Abstract
Background
Cutaneous melanoma (CM) is a significant contributor to skin cancer-related mortality globally and in Canada. Despite the well-established link between ultraviolet (UV) radiation exposure and skin cancer risk, there remains a gap in population-level interventions and persistent misconceptions about sun exposure and impact of environment on individual behavior.
Objective
The current study provides an ecological analysis using latest available data (2011-2017) to define geographic/environmental contributors to the CM landscape in Canada.
Methods
Utilizing Canadian Cancer Registry and Canadian Urban Environmental Health Research Consortium data, we analyzed 39,605 CM cases occurring in Canada from 2011 to 2017. Environmental data, including UV radiation, greenspace (normalized difference vegetation index), temperature, heat events, and precipitation was used to evaluate the effect of environment on CM incidence rates across Forward Sortation Area postal codes.
Results
Forward Sortation Areas with increased CM incidence were associated with higher annual average temperature, snowfall, heat events, normalized difference vegetation index, and vitamin D-weighted UV exposure. Conversely, factors associated with decreased incidence included an increased annual highest temperature, rain precipitation, and a longer duration of heat events.
Limitations
This study is subject to ecological bias and findings should be interpreted with caution.
Conclusion
This study further substantiates associations between specific environmental factors and CM incidence.
Key words: Canadian cancer registry, climate, cutaneous melanoma, epidemiology, geography, risk factors
Capsule Summary.
-
•
Previous data from 1992 to 2010 linked specific environmental and geographic factors to CM incidence rates. The current study defines/validates associations and indicates increasing CM incidence in Canada.
-
•
Results of this work detail the interaction of environment with CM incidence in Canada and its potential impact on high-risk sun exposure behaviors.
Introduction
Cutaneous melanoma (CM) is one of the deadliest skin cancers.1 As of 2019, it represented approximately 3.8% of new cancer cases in Canada, as well as 1.9% and 1.2% of cancer deaths in males and females, respectively.1 In the United States, estimates predict that 100,640 new melanomas will be diagnosed in 2024, with roughly 8300 deaths. Assessments of the global impact of melanoma estimate that 325,000 new CM cases and 57,000 new CM deaths occurred globally in 2020, and if trends remain unchanged, the burden of CM will increase to an estimated 510,000 new cases and 96,000 deaths by 2040.2 Globally, CM cases are concentrated in Central and Eastern Europe (16.3%), followed by North America (14.7%) and Western Europe (13.0%).2 Oceania, including Australia/New Zealand, which have the highest age-standardized CM incidence in the world, accounts for 5.9% of global CM cases.
A number of skin cancers are known to be driven by external triggers (eg, ultraviolet (UV) radiation, human papillomavirus, Merkel cell polyomavirus, etc).3, 4, 5, 6 In particular, the association between UV radiation exposure and skin cancer risk is well-established,1,7 and several causal mechanisms have been detailed.8 The interplay of UV or solar radiation with host factors and other determinants establishes an individual's CM risk.9, 10, 11, 12, 13, 14, 15 Despite extensive knowledge regarding the detrimental impact of UV radiation, with notable exceptions in Australia/New Zealand,10 policymakers have not adopted adequate population-level interventions to promote sun protection/sun avoidance as was achieved for smoking, human papillomavirus vaccination and other cancer preventative measures. There are also persistent misconceptions related to the dangers of sun exposure at the individual level16 and in colder climates.17 Studies indicate that the aesthetic of tanned skin remains highly valued and a key motivation of intentional tanning behaviours.18, 19, 20
Previous studies conducted by our group have provided a detailed analysis of Canadian CM epidemiologic trends and the associated disease burden between 1992 and 2010.6,11,21 Our group also evaluated the relationship between several geographic and environmental factors and their association with CM incidence for 1992-2010 across Canada,22 concluding that increases in annual average temperature, summer UV radiation, and greenspace/vegetation were associated with a higher expected incidence of CM cases.22 This work was part of a larger project studying incidence of various malignancies in Canada during this period.
When CM rates in Canada between 1992 and 2010 and 2011 and 2017 were compared, our group reported an increase in age-adjusted CM incidence nationally and across each individual province.23 In the current ecological study, we sought to update and validate our analysis examining environmental and geographic factors associated with CM using the latest available data from 2011 to 2017. This is important as the medical and information technologies evolved dramatically since 1990s improving our ability to detect and report/track incidence of melanoma. Since CM incidence rates continue to increase it is important to reevaluate our current model. We hypothesize the previously identified associations will continue to be significant, and that other potential contributing factors may be identified. Our objective is to determine robust region-specific associations between CM incidence and weather events (rain, snow, heat), temperature, greenspace (quantified by the normalized difference vegetation index), and UV radiation index. This data provide an up-to-date understanding of the CM landscape in North America and will help in the development of targeted interventions to reduce the burden of CM.
Methods
This study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology checklist.23, 24, 25, 26 This study received an exemption from the McGill University Research Ethics Board review.
Study variables - melanoma incidence
Data sources and methodology for this study have been previously described in detail in.22 Briefly, this study utilises age-standardized CM incidence per 100,000 individuals as the primary outcome variable, with data on CM incidence spanning 2011-2017 from the Canadian Cancer Registry.27 CM diagnoses were classified according to the International Classification of Diseases (ICD) for Oncology -3, ICD-9, and ICD-10 codes for all CM subtypes, consistent with previous reporting.22 Only Canadian Cancer Registry data were used for this study, as Québec's Le Registre Québécois du Cancer employed distinct criteria when assessing CM cases that were not directly comparable to data from other Canadian provinces.6,21,23 Incidence analyses were conducted at the Forward Sortation Area (FSA) level, with Canadian postal codes providing the geographical basis for these analyses available publicly through Statistics Canada. Population counts for FSAs were derived from Statistics Canada Census of Population data for 2011-2017.28 In Canada, postal codes consist of letters and numbers (eg, H4A 3J1), where the first 3 entries represent an FSA, designating specific areas within larger geographic regions.
Study variables – environmental data
The Canadian Urban Environmental Health Research Consortium is funded by the Canadian Institutes of Health Research and provides comprehensive geospatial exposure data on various environmental factors.29 Environmental data were averaged across the time period studied. The variables included UV radiation, measured by taking the average daily dose of vitamin D-weighted UV (J/m2) for summer months (June, July, and August), average greenspace measured by the normalized difference vegetation index, annual highest temperature, absolute number and average length of annual heat events, annual total precipitation (rain and snow), and absolute number and average length of precipitation events (rain and snow).
Procedure and statistical analysis
All analyses were conducted using R statistical software, version 4.3.2.30 Incidence rates of CM per 100,000 individuals by FSA were extracted for the 2011-2017 period. To determine factors associated with increased CM incidence, variables were chosen on a conceptual basis and in line with previous studies.22 Each FSA was categorised as being high- or low-risk based on statistical comparisons with the national average CM incidence rate and compared by several independent environmental factors as described above. A two-sided t-test with Welch-Satterthwaite variance approximation was employed for these comparisons.22
Variables were chosen on a conceptual and statistic basis for further multivariate analysis. Stepwise variable selection using Akaike Information Criterion, and/or variables that were deemed to be conceptually relevant based on previously acknowledged associations were employed for modelling.22 Variance inflation factors were assessed to identify collinearity.22 Due to small sample size, northern territories were excluded in regression analyses.
As in our previous 1992-2010 study, given the noncontinuous and right-skewed nature of CM incidence data, a negative binomial regression model in R was employed.22 This modeling approach is particularly suited for count data exhibiting skewness and potential overdispersion. P values less than .05 were considered statistically significant. Complete case analysis was used for the primary analyses in this study.22
Results
Table I presents data on CM incidence in Canada across the study period, adapted from Conte et al.11 There was a total of 39,605 incident cases of CM in Canada between 2011 and 2017, representing an age-adjusted rate of 20.8 cases per 100,000 individuals. The provinces that documented the highest rates included Prince Edward Island (33.9), Nova Scotia (30.8), and New Brunswick (22.5), while those with the lowest rates were Saskatchewan (14.5), Alberta (15.5), and Manitoba (16.3). The Northern Territories (Nunavut, Yukon, and Northwest Territories) had an overall reported rate of 6.1 cases per 100,000 individuals, however the sample size was small across the period of interest (n = 50). Our data included a total of 1063 Forward Sortation Areas (FSAs), 470 (44.2%) of which were assigned into the “high-risk” category and had a CM incidence above the national average, while 593 (55.8%) were assigned to the ‘low-risk’ category with a CM incidence below the national average.
Table I.
Cutaneous melanoma cases and incidence rate per 100,000 individuals for studied Canadian provinces
| Province | Cases | Average population | Incidence per hundred thousand per year |
|---|---|---|---|
| Canada | 39,615 | 27,271,765 | 20.75 |
| Newfoundland and Labrador | 690 | 527,502 | 18.69 |
| Prince Edward Island | 345 | 145,553 | 33.86 |
| Nova Scotia | 2030 | 942,372 | 30.77 |
| New Brunswick | 1200 | 760,080 | 22.55 |
| Ontario | 21,445 | 13,633,639 | 22.47 |
| Manitoba | 1460 | 1,281,235 | 16.28 |
| Saskatchewan | 1125 | 1,110,054 | 14.48 |
| Alberta | 4385 | 4,044,619 | 15.49 |
| British Columbia | 6885 | 4,709,418 | 20.89 |
| Northern Territories | 50 | 117,294 | 6.09 |
The table adapted from Conte et al.11
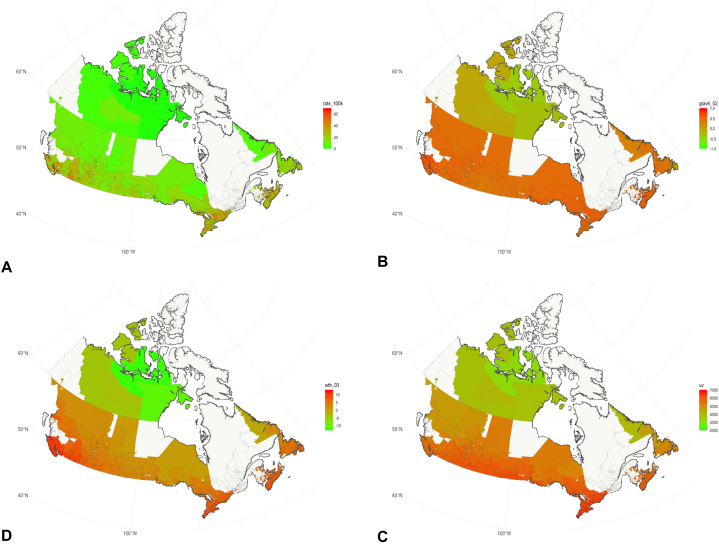
Using age-standardized rates at the FSA level, various geographic and environmental variables were correlated with 2011-2017 CM incidence rates. It is important to state that most environmental variables have remained stable/have not changed over decades based on the the Canadian Urban Environmental Health Research Consortium data. Fig 1 presents maps of CM cases per 100,000 individuals (A), average NVDI (B), annual average temperature (C), and mean daily dose of vitamin D-weighted UV J/m2 for summer months (D), by FSA, with data averaged over 2011-2017. A general increasing trend is observed for the studied variables from north to south. Table II presents comparisons between the high- and low-risk regions by environmental variables deemed statistically or conceptually relevant to the analysis. A two-tailed t-test with Welch-Satterthwaite variance approximation was used with alpha = 0.05. High-risk FSAs were significantly different from low-risk FSAs for all but 2 variables - annual number of days with heat events based on maximum temperature, and annual average length of heat events based on maximum temperature. These variables were conserved in modelling based on stepwise variable selection and based on conceptual relevance.
Fig 1.
Map of CM incidence per 100,000 individuals (A), average normalized difference vegetation index (NVDI) (B), Annual average temperature (C), and mean daily dose of vitamin D-weighted UV J/m2 for summer months (D), by Forward Sortation Area (FSA); data averaged between 2011 and 2017.
Table II.
National differences in environmental conditions/variables that significantly contribute to increased melanoma incidence between high-risk and low-risk FSAs
| National (excluding Quebec and Northern Territories) |
t-test∗ (P-value) | ||
|---|---|---|---|
| FSAs with higher than national average (n = 470), mean (range) | FSAs with lower than national average (n = 593), mean (range) | ||
| Average annual temperature, Celsius | 7.93 (0.36-11.02) | 6.57 (−12.23 to 11.81) | <.01 |
| Average NVDI at 1000 m | 0.44 (−0.09 to 0.63) | 0.37 (−0.38 to 0.64) | <.01 |
| Mean daily dose of vitD-weighted UV (J/m2) for summer months† | 6032.17 (5003.78-6704.31) | 5867.84 (3243.56-6704.31) | <.01 |
| Annual highest temperature, Celsius | 31.79 (25.69-37.49) | 32.40 (21.54-36.77) | <.01 |
| Annual total precipitation as rain (mm) | 699.22 (210.76-1437.39) | 572.62 (81.50-1636.45) | <.01 |
| Annual number of days with heat events based on maximum temperature | 10.32 (2.71-19.80) | 10.28 (3.99-19.80) | .78 |
| Annual average length of heat events based on max temperature | 4.08 (1.63-6.01) | 4.11 (2.57-6.06) | .54 |
High-risk FSAs were compared to low-risk FSAs via a two-tailed t test and significance was determined (alpha = 0.05).
J/m, Joules per meter squared; NDVI, normalized difference vegetation index; vitD, vitamin D.
Wilcoxon Rank Test performed if one or both displayed a non-normal distribution. FSA- Forward Sortation Area.
Summer months include June, July, and August.
Table III documents the unadjusted incidence rate ratios (IRRs) and 95% confidence intervals (CIs) for correlates of CM, and Table IV presents the results of the adjusted regression model. Several other variables were included in initial data exploration phases but later excluded due to collinearity. Stepwise variable selection was used to include the 7 final variables presented in Table IV. Each variable was centered and scaled by the standard deviation (SD) and converted to z-scores. For instance, with regards to annual average temperature, 1 SD increase in average annual temperature, or 1.5 °C, correlated with a 26.3% increase in the expected number of CM cases in a given area. The direction of each univariate model was conserved in the final multivariate model except for average total precipitation as rain, which was positively associated with increased CM in the univariate analysis (IRR 1.08, 95% CI 1.07-1.09) and negatively associated with CM after adjustment (IRR 0.82, 95% CI 0.78-0.84).
Table III.
Unadjusted IRR, 95% confidence intervals, and standard errors for correlates of cutaneous melanoma rates
| IRR (95% CI) | Std Error | P value | |
|---|---|---|---|
| Average highest annual temperature, °C | 0.9047 (0.8951-0.9144) | 0.0051 | <.001 |
| Annual average temperature, °C | 1.0991 (1.0870-1.1113) | 0.0056 | <.001 |
| Average total precipitation as rain, mm | 1.0753 (1.0648-1.0858) | 0.0049 | <.001 |
| Average annual number of days with heat events | 1.0050 (0.9944-1.0157) | 0.0054 | .355 |
| Average length of heat events | 0.9559 (0.9459-0.9659) | 0.0053 | <.001 |
| Mean daily dose of vitD-weighted UV J/m2 for summer months | 1.0643 (1.0524-1.0764) | 0.0058 | <.001 |
| Average NDVI at 1000 m | 1.2727 (1.256-1.2887) | 0.0064 | <.001 |
AIC: 9403.2. Stepwise variable selection was performed, and variables selected according to best model fit as assessed by the Akaike Information Criterion (AIC). J/m2, joules per meter squared; NDVI, normalized difference vegetation index; vitD, vitamin D. All variables are presented in “Z-score” units. Coefficients represent the change in the number of melanoma cases that could be expected in an FSA for a one standard deviation increase above the variable’s mean. For instance, (based on IRR value) one might expect a 26.3% increase in the number of cases in an FSA where the annual average temperature exceeded the national mean by one standard deviation.
CI, Confidence interval; IRR, incidence rate ratio; J/m2, joules per meter squared; NDVI, normalized difference vegetation index; Std Error, standard error; vitD, vitamin D.
Table IV.
Adjusted IRR, 95% confidence intervals, and standard errors for correlates of cutaneous melanoma rates
| IRR (95% CI) | Std Error | P value | |
|---|---|---|---|
| Intercept | - | 0.0053 | <.001 |
| Average highest annual temperature, °C | 0.9038 (0.8864-0.9216) | 0.0099 | <.001 |
| Annual average temperature, °C | 1.2634 (1.2275-1.3004) | 0.0147 | <.001 |
| Average total precipitation as rain, mm | 0.8159 (0.7965-0.8358) | 0.0123 | <.001 |
| Average annual number of days with heat events | 1.0659 (1.0499-1.0822) | 0.0077 | <.01 |
| Average length of heat events | 0.8596 (0.8472-0.8722) | 0.0074 | <.01 |
| Mean daily dose of vitD-weighted UV J/m2 for summer months | 1.0393 (1.0170-1.0622) | 0.0111 | <.01 |
| Average NDVI at 1000 m | 1.2735 (1.2560-1.2913) | 0.0071 | <.01 |
AIC: 9403.2. Stepwise variable selection was performed, and variables selected according to best model fit as assessed by the Akaike Information Criterion (AIC). J/m2, joules per meter squared; NDVI, normalized difference vegetation index; vitD, vitamin D. All variables are presented in “Z-score” units. Coefficients represent the change in the number of melanoma cases that could be expected in an FSA for a 1 standard deviation increase above the variable’s mean. For instance, (based on IRR value) one might expect a 26.3% increase in the number of cases in an FSA where the annual average temperature exceeded the national mean by one standard deviation.
CI, Confidence interval; IRR, incidence rate ratio; J/m2, joules per meter squared; NDVI, normalized difference vegetation index; Std Error, standard error; vitD, vitamin D.
Our final multivariate model found 4 variables that correlated with increased expected CM cases after adjustment for all predictor variables.
Specifically, annual average temperature (IRR 1.26, 95% CI 1.23-1.30), average annual number of days with heat events (IRR 1.07, 95% CI 1.05-1.08), mean daily dose of vitamin-D weighted UV in the summer months (IRR 1.04, 95% CI 1.02-1.06), and higher average NVDI (IRR 1.27, 95% CI 1.26-1.29) (ie, greenspace) were associated with increases in CM rates. In other words, taking, for example, annual average temperature, 1 SD increase in average annual temperature, or 1.5 °C, correlated with a 26.3% increase in the expected number of CM cases for the region. In contrast, 3 variables were negatively associated with CM rates, namely the average highest annual temperature (IRR 0.90, 95% CI 0.88-0.92), average total precipitation in the form of rain (IRR 0.82, 95% CI 0.80-0.84), and average length of heat events in days (IRR 0.86, 95% CI 0.85-0.87).
Discussion
The current ecological study validates a previous analysis conducted by our group22 evaluating the correlation between several climate and environmental variables and CM rates by FSA in Canada. Our previous analysis using data for 1992-2010 for CM and environmental factors indicated that higher average annual temperature, availability of green spaces, and the UV radiation index were the 3 main factors associated with higher CM risk in Canada, while the number of annual heat events together with highest annual temperature and higher average number of annual rain events were associated with a decrease in CM incidence rates. This study, using updated CM incidence values which have increased across all jurisdictions, validates these associations, and additionally suggests a new correlation between the average number of days of heat events and decreased risk of CM incidence.
As we hypothesized previously, these associations may be explained by the impact of climate and environmental factors on individual habits and community norms/behaviours.9,31 More greenspace, higher average annual temperatures, and a higher summer UV radiation index may indicate locations that are more temperate for longer periods of the year, leading individuals to enjoy the outdoors more often and for longer, thus exposing themselves more frequently to UV.9,31 Conversely, long periods of heat events, more rain, and extreme heat may prompt individuals to stay indoors and thus reduce their cumulative sun exposure.
Corroborating these results, research from Australia has demonstrated a correlation between increasing temperatures and increased outdoor activity among adults, coupled with a decreased tendency to use protective clothing and an increased likelihood of sunburns. With temperatures surpassing 27 °C, the risk of sunburn is diminished. This reduction was attributed to individuals actively seeking shade for comfort, presenting a contrasting scenario where higher temperatures correlated with a protective behavioral response.32
Sun exposure remains the primary modifiable risk factor for melanoma and addressing certain prevalent beliefs in Canada and the Unites States could present actionable avenues to increase sun protection practices. The notion that Canadians and Americans residing in northern states do not get enough vitamin D because of the weather or northern climate is often discussed and prompts individuals to actively seek sun exposure.10,33 Furthermore, the presence of diverging recommendations on the use sunscreen and sun protective clothing is still publicised through official and reputable channels.10,33 Both may contribute to diminished sun protection practices explaining in part the North American “sunscreen paradox” observation.9 Clear and consistent guidelines related to vitamin D requirements and sources should be published. Additionally, beliefs that residing in cold-climate countries like Canada or the northern United States lowers CM risk have been documented,17 and should be addressed through public health messaging and campaigns.
This study has several strengths and limitations. As with any ecological study, it is crucial to interpret these findings with caution and to acknowledge that aggregated group-level data cannot be directly extrapolated to individual-level associations. The ecological design also poses challenges in controlling for individual-level confounding variables, as the observed associations between environmental factors and CM rates may be confounded by unmeasured individual characteristics. The specific associations outlined do not consider Fitzpatrick skin types, which, as highlighted in our prior publication, exhibit variations across provinces6 and interplay with the environment in determining individual CM risk. While the accuracy and reliability of the Canadian Cancer Registry has been extensively documented,34, 35, 36 a number of other individual factors, including sex, gender, race, occupation, and socioeconomic status, are crucial determinants of health and cancer incidence and are not captured in the current study or databases used for analysis. There is an important, potentially decades-long latency period between sun exposure and the development of CM, as with other skin cancers. Hence, while the analysis of data for 2011-2017 does not take into account this latency period, it is important to highlight that climate variables tracked by the Canadian Urban Environmental Health Research Consortium since 1983 have not changed significantly over time. Moreover, it is reassuring that our prior study documented the same trends for a much longer study period (1992-2010), indicating the robustness of our results.
This study provides important insight into the increasing trends in CM incidence and their association with environmental and climate factors. With climate change being an increasing concern worldwide, its impact on CM incidence cannot be overlooked. As global temperatures continue to gradually rise and the ozone layer is depleted, there is an increasing risk of prolonged sun exposure, altering UV radiation patterns and intensities.37 Changes in climate may lead to shifts in behavioral patterns, with individuals spending more time outdoors and experiencing extended periods of UV exposure, as detailed in our model. Additionally, alterations in environmental factors such as temperature, precipitation, and greenspace will influence the geographical distribution of CM risk. The intricate interplay between climate change and CM incidence necessitates continued monitoring and careful consideration in public health strategies.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: Author AMB was supported by the Dr Clarke K. McLeod Memorial Scholarship to undertake this research. This research is funded by a Proof-of-Concept Intervention Grant in Primary Prevention of Cancer (Action Grant) of the Canadian Cancer Society and the Canadian Institutes of Health Research (CIHR)-Institute for Cancer Research (CCS grant #707233/CIHR-ICR grant #478510). This work was further supported by the CIHR Project Scheme Grant #426655 to Dr Litvinov, CIHR Catalyst Grant #428712, Merck Canada Inc Grant # MCA-23-167375 to Dr Litvinov and by the Fonds de la recherche du Québec – Santé (#296643).
Patient consent: Not applicable.
IRB approval status: This study received an exemption from the McGill University Research Ethics Board review.
References
- 1.Public Health Agency of Canada Melanoma skin cancer [internet] 2009. https://www.canada.ca/en/public-health/services/chronic-diseases/cancer/melanoma-skin-cancer.html
- 2.Arnold M., Singh D., Laversanne M., et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158(5):495–503. doi: 10.1001/jamadermatol.2022.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drucker A.M., Kleiner O., Manion R., et al. Top ten research priorities for psoriasis, atopic dermatitis and hidradenitis suppurativa: the SkIN Canada priority setting initiative. J Cutan Med Surg. 2023;27(2):133–139. doi: 10.1177/12034754231156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darwich R., Ghazawi F.M., Rahme E., et al. Retinoblastoma incidence trends in Canada: a national comprehensive population-based study. J Pediatr Ophthalmol Strabismus. 2019;56(2):124–130. doi: 10.3928/01913913-20190128-02. [DOI] [PubMed] [Google Scholar]
- 5.Litvinov I.V., Shtreis A., Kobayashi K., et al. Investigating potential exogenous tumor initiating and promoting factors for Cutaneous T-Cell Lymphomas (CTCL), a rare skin malignancy. Oncoimmunology. 2016;5(7) doi: 10.1080/2162402X.2016.1175799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghazawi F.M., Le M., Lagacé F., et al. Incidence, mortality, and spatiotemporal distribution of cutaneous malignant melanoma cases across Canada. J Cutan Med Surg. 2019;23(4):394–412. doi: 10.1177/1203475419852048. [DOI] [PubMed] [Google Scholar]
- 7.D'Orazio J., Jarrett S., Amaro-Ortiz A., Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sample A., He Y.Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol Photoimmunol Photomed. 2018;34(1):13–24. doi: 10.1111/phpp.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alli S., LeBeau J., Hasbani A., Lagacé F., Litvinov I.V., Peláez S. Understanding the perceived relationship between sun exposure and melanoma in Atlantic Canada: a consensual qualitative study highlighting a ‘sunscreen paradox’. Cancers. 2023;15(19):4726. doi: 10.3390/cancers15194726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conte S., Aldien A.S., Jetté S., et al. Skin cancer prevention across the G7, Australia and New Zealand: a review of legislation and guidelines. Curr Oncol. 2023;30(7):6019–6040. doi: 10.3390/curroncol30070450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conte S., Ghazawi F.M., Le M., et al. Population-based study detailing cutaneous melanoma incidence and mortality trends in Canada. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.830254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeremian R., Lytvyn Y., Fotovati R., et al. Distinct signatures of mitotic age acceleration in cutaneous melanoma and acquired melanocytic nevi. J Invest Dermatol. 2024 doi: 10.1016/j.jid.2024.01.012. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Jeremian R., Malinowski A., Lytvyn Y., et al. Skin photoaging following sun exposure is associated with decreased epigenetic and biological age and correlates with basal cell carcinoma phenotype. Br J Dermatol. 2023;22 doi: 10.1093/bjd/ljad527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeremian R., Xie P., Fotovati M., Lefrançois P., Litvinov I.V. Gene-environment analyses in a UK biobank skin cancer cohort identifies important SNPs in DNA repair genes that may help prognosticate disease risk. Cancer Epidemiol Biomarkers Prev. 2023;32(11):1599–1607. doi: 10.1158/1055-9965.EPI-23-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang E.R., Ghezelbash S., Xie P., Fotovati M., Litvinov I.V., Lefrançois P. Comparison of the basal cell carcinoma (BCC) tumour microenvironment to other solid malignancies. Cancers (Basel) 2023;15(1):305. doi: 10.3390/cancers15010305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee T.K., Brazier A.S.A., Shoveller J.A., Gallagher R.P. Sun-related behavior after a diagnosis of cutaneous malignant melanoma. Melanoma Res. 2007;17(1):51–55. doi: 10.1097/CMR.0b013e3280112b98. [DOI] [PubMed] [Google Scholar]
- 17.Skin cancer risk in Alberta on par with warmer countries | Folio [internet] https://www.ualberta.ca/folio/2017/06/skin-cancer-risk-in-alberta-on-par-with-warmer-countries.html
- 18.Banerjee S.C., Hay J.L., Geller A.C., Gagne J.J., Frazier A.L. Quitting the “Cancer Tube”: a qualitative examination of the process of indoor tanning cessation. Transl Behav Med. 2014;4(2):209–219. doi: 10.1007/s13142-014-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glanz K., Jordan A., Lazovich D., Bleakley A. Frequent indoor tanners' beliefs about indoor tanning and cessation. Am J Health Promot. 2019;33(2):293–299. doi: 10.1177/0890117118784235. [DOI] [PubMed] [Google Scholar]
- 20.Shoveller J., Lovato C., Peters L., Rivers J. Canadian national survey on sun exposure & protective behaviours: outdoor workers. Can J Public Health. 2000;91:34–35. doi: 10.1007/BF03404250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghazawi F.M., Cyr J., Darwich R., et al. Cutaneous malignant melanoma incidence and mortality trends in Canada: a comprehensive population-based study. J Am Acad Dermatol. 2019;80(2):448–459. doi: 10.1016/j.jaad.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 22.Berman-Rosa M., Logan J., Ghazawi F.M., et al. Analysis of geographic and environmental factors and their association with cutaneous melanoma incidence in Canada. Dermatology. 2022;238(6):1006–1017. doi: 10.1159/000524949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghazawi F.M., Le M., Alghazawi N., et al. Trends in incidence of cutaneous malignant melanoma in Canada: 1992-2010 versus 2011-2015. J Am Acad Dermatol. 2019;80(4):1157–1159. doi: 10.1016/j.jaad.2018.10.055. [DOI] [PubMed] [Google Scholar]
- 24.Muntyanu A., Nechaev V., Pastukhova E., et al. Risk factors and communities disproportionately affected by cervical cancer in the Russian Federation: a national population-based study. Lancet Reg Health Eur. 2022;20 doi: 10.1016/j.lanepe.2022.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghazawi F.M., Darwich R., Le M., et al. Incidence trends of conjunctival malignant melanoma in Canada. Br J Ophthalmol. 2020;104(1):23–25. doi: 10.1136/bjophthalmol-2019-313977. [DOI] [PubMed] [Google Scholar]
- 26.Lagacé F., Ghazawi F.M., Le M., et al. Analysis of incidence, mortality trends, and geographic distribution of breast cancer patients in Canada. