Highlights
-
•
Observational study on cardiac sarcoidosis outcomes using nonsteroidal immunosuppressants.
-
•
Of the total cohort of 512 patients, only 26 patients underwent nonsteroidal immunosuppressive therapy.
-
•
Clinical events: all-cause death (19.2 %), fatal ventricular arrhythmias (15.4 %), and heart failure hospitalization (19.2 %).
Keywords: Cardiac sarcoidosis, Nonsteroidal immunosuppressive therapy, Fluorine-18 fluorodeoxyglucose positron emission tomography, Cardiac magnetic resonance, Late gadolinium enhancement
Abstract
Background
Nonsteroidal immunosuppressive therapy is a potential therapeutic strategy for cardiac sarcoidosis. However, it is not recommended as an established treatment option. This study aimed to demonstrate the clinical outcomes of patients with cardiac sarcoidosis using nonsteroidal immunosuppressants through the ILLUstration of the Management and PrognosIs of JapaNese PATiEnts with Cardiac Sarcoidosis multicenter retrospective registry.
Methods
From a cohort of 512 patients, 426 who received corticosteroid therapy and 26 who received other immunosuppressive therapy were included for analysis. Clinical outcomes included all-cause death, fatal ventricular arrhythmic events (FVAE), and worsening heart failure with hospitalization.
Results
Nonsteroidal immunosuppressants were used for retained fluorodeoxyglucose uptake in the heart (n = 14), corticosteroid side effects (n = 7), ventricular arrhythmia (n = 4), complete atrioventricular block (n = 2), worsened extracardiac sarcoidosis (n = 2), and other reasons (n = 2). They comprised of methotrexate (n = 20), cyclosporine (n = 2), cyclophosphamide (n = 2), and azathioprine (n = 3). After the addition of a nonsteroidal immunosuppressant, corticosteroids were reduced in 14 of 26 patients (5 [5–17] mg), although no patient discontinued corticosteroids. Of the 14 patients, decreased fluorodeoxyglucose uptake was observed in seven at follow-up. Clinical outcomes were observed in 11 patients (42.3 %). Detected events included all-cause death in five patients (19.2 %), FVAE in four (15.4 %), and worsening heart failure with hospitalization in five (19.2 %), with some overlap.
Conclusions
Nonsteroidal immunosuppressive therapy may be a possible treatment option for patients who are not stabilized with corticosteroids alone or develop corticosteroid side effects.
1. Introduction
Cardiac sarcoidosis (CS) is a life-threatening condition that results in a range of manifestations including electrophysiological [1], morphological [2], [3], and motion [4] abnormalities of the heart. Typical histopathological findings in the involved organs are multinucleated giant cells in non-caseating epithelioid granulomas [5]. In general, CD4 + lymphocytes accumulate in the tissues adjacent to granulomas, suggesting an active inflammatory condition. Therefore, catheter-based endomyocardial biopsy (EMB) is performed to diagnose CS [6]. Other diagnostic tools to detect local inflammation, morphological abnormalities, and scar tissue in CS include Gallium-67 citrate scintigraphy, fluorine-18 fluorodeoxyglucose positron emission tomography (18F-FDG PET), and cardiac magnetic resonance imaging (CMR). These imaging techniques have also been used for the follow-up of CS and to guide the management of patients treated with immunosuppressive therapy [7], [8], [9].
Immunosuppressive therapy using corticosteroids is recommended as first-line treatment in the CS guidelines [10]. Given variations in the patterns of disease activity, disease progression, and response to corticosteroid therapy, the required intensity of corticosteroid therapy varies among patients. The occurrence of side effects associated with corticosteroid therapy also varies. Therefore, alternative therapies are needed to strengthen immunosuppression and avoid corticosteroid-related adverse effects. Second-line immunosuppressive therapies, including methotrexate, cyclosporine, cyclophosphamide, and azathioprine have been tried for non-cardiac sarcoidosis [11], [12], [13]. However, nonsteroidal immunosuppressive therapy is still not yet been established.
In this sub-analysis of a multicenter registration of Japanese patients with CS, we aimed to elucidate clinical characteristics of patients with CS who had been treated with nonsteroidal immunosuppressive therapy. Specifically, our objectives included describing the clinical indications for nonsteroidal immunosuppressant use, evaluating therapeutic effects through various imaging tests, and assessing clinical outcomes.
2. Methods
2.1. Patients
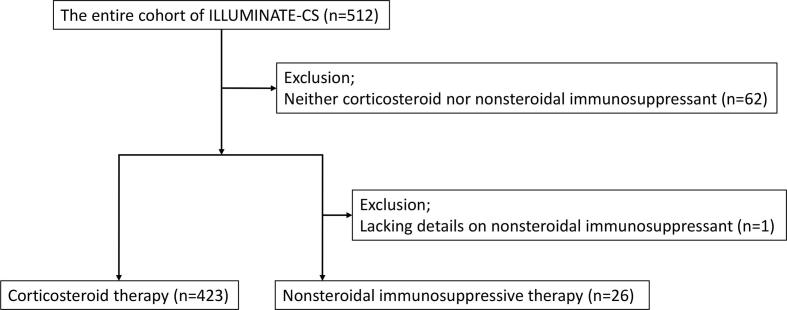
The patients in this study were selected from the existing registry of cardiac sarcoidosis entitled ILLUstration of the Management and prognosis of JapaNese PATiEnts with Cardiac Sarcoidosis (ILLUMINATE-CS) [14]. The details of the registry have been published in the publicly available University Hospital Information Network (UMIN000034974). Briefly, the participants were patients with CS diagnosed based on the 2016 guidelines of the Japanese Circulation Society [10] or 2014 expert consensus statement by the Heart Rhythm Society [15], and registered at 33 facilities across Japan. The study complied with the principles of the Declaration of Helsinki. The study protocol was approved by the ethics committee of each facility and the requirement for informed consent was waived. Of the total cohort of 512 patients, 62 who did not receive corticosteroid or nonsteroidal immunosuppressive therapy were excluded. After excluding patients with missing data on nonsteroidal immunosuppressant use, 26 patients who underwent nonsteroidal immunosuppressive therapy and 423 patients who underwent corticosteroid therapy were selected for further analysis (Fig. 1).
