Abstract
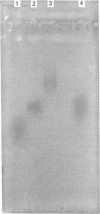
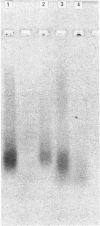
A glycosaminoglycan-bearing polypeptide (S.GP), present in human seminal plasma, was purified to homogeneity by a combination of CsCl density-gradient centrifugation, f.p.l.c. ion-exchange chromatography on a Mono Q HR column and Superose 6 gel filtration. The observed polydispersity of S.GP was attributed to the heterogeneity of its glycosaminoglycan content. Enzymic deglycosylation experiments and N-terminal amino-acid sequence determination indicate that it consists of a polypeptide (apparent molecular mass approx. 18 kDa) bearing both chondroitin and heparan sulphate chains. Evidence is given that S.GP contains a glycosaminoglycan-linkage domain of a so far uncharacterized gene product, proteolytically processed in the genital tract.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Gusse M., Sautière P., Bélaiche D., Martinage A., Roux C., Dadoune J. P., Chevaillier P. Purification and characterization of nuclear basic proteins of human sperm. Biochim Biophys Acta. 1986 Oct 29;884(1):124–134. doi: 10.1016/0304-4165(86)90235-7. [DOI] [PubMed] [Google Scholar]
- Hadley M. A., Byers S. W., Suárez-Quian C. A., Kleinman H. K., Dym M. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J Cell Biol. 1985 Oct;101(4):1511–1522. doi: 10.1083/jcb.101.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt C. A., Muir H. Gel electrophoresis of proteoglycans and glycosaminoglycans on large-pore composite polyacrylamide-agarose gels. Anal Biochem. 1971 Dec;44(2):612–622. doi: 10.1016/0003-2697(71)90250-8. [DOI] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Périn J. P., Bonnet F., Maillet P., Jollès P. Characterization and N-terminal sequence of human platelet proteoglycan. Biochem J. 1988 Nov 1;255(3):1007–1013. doi: 10.1042/bj2551007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez J. P., Minguell J. J. Synthesis of extracellular matrix components by somatic testicular cells from immature and pubertal rats. Int J Androl. 1989 Jun;12(3):231–239. doi: 10.1111/j.1365-2605.1989.tb01308.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez J. P., Minguell J. J. Synthesis of proteoglycans and hyaluronic acid by long-term cultures of testicular cells from immature and pubertal rats. Cell Biochem Funct. 1989 Oct;7(4):293–300. doi: 10.1002/cbf.290070408. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989 Aug 15;264(23):13369–13372. [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Sanderson R. D., Bernfield M. Molecular polymorphism of a cell surface proteoglycan: distinct structures on simple and stratified epithelia. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9562–9566. doi: 10.1073/pnas.85.24.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders S., Jalkanen M., O'Farrell S., Bernfield M. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989 Apr;108(4):1547–1556. doi: 10.1083/jcb.108.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Fritz I. B. Structural characterization of proteoglycans produced by testicular peritubular cells and Sertoli cells. J Biol Chem. 1985 Sep 25;260(21):11874–11883. [PubMed] [Google Scholar]
- Skinner M. K., Tung P. S., Fritz I. B. Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol. 1985 Jun;100(6):1941–1947. doi: 10.1083/jcb.100.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantravahi R. V., Stevens R. L., Austen K. F., Weis J. H. A single gene in mast cells encodes the core peptides of heparin and chondroitin sulfate proteoglycans. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9207–9210. doi: 10.1073/pnas.83.23.9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishler P. V., Epstein C. J. A convenient method of preparing polyacrylamide gels for liquid scintillation spectrometry. Anal Biochem. 1968 Jan;22(1):89–98. doi: 10.1016/0003-2697(68)90262-5. [DOI] [PubMed] [Google Scholar]
- Tung P. S., Fritz I. B. Interactions of sertoli cells with myoid cells in vitro. Biol Reprod. 1980 Aug;23(1):207–217. doi: 10.1093/biolreprod/23.1.207. [DOI] [PubMed] [Google Scholar]