling and laser suture lysis if needed.
The use of AMG and fibrin glue in a chronically overfiltering bleb can also be considered. Intrableb insertion of AMG has been reported to improve bleb morphology in avascular blebs.23 However, given its temporary nature, AMG alone may not provide adequate or durable support in settings of overfiltration with underlying scleral necrosis. In these cases, an open revision with additional 10-0 nylon sutures or use of a scleral patch graft may provide a more durable solution depending on the state of the scleral flap.
In tube shunt surgeries, fibrin glue and AMG has been utilized in patching tube erosions in situations where conjunctival closure is difficult.24 Additionally, fibrin glue has been used in combination with scleral patch material as a plug for leaking sclerostomy tracks in tube repositioning procedures.25 AMG may not offer advantages over a scleral patch in these cases where long term closure of the leaking track is often desired.
4. Conclusions
Our case report describes the successful utilization of AMG in addressing an overfiltering trabeculectomy flap in the acute intra-operative setting, ultimately leading to favorable postoperative outcomes in a patient with severe juvenile open angle glaucoma. By bolstering the scleral flap with AMG and fibrin sealant, we were able to effectively regulate aqueous flow efflux, maintain anterior chamber stability and mitigate the risk of postoperative hypotony without significant compromise to surgical success.
Patient consent
Consent was not obtained as the Stanford Institutional Review Board does not require consent for case reports.
Funding
Support was provided by NIH grants KL2TR003143 (WWL), K08EY034600 (WWL), a Research to Prevent Blindness Career Development Award (WWL), the NIH NEI P30 Vision Research Core (EY026877, Stanford Ophthalmology), and an unrestricted grant from Research to Prevent Blindness (Stanford Ophthalmology).
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
CRediT authorship contribution statement
Michael C. Yang: Data curation, Writing – original draft, Writing – review & editing. Michelle T. Sun: Data curation, Writing – review & editing. Wendy W. Liu: Conceptualization, Data curation, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements and Disclosures
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2024.102128.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Quigley H.A., Broman A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon M.O., Beiser J.A., Brandt J.D., et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–720. doi: 10.1001/archopht.120.6.714. ; discussion 829-830. [DOI] [PubMed] [Google Scholar]
- 3.Cairns J.E. Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol. 1968;66(4):673–679. [PubMed] [Google Scholar]
- 4.Gedde S.J., Feuer W.J., Lim K.S., et al. Treatment outcomes in the primary tube versus trabeculectomy study after 5 years of follow-up. Ophthalmology. 2022;129(12):1344–1356. doi: 10.1016/j.ophtha.2022.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jampel H.D., Musch D.C., Gillespie B.W., et al. Perioperative complications of trabeculectomy in the collaborative initial glaucoma treatment study (Cigts) Am J Ophthalmol. 2005;140(1):16–22. doi: 10.1016/j.ajo.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Bochmann F., Azuara-Blanco A. Interventions for late trabeculectomy bleb leak. Cochrane Database Syst Rev. 2012;(9) doi: 10.1002/14651858.CD006769.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alwitry A., Rotchford A., Patel V., Abedin A., Moodie J., King A.J. Early bleb leak after trabeculectomy and prognosis for bleb failure. Eye. 2009;23(4):858–863. doi: 10.1038/eye.2008.130. [DOI] [PubMed] [Google Scholar]
- 8.Kawai M., Nakabayashi S., Shimizu K., Hanada K., Yoshida A. Autologous transplantation of a free tenon's graft for repairing excessive bleb leakage after trabeculectomy: a case report. Case Rep Ophthalmol. 2014;5(3):297–301. doi: 10.1159/000368159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friberg T.R., Lace J.W. A comparison of the elastic properties of human choroid and sclera. Exp Eye Res. 1988;47(3):429–436. doi: 10.1016/0014-4835(88)90053-x. [DOI] [PubMed] [Google Scholar]
- 10.Davis J.S. Ii. Skin grafting at the johns hopkins hospital. Ann Surg. 1909;50(3):542–549. doi: 10.1097/00000658-190909000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Röck T., Bartz-Schmidt K.U., Landenberger J., Bramkamp M., Röck D. Amniotic membrane transplantation in reconstructive and regenerative ophthalmology. Ann Transplant. 2018;23:160–165. doi: 10.12659/AOT.906856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jirsova K., Jones G.L.A. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank. 2017;18(2):193–204. doi: 10.1007/s10561-017-9618-5. [DOI] [PubMed] [Google Scholar]
- 13.Budenz D.L., Barton K., Tseng S.C. Amniotic membrane transplantation for repair of leaking glaucoma filtering blebs. Am J Ophthalmol. 2000;130(5):580–588. doi: 10.1016/s0002-9394(00)00600-0. [DOI] [PubMed] [Google Scholar]
- 14.Kee C., Hwang J.M. Amniotic membrane graft for late-onset glaucoma filtering leaks. Am J Ophthalmol. 2002;133(6):834–835. doi: 10.1016/s0002-9394(02)01415-0. [DOI] [PubMed] [Google Scholar]
- 15.Li G., O'Hearn T., Yiu S., Francis B.A. Amniotic membrane transplantation for intraoperative conjunctival repair during trabeculectomy with mitomycin C. J Glaucoma. 2007;16(6):521–526. doi: 10.1097/IJG.0b013e3180408ddb. [DOI] [PubMed] [Google Scholar]
- 16.Stavrakas P., Georgopoulos G., Milia M., et al. The use of amniotic membrane in trabeculectomy for the treatment of primary open-angle glaucoma: a prospective study. Clin Ophthalmol. 2012;6:205–212. doi: 10.2147/OPTH.S27187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H., Moon S., Kim J., Lee J. The effect of amniotic membrane transplantation on trabeculectomy in patients with pseudoexfoliation glaucoma. Journal of Ophthalmology. 2022;2022 doi: 10.1155/2022/9355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujishima H., Shimazaki J., Shinozaki N., Tsubota K. Trabeculectomy with the use of amniotic membrane for uncontrollable glaucoma. Ophthalmic Surg Laser. 1998;29(5):428–431. [PubMed] [Google Scholar]
- 19.Nakamura M., Naka M., Tatsumi Y., et al. Filtering bleb structure associated with long-term intraocular pressure control after amniotic membrane-assisted trabeculectomy. Curr Eye Res. 2012;37(3):239–250. doi: 10.3109/02713683.2011.635403. [DOI] [PubMed] [Google Scholar]
- 20.Roque J., Vaz F.T., Basto R., et al. Use of amniotic membrane in MMC-augmented trabeculectomy: a retrospective comparative study. Clin Ophthalmol. 2021;15:4527–4533. doi: 10.2147/OPTH.S342593. PMID: 34866897; PMCID: PMC8636844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheha H., Kheirkhah A., Taha H. Amniotic membrane transplantation in trabeculectomy with mitomycin C for refractory glaucoma. J Glaucoma. 2008;17(4):303–307. doi: 10.1097/IJG.0b013e31815c3a47. PMID: 18552616. [DOI] [PubMed] [Google Scholar]
- 22.Kim J.C., Tseng S.C. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14(5):473–484. [PubMed] [Google Scholar]
- 23.Gupta V., Shaikh N.F., Gupta S. Remodeling an avascular bleb. J Glaucoma. 2019;28(7):e126–e127. doi: 10.1097/IJG.0000000000001212. PMID: 31274705. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth G., Rotchford A., Dua H.S., King A.J. A novel use of amniotic membrane in the management of tube exposure following glaucoma tube shunt surgery. Br J Ophthalmol. 2006;90(4):417–419. doi: 10.1136/bjo.2005.084905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panarelli J.F., Banitt M.R., Sidoti P.A. Scleral fistula closure at the time of glaucoma drainage device tube repositioning: a novel technique. Arch Ophthalmol. 2012;130(11):1447–1451. doi: 10.1001/archophthalmol.2012.2219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.