Fig. 1.
Patient selection. Of the 512 patients enrolled in the study, 62 without corticosteroid or nonsteroidal immunosuppressive therapy data and one with missing data on nonsteroidal immunosuppressant use were excluded. Finally, 26 patients with nonsteroidal immunosuppressive therapy and 423 patients with corticosteroid therapy data were enrolled in this study.
2.2. Electrocardiography and imaging studies for cardiac sarcoidosis
Electrocardiography (ECG) and various imaging studies required for CS diagnosis were performed at multiple time points. Briefly, basic electrocardiographic characteristics, including parameters reflecting intracardiac conduction disturbances, were collected. Transthoracic echocardiography parameters included basic morphological measurements of the left ventricle and the prevalence of septal thinning and aneurysms. CMR parameters included the prevalence of late gadolinium enhancement (LGE) and its localization in the American Heart Association (AHA) 17-segments model. The uptake of 18F-FDG PET is also evaluated using the AHA 17-segments model. ECGs and other imaging studies performed at diagnosis (baseline) and when the therapeutic drug reached the maintenance dose (follow up) were selected for the analysis.
2.3. Nonsteroidal immunosuppressants
In addition to the basic datasets of the ILLIMINATE-CS, clinical information regarding nonsteroidal immunosuppressants was collected for analysis. Additional investigations included the use of nonsteroidal immunosuppressants, side effects of corticosteroids, details of nonsteroidal immunosuppressants, side effects of nonsteroidal immunosuppressants, and the weaning of patients from corticosteroid therapy.
2.4. Clinical outcomes
Clinical outcomes included all-cause death, fatal ventricular arrhythmic events (FVAE), and worsening heart failure with hospitalization. FVAE included sudden cardiac death, documented ventricular fibrillation (VF), sustained ventricular tachycardia (VT) lasting > 30 s, and appropriate implantable cardioverter-defibrillator therapy.
2.5. Statistical analysis
Categorical parameters are presented as numbers and percentages. Continuous variables with a normal distribution are presented as means and standard deviations, whereas those with a non-normal distribution are described as medians and interquartile ranges. Student’s t-test for nominal distribution, Mann–Whitney U test for non-nominal distribution, and chi-square test for categorical parameters were used for intergroup comparison. A paired t-test for nominal distribution and McNemar’s test for categorical parameters were used for intragroup comparisons. Because the number of selected patients who received nonsteroidal immunosuppressive therapy was relatively small, statistical tests for clinical outcomes were not performed.
3. Results
3.1. Baseline characteristics
Baseline patient characteristics are summarized in Table 1. In patients who received nonsteroidal immunosuppressive therapy, the mean age at the diagnosis was 60.3 ± 11.7 years and 50 % were male. The initial manifestations of CS at diagnosis were impaired wall motion (42.3 %), atrioventricular block (34.6 %), VT or VF (19.2 %), and heart failure (19.2 %), with some overlap. Histories that may be related to sarcoidosis at the time of CS diagnosis were VF in 4.3 %, sustained VT in 23.1 %, any VT in 30.7 %, and atrioventricular block in 39.1 %. A New York Heart Association classification of heart failure III or IV was observed in 11.5 % of the patients. Although there were no significant differences, patients treated with nonsteroidal immunosuppressants showed a trend toward more male, more isolated CS, and less history of atrioventricular block than patients treated with corticosteroids. Corticosteroid treatment just before the start of nonsteroidal immunosuppressants included pulse corticosteroid therapy in two patients or 20 [8.75–30] mg of prednisolone in the other patients.
Table 1.
Patient characteristics.
| Corticosteroid therapy (n = 423) | Nonsteroidal immunosuppressive therapy (n = 26) | P-value | |
|---|---|---|---|
| Age, years | 61.2 ± 10.8 | 60.3 ± 11.7 | 0.671 |
| Male, n (%) | 148 (35.0) | 13 (50.0) | 0.121 |
| Histological diagnosis, n (%) | 261 (61.7) | 16 (61.5) | 0.975 |
| Isolated CS, n (%) | 119 (28.1) | 10 (38.5) | 0.259 |
| Isolated CS with histology, n (%) | 18 (4.3) | 3 (11.5) | 0.114 |
| Lung sarcoidosis, n (%) | 243 (57.4) | 12 (46.2) | 0.214 |
| Ocular sarcoidosis, n (%) | 117 (27.7) | 7 (26.9) | 0.954 |
| Cutaneous sarcoidosis, n (%) | 86 (20.3) | 5 (19.2) | 1.000 |
| EMB-performed, n (%) | 219 (51.8) | 12 (46.2) | 0.578 |
| EMB-positive, n (%) | 47 (11.1) | 4 (15.4) | 0.475 |
| First manifestation | |||
| AV block, n (%) | 176 (41.6) | 9 (34.6) | 0.734 |
| VT or VF, n (%) | 82 (19.4) | 5 (19.2) | 0.888 |
| HF, n (%) | 80 (18.9) | 5 (19.2) | 0.884 |
| Impaired wall motion, n (%) | 199 (47.0) | 11 (42.3) | 0.830 |
| Thinned basal septum, n (%) | 18 (4.3) | 1 (3.8) | 0.886 |
| ECG abnormality, n (%) | 104 (24.6) | 6 (23.1) | 0.931 |
| History | |||
| HTN, n (%) | 151 (35.7) | 5 (19.2) | 0.255 |
| DM, n (%) | 103 (24.3) | 9 (34.6) | 0.106 |
| DL, n (%) | 64 (15.1) | 3 (11.5) | 1.000 |
| CAD, n (%) | 20 (4.7) | 0 (0.0) | 0.614 |
| HF admission, n (%) | 83 (19.6) | 3 (11.5) | 0.592 |
| VF, n (%) | 14 (3.3) | 1 (4.3) | 0.571 |
| Sustained VT, n (%) | 55 (13.0) | 6 (23.1) | 0.093 |
| Any VT, n (%) | 115 (27.2) | 8 (30.8) | 0.691 |
| AV block, n (%) | 187 (44.2) | 9 (39.1) | 0.544 |
| NYHA class III or IV, n (%) | 52 (12.3) | 3 (11.5) | 1.000 |
| Creatinine, mg/dL | 0.78 [0.65–0.94] | 0.91 [0.70–1.13] | 0.052 |
| ACE, IU/L | 16.7 [11.6–21.9] | 20.6 [11.8–24.3] | 0.394 |
| Lysozyme, µg/mL | 9.6 [7.0–13.1] | 8.8 [5.4–30.7] | 0.716 |
| s-IL2R, IU/mL | 521.0 [380.0–809.5] | 524.0 [353.5–928.5] | 0.953 |
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; AV, atrioventricular; BNP, B-type natriuretic peptide; CAD, coronary artery disease; CS, cardiac sarcoidosis; DL, dyslipidemia; DM, diabetes mellitus; EMB, endomyocardial biopsy; HF, heart failure; HTN, hypertension; NT-proBNP, N-terminal prohormone of B-type natriuretic peptide; NYHA, New York Heart Association; sIL-2R, soluble interleukin 2 receptor; VF, ventricular fibrillation; VT, ventricular tachycardia. Data are presented as mean ± standard deviation, median [interquartile range], or n (%).
3.2. Electrocardiography and transthoracic echocardiography
The electrocardiographic and transthoracic echocardiographic parameters are summarized in Table 2. There were no differences in the baseline parameters between patients who received corticosteroid therapy and nonsteroidal immunosuppressive drugs. Notably, electrocardiography in the corticosteroid therapy group changed significantly, comparing baseline and follow-up, as follows: heart rate (68.4 ± 16.1 bpm vs. 70.9 ± 12.5 bpm, P = 0.002), QT interval (448.7 ± 63.7 ms vs. 414.8 ± 95.9 ms, P = 0.003), frequency of normal atrioventricular conduction (59.2 % vs. 74.4 %, P < 0.001), frequency of 3rd degree atrioventricular block (23.1 % vs. 12.6 %, P < 0.001), frequency of right bundle branch block (37.4 % vs. 26.3 %, P = 0.003), frequency of pacing (27.7 % vs. 36.8 %, P = 0.015), basal interventricular septal thickness (8.4 ± 2.9 mm vs. 7.6 ± 3.7 mm, P < 0.001), middle interventricular septal thickness (9.4 ± 2.6 mm vs. 8.9 ± 3.5 mm, P < 0.001), frequency of septal thinning (42.3 % vs. 51.9 %, P = 0.007), and aneurysm (10.3 % vs. 15.3 %, P = 0.039). In contrast, the nonsteroidal immunosuppressant group revealed significant changes only in left atrial dimension (36.7 ± 10.1 mm vs. 38.4 ± 10.2 mm, P = 0.043) and frequency of aneurysm (20.8 % vs. 23.1 %, P = 0.035).
Table 2.
Electrocardiographic and echocardiographic characteristics.
| Corticosteroid therapy (n = 423) |
Nonsteroidal immunosuppressive therapy (n = 26) |
|||
|---|---|---|---|---|
| Baseline | Follow up | Baseline | Follow up | |
| Electrocardiography | ||||
| Heart rate, bpm | 68.4 ± 16.1 | 70.9 ± 12.5† | 68.1 ± 17.4 | 67.5 ± 9.3 |
| PQ, ms | 198.6 ± 109.0 | 170.2 ± 92.8 | 185.2 ± 32.7 | 172.6 ± 29.1 |
| QRS, ms | 133.4 ± 34.9 | 125.6 ± 38.8 | 130.7 ± 30.2 | 131.8 ± 29.1 |
| QT, ms | 448.7 ± 63.7 | 414.8 ± 95.9† | 454.6 ± 60.2 | 442.3 ± 29.3 |
| Normal AV conduction, n (%) | 228 (59.2) | 212 (74.4) ‡ | 11 (50.0) | 13 (76.5) |
| 1st or 2nd degree AV block, n (%) | 64 (16.6) | 37 (13.0) | 5 (7.2) | 1 (5.9) |
| 3rd degree AV block, n (%) | 89 (23.1) | 36 (12.6) | 6 (27.2) | 3 (17.6) |
| RBBB, n (%) | 130 (37.4) | 76 (26.3) † | 6 (33.3) | 4 (22.2) |
| LBBB, n (%) | 16 (4.7) | 9 (3.2) | 0 (0.0) | 0 (0.0) |
| LAHB, n (%) | 35 (10.4) | 27 (9.5) | 4 (22.2) | 4 (22.2) |
| LPHB, n (%) | 4 (1.2) | 2 (0.7) | 0 (0.0) | 0 (0.0) |
| Pacing, n (%) | 94 (27.7) | 105 (36.8) * | 4 (22.2) | 8 (44.4) |
| Echocardiography | ||||
| LVEF, % | 49.1 ± 15.7 | 49.3 ± 14.9 | 46.7 ± 16.3 | 43.1 ± 16.6 |
| LVEDD, mm | 53.0 ± 9.5 | 53.9 ± 23.2 | 55.5 ± 7.7 | 57.6 ± 9.6 |
| LVESD, mm | 39.7 ± 12.1 | 39.3 ± 12.7 | 42.5 ± 11.3 | 47.2 ± 14.0 |
| Basal IVST, mm | 8.4 ± 2.9 | 7.6 ± 3.7‡ | 7.4 ± 3.1 | 6.9 ± 2.9 |
| Mid IVST, mm | 9.4 ± 2.6 | 8.9 ± 3.5‡ | 8.4 ± 2.3 | 7.8 ± 1.8 |
| LAD, mm | 37.7 ± 7.4 | 38.0 ± 7.9 | 36.7 ± 10.1 | 38.4 ± 10.2 |
| Septal thinning, n (%) | 173 (42.3) | 190 (51.9) † | 12 (50.0) | 13 (50.0) |
| Aneurysm, n (%) | 42 (10.3) | 56 (15.3) * | 5 (20.8) | 6 (23.1) * |
AV, atrioventricular; IVST, interventricular septal thickness; LAD, left atrial dimension; LAHB, left anterior hemiblock; LPHB, left posterior hemiblock; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; RBBB, right bundle branch block. Data are presented as mean ± standard deviation or n (%). * means P < 0.05 compared to baseline. † means P < 0.01 compared to baseline. ‡ means P < 0.001 compared to baseline.
3.3. CMR and 18F-FDG PET
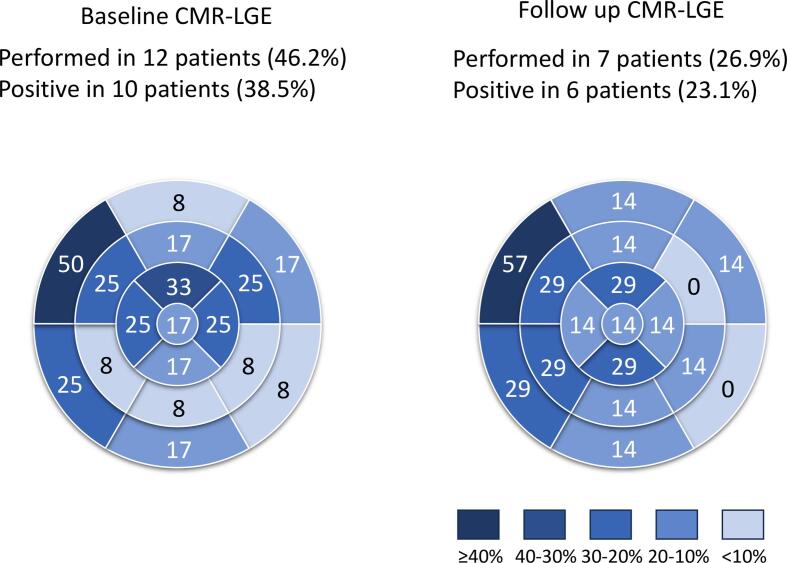
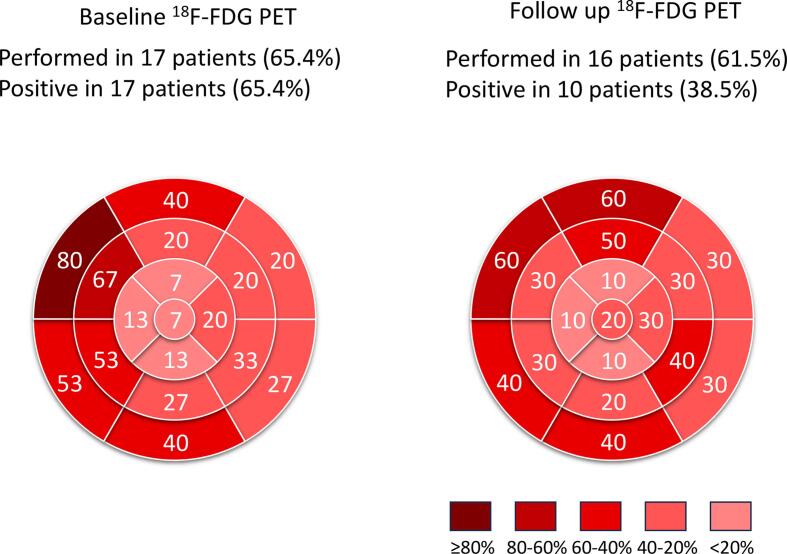
The local distributions of LGE on CMR in patients who received nonsteroidal immunosuppressive therapy were exhibited at baseline (at diagnosis) and follow-up (at the final scan) (Fig. 2). CMR was performed in 12 patients (46.2 % of the enrolled patients) at baseline and in seven patients (26.9 %) at follow-up with a mean duration of 6.7 ± 8.3 years. LGE was observed in 10 patients (38.5 % enrolled, 83.3 % performed) at baseline and in six patients (23.1 % enrolled, 85.7 % performed) at follow-up with a mean duration of 7.4 ± 5.2 months. LGE was most frequently observed in the basal anteroseptal segment at baseline and follow-up. The local distribution of 18F-FDG uptake in patients who received nonsteroidal immunosuppressive therapy was also displayed at baseline (diagnosis) and follow-up (final scan) (Fig. 3). 18F-FDG PET was performed in 17 patients (65.4 %) at baseline and in 16 patients (61.5 %) at follow-up. 18F-FDG uptake was observed in 17 patients (65.4 % enrolled, 100 % performed) at baseline and in 10 patients (38.5 % enrolled, 62.5 % performed) at follow-up. The uptake was frequently observed in the basal and middle segments of the anterior, septal, and inferior walls.
Fig. 2.
Late gadolinium enhancement in cardiac magnetic resonance at baseline and follow up. Cardiac magnetic resonance imaging was performed in 12 patients (46.2% of the enrolled patients) at baseline and was repeated after the administration of nonsteroidal immunosuppressants in seven patients (26.9%). Late gadolinium enhancement was observed in 10 patients (38.5% of 26 patients) at baseline and in six patients (23.1%) at follow-up. CMR-LGE: late gadolinium enhancement on cardiac magnetic resonance.
Fig. 3.
Fluorine-18 fluorodeoxyglucose positron emission tomography at baseline and follow up. Fluorine-18 fluorodeoxyglucose positron emission tomography was performed in 17 patients (65.4% of enrolled patients) at baseline and was repeated after administering nonsteroidal immunosuppressants in 16 patients (61.5% of enrolled patients). Fluorodeoxyglucose uptake was observed in 17 patients (65.4% enrolled, 100% performed) and 10 patients (38.5% enrolled, 62.5% performed) at follow-up.
3.4. Clinical course of patients on nonsteroidal immunosuppressive therapy
The reasons for using nonsteroidal immunosuppressants are summarized in Table 3 and comprised of retained FDG heart uptake, suggesting uncontrolled inflammation (n = 14), side effects of corticosteroids (n = 7), VT or frequent ventricular premature contraction (VPC) (n = 4), complete atrioventricular block (n = 2), worsened symptoms relating to non-cardiac sarcoidosis including neuro and pulmonary sarcoidosis (n = 2), and the other reasons (n = 2), with some overlap. Details of the side effects of corticosteroids included myopathy (n = 3), blood glucose deterioration (n = 3), epigastralgia suggestive of gastroduodenitis (n = 2), and osteonecrosis of the femoral head (n = 1), with some overlap. The deterioration of blood glucose was not an independent reason but was accompanied by other reasons in nonsteroidal immunosuppressive therapy. The nonsteroidal immunosuppressants administered included methotrexate (n = 20, 6.9 ± 2.1 mg/week), cyclosporine (n = 2, 75 or 100 mg/day), cyclophosphamide (n = 2, 25 or 50 mg/day), azathioprine (n = 3, 100 mg/day in each), and adalimumab (n = 1, 40 mg in every 14 days), with some overlap (Table 4). After the addition of nonsteroidal immunosuppressants, corticosteroids were reduced in 14 of 26 patients, with a median maintenance dose of 5 mg (interquartile range, 5–17 mg), although no patients discontinued corticosteroids. Of the 14 patients who started nonsteroidal immunosuppressants for retained FDG uptake, the total segment of FDG uptake decreased significantly (5 [4], [5], [6] vs. 2 [0–5.5], P = 0.016) and decreased FDG uptake was observed in seven patients (50 %) at the follow-up scan. Notably, FDG uptake was negative in six of the 16 patients at the follow-up scan, although no patient was negative at the baseline scan (Fig. 3). In patients who developed corticosteroid side effects, epigastralgia rapidly improved in two patients after corticosteroid dose reduction. Corticosteroid-induced hyperglycemia requires continued administration of oral hypoglycemic agents or insulin even after corticosteroid reduction. Of the four patients with VT or frequent VPC, VT was not suppressed even after the addition of methotrexate. In a patient who initially received corticosteroids, frequent antiarrhythmic agent-resistant VPC was suppressed by the addition of cyclophosphamide and corticosteroid reduction. In two patients with complete atrioventricular block during corticosteroid maintenance therapy, nonsteroidal immunosuppressants were added after pacemaker or implantable cardioverter defibrillator (ICD) implantation. Side effects of nonsteroidal immunosuppressants were observed in six cases (Table 5). One patient with increased serum KL-6 levels 30 months after the administration of methotrexate showed possible methotrexate-associated interstitial pneumonia and was changed from methotrexate to azathioprine. A patient with increased soluble interleukine-2 receptor 40 months after the administration of methotrexate developed methotrexate-associated lymphoproliferative disorder which resolved after the discontinuation of methotrexate. In a patient who developed septic coxitis during methotrexate administration, the drug was stopped for 4 months and subsequently resumed. Methotrexate was discontinued in one patient who showed worsened renal function during administration. One patient who developed hepatic dysfunction during methotrexate therapy showed improved hepatic function after its discontinuation. A patient who developed anemia during azathioprine treatment underwent red blood cell transfusion and discontinuation of therapy, which improved the anemia.
Table 3.
Reasons for the use of nonsteroidal immunosuppressants.
| Reason | n (%) |
|---|---|
| Retained FDG uptake, n (%) | 13 (50) |
| Side effects of steroids, n (%) | 7 (26.9) |
| VT or frequent VPC, n (%) | 4 (15.4) |
| Complete AV block, n (%) | 2 (7.7) |
| Worsened extra-cardiac sarcoidosis, n (%) | 2 (7.7) |
| Other reasons, n (%) | 2 (7.7) |
AV, atrioventricular; FDG, fluorodeoxyglucose; VF, ventricular fibrillation; VPC, ventricular premature contraction; VT, ventricular tachycardia.
Table 4.
Details of nonsteroidal immunosuppressants.
| n (%) | dosage | |
|---|---|---|
| Methotrexate | 20 (76.9) | 6.9 ± 2.1 mg/week |
| Cyclosporine | 2 (7.7) | 75 or 100 mg/day |
| Cyclophosphamide | 2 (7.7) | 25 or 50 mg/day |
| Azathioprine | 3 (11.5) | 100 mg/day |
| Adalimumab | 1 (3.8) | 40 mg/14 days |
Table 5.
Side effects of nonsteroidal immunosuppressants.
| Overall, n | 6 |
|---|---|
| Methotrexate-associated interstitial pneumonia, n | 1 |
| Methotrexate-associated lymphoproliferative disorder, n | 1 |
| Methotrexate-induced septic coxitis, n | 1 |
| Methotrexate-induced acute kidney injury, n | 1 |
| Methotrexate-induced hepatic dysfunction, n | 1 |
| Azathioprine-induced anemia, n | 1 |
3.5. Clinical outcomes
As summarized in Table 6, clinical outcome events were observed in 11 patients (42.3 %), with a mean follow-up duration of 1850 days after the initiation of nonsteroidal immunosuppressive therapy. Detected events included all-cause death in five patients (19.2 %), FVAE in four patients (15.4 %), and worsening heart failure with hospitalization in five patients (19.2 %), with some overlap. Of the seven patients who started nonsteroidal immunosuppressants for retained FDG uptake and reduced uptake after additional therapy, clinical outcome events occurred only in one patient. In contrast, of the two patients whose FDG uptake did not improve even after additional immunosuppressive therapy, clinical outcome events occurred.
Table 6.
Clinical outcome events.
| Overall, n (%) | 11 (42.3) |
|---|---|
| All-cause death, n (%) | 5 (19.2) |
| FVAE, n (%) | 4 (15.4) |
| Worsening heart failure with hospitalization, n (%) | 5 (19.2) |
FVAE, fatal ventricular arrhythmic event.
4. Discussion
In this sub-study of ILLUMINATE-CS, 26 patients treated with nonsteroidal immunosuppressive therapy were investigated. Regarding nonsteroidal immunosuppressants, methotrexate, cyclosporine, cyclophosphamide, and azathioprine were administered to 20, 2, 2, and 3 patients, respectively. Clinical outcome events were observed in 11 patients (42.3 %), including all-cause death in five patients (19.2 %), FVAE in four patients (15.4 %), and worsening heart failure with hospitalization in five patients (19.2 %).
First, the number of patients selected for this sub-study in which nonsteroidal immunosuppressive therapy was performed was 26 (5.1 % of registered patients in the ILLUMINATE-CS registry), which seemed relatively small. This low frequency suggests that most patients treated with corticosteroids might be well-controlled without serious side effects. However, in contrast, this frequency might also indicate that cardiologists may not be familiar with nonsteroidal immunosuppressants and may thus be hesitant to use them, resulting in some patients not receiving adequate therapeutic regimens. The nonsteroidal immunosuppressants administered in our study’s participants were diverse, encompassing a wide range of medications mentioned in previous literature and guidelines. Within them, the most frequently used nonsteroidal immunosuppressant in the study is methotrexate, which is the same as reported in a recent systematic review [16].
In our study cohort, the two main reasons why patients were treated with nonsteroidal immunosuppressants were as follows: the patients were not fully controlled by corticosteroid therapy alone and the patients experienced corticosteroid-related side effects. In those not fully controlled on corticosteroid therapy alone, some exhibited worsened rhythm conditions, including atrioventricular block or ventricular tachyarrhythmia, as well as concomitant shock therapy with an ICD or cardiac resynchronization therapy with a defibrillator. However, other patients exhibited worsened regional wall motion or elevated levels of B-type natriuretic peptide. Notably, despite corticosteroid therapy, most patients exhibited FDG uptake on 18F-FDG PET imaging. In relation to side effects, development of corticosteroid-related skeletal myopathy and hyperglycemia occurred. Notably, 71 % of the patients who experienced corticosteroid-related side effects were tapered to 5 mg prednisolone. Thus, the addition of nonsteroidal immunosuppressants seemed reasonable. Whether to stop or continue the minimum dose of corticosteroids remains controversial.
The efficacy and safety of additional nonsteroidal immunosuppressive therapies are summarized in the Results section. Briefly, nonsteroidal immunosuppressive therapy achieved corticosteroid dose reduction and reduced FDG uptake on 18F-FDG PET imaging. FDG uptake was observed in every patient who underwent 18F-FDG PET, even after treatment with corticosteroids. In contrast, after the initiation of nonsteroidal immunosuppressive therapy, FDG uptake was observed in only 62.5 % of the patients during the follow-up scan. Similar to the regional distribution of FDG uptake in the main study [14], the present study revealed a relatively higher prevalence in the basal or mid-septal wall before immunosuppressive therapy, and a trend toward reduction in the same segment at follow-up. Regarding safety, nonsteroidal immunosuppressive therapy-associated side effects were observed in six cases. Because some of these complications can be fatal, patients undergoing nonsteroidal immunosuppressive therapy should be followed up with care.
In previous studies from European countries and the USA, methotrexate was started at 10–15 mg/week and then increased to 15–20 mg/week [17], [18]. In contrast, some studies from Japan have reported a lower methotrexate dose of 6 mg/week [19], [20], [21]. In the present study, the methotrexate dose was similar to that reported in Japan. Although the body size is generally smaller in the Japanese population than in the European or US populations, the reported methotrexate dose might be too low to achieve sufficient efficacy.
The overall clinical event rate was 42.3 %, with a mean follow-up period of approximately 5 years. Compared to the estimated 5-year event rate at the primary endpoint of 31.0 % in the ILLUMINATE-CS [14], the one reported in this sub-study seemed relatively high. This difference may be attributed to relatively more worsened left ventricular sizes and the prevalence of aneurysms in patients on nonsteroidal immunosuppressive therapy as shown in Table 2.
Considering all the data, nonsteroidal immunosuppressive therapy seems a favorable alternative strategy for patients with corticosteroid-related side effects. Since the intracardiac conduction after nonsteroidal immunosuppressive therapy showed a trend toward decreased atrioventricular block and increased normal atrioventricular conduction (Table 2), those who showed conduction deterioration may also benefit from the therapy. Because FVAE was not suppressed even after the addition of nonsteroidal immunosuppressive therapy, a comprehensive therapeutic strategy including ICD implantation and catheter ablation may be required. Although FDG uptake was decreased after the addition of nonsteroidal immunosuppressive therapy, clinical events occurred in 5 of 13 patients who used nonsteroidal immunosuppressants for retained FDG uptake. Therefore, further investigations are needed for this cluster.
This study has some limitations. First, this was a retrospective, observational study. All data, including clinical examinations performed in clinical practice, were collected after enrollment. Thus, some data were missing, especially during follow-up examinations. Second, although the mother database of the ILLUMINATE-CS consisted of 512 patients, the number of patients selected for this sub-study was small. Additionally, there was a sample size imbalance between patients receiving corticosteroid therapy and those receiving nonsteroidal immunosuppressive therapy, which may introduce confounding factors between the groups. Therefore, we decided that statistical comparisons were not appropriate for this study. Finally, we also attempted to compare the clinical events and imaging studies between different types of nonsteroidal immunosuppressive therapy. However, this was impossible due to the small sample size.
In conclusion, nonsteroidal immunosuppressive therapy may be a viable treatment option for patients who are not stabilized on corticosteroid therapy alone or who experience corticosteroid side effects, as it can reduce FDG uptake and avoid corticosteroid side effects. However, this study was only a descriptive analysis without group comparisons. Therefore, a large-scale interventional trial is warranted to determine the efficacy and safety of nonsteroidal immunosuppressive therapy.
Funding
ILLUstration of the Management and prognosIs of JapaNese PATiEnts with Cardiac Sarcoidosis (ILLUMINATE-CS) was partially supported by a Novartis Pharma Research Grant.
CRediT authorship contribution statement
Kenichiro Suwa: Writing – original draft, Methodology, Investigation, Formal analysis. Yoshihisa Naruse: Writing – review & editing, Methodology, Investigation. Takeru Nabeta: Writing – review & editing, Investigation, Conceptualization. Takeshi Kitai: Writing – review & editing, Investigation. Tatsunori Taniguchi: Writing – review & editing, Investigation. Kenji Yoshioka: Writing – review & editing, Investigation. Hidekazu Tanaka: Writing – review & editing, Investigation. Takahiro Okumura: Writing – review & editing, Investigation. Yuichi Baba: Writing – review & editing, Investigation. Yuya Matsue: Writing – review & editing, Investigation, Funding acquisition, Data curation, Conceptualization. Yuichiro Maekawa: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Y.M. received honorariums from Otsuka Pharmaceutical Co. and Novartis, Japan. The other authors declare no conflicts of interest.
Acknowledgement
We thank all the investigators of the ILLUMINATE-CS who generously provided us with their patient data.
References
- 1.Koplan B.A., Soejima K., Baughman K., Epstein L.M., Stevenson W.G. Refractory ventricular tachycardia secondary to cardiac sarcoid: electrophysiologic characteristics, mapping, and ablation. Heart Rhythm. 2006;3:924–929. doi: 10.1016/j.hrthm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 2.Tanizawa K., Handa T., Nagai S., Yokomatsu T., Ueda S., Ikezoe K., et al. Basal interventricular septum thinning and long-term left ventricular function in patients with sarcoidosis. Respir Investig. 2022;60:385–392. doi: 10.1016/j.resinv.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Kurmann R., Mankad S.V., Mankad R. Echocardiography in Sarcoidosis. Curr Cardiol Rep. 2018;20:118. doi: 10.1007/s11886-018-1065-9. [DOI] [PubMed] [Google Scholar]
- 4.Valantine H., McKenna W.J., Nihoyannopoulos P., Mitchell A., Foale R.A., Davies M.J., et al. Sarcoidosis: a pattern of clinical and morphological presentation. Br Heart J. 1987;57:256–263. doi: 10.1136/hrt.57.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fathima S., Roberts W.C. Comparison of Clinical and Morphologic Findings in Patients With Cardiac Sarcoidosis Severe Enough to Warrant Heart Transplantation in Those With -vs- Those Without Non-Caseating Granulomas in the Explanted Heart (Burnt-Out Sarcoid) Am J Cardiol. 2019;124:599–603. doi: 10.1016/j.amjcard.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Sohn D.W., Park J.B. Cardiac sarcoidosis. Heart. 2023;109:1132–1138. doi: 10.1136/heartjnl-2022-321379. [DOI] [PubMed] [Google Scholar]
- 7.Lee P.I., Cheng G., Alavi A. The role of serial FDG PET for assessing therapeutic response in patients with cardiac sarcoidosis. J Nucl Cardiol. 2017;24:19–28. doi: 10.1007/s12350-016-0682-1. [DOI] [PubMed] [Google Scholar]
- 8.Chareonthaitawee P., Beanlands R.S., Chen W., Dorbala S., Miller E.J., Murthy V.L., et al. Joint SNMMI-ASNC expert consensus document on the role of (18)F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Cardiol. 2017;24:1741–1758. doi: 10.1007/s12350-017-0978-9. [DOI] [PubMed] [Google Scholar]
- 9.Birnie D.H., Nery P.B., Ha A.C., Beanlands R.S. Cardiac Sarcoidosis. J Am Coll Cardiol. 2016;68:411–421. doi: 10.1016/j.jacc.2016.03.605. [DOI] [PubMed] [Google Scholar]
- 10.Terasaki F., Azuma A., Anzai T., Ishizaka N., Ishida Y., Isobe M., et al. JCS 2016 Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis - Digest Version - Circ J. 2019;83:2329–2388. doi: 10.1253/circj.CJ-19-0508. [DOI] [PubMed] [Google Scholar]
- 11.Vorselaars A.D.M., Wuyts W.A., Vorselaars V.M.M., Zanen P., Deneer V.H.M., Veltkamp M., et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805–812. doi: 10.1378/chest.12-1728. [DOI] [PubMed] [Google Scholar]
- 12.Fang C., Zhang Q., Wang N., Jing X., Xu Z. Effectiveness and tolerability of methotrexate in pulmonary sarcoidosis: a single center real-world study. Sarcoidosis Vasc Diffuse Lung Dis. 2019;36:217–227. doi: 10.36141/svdld.v36i3.8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baughman R.P., Lower E.E. A clinical approach to the use of methotrexate for sarcoidosis. Thorax. 1999;54:742–746. doi: 10.1136/thx.54.8.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nabeta T., Kitai T., Naruse Y., Taniguchi T., Yoshioka K., Tanaka H., et al. Risk stratification of patients with cardiac sarcoidosis: the ILLUMINATE-CS registry. Eur Heart J. 2022;43:3450–3459. doi: 10.1093/eurheartj/ehac323. [DOI] [PubMed] [Google Scholar]
- 15.Birnie D.H., Sauer W.H., Bogun F., Cooper J.M., Culver D.A., Duvernoy C.S., et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 16.Gallegos C., Oikonomou E.K., Grimshaw A., Gulati M., Young B.D., Miller E.J. Non-steroidal treatment of cardiac sarcoidosis: a systematic review. Int J Cardiol Heart Vasc. 2021;34 doi: 10.1016/j.ijcha.2021.100782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthal D.G., Parwani P., Murray T.O., Petek B.J., Benn B.S., De Marco T., et al. Long-term corticosteroid-sparing immunosuppression for cardiac sarcoidosis. J Am Heart Assoc. 2019;8:e010952. doi: 10.1161/JAHA.118.010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vis R., Mathijssen H., Keijsers R.G.M., van de Garde E.M.W., Veltkamp M., Akdim F., et al. Prednisone vs methotrexate in treatment naive cardiac sarcoidosis. J Nucl Cardiol. 2023;30:1543–1553. doi: 10.1007/s12350-022-03171-6. [DOI] [PubMed] [Google Scholar]
- 19.Nagai S., Yokomatsu T., Tanizawa K., Ikezoe K., Handa T., Ito Y., et al. Treatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern Med. 2014;53:427–433. doi: 10.2169/internalmedicine.53.0794. [DOI] [PubMed] [Google Scholar]
- 20.Dotare T., Maeda D., Matsue Y., Minamino T. Effectiveness of methotrexate as a second-line treatment for cardiac sarcoidosis assessed via (18)F-FDG PET: a case report. Eur Heart J Case Rep. 2022;6 doi: 10.1093/ehjcr/ytac226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morimoto R., Unno K., Fujita N., Sakuragi Y., Nishimoto T., Yamashita M., et al. Prospective analysis of immunosuppressive therapy in cardiac sarcoidosis with fluorodeoxyglucose myocardial accumulation: PRESTIGE study. JACC Cardiovasc Imaging. 2023 doi: 10.1016/j.jcmg.2023.05.017. [DOI] [PubMed] [Google Scholar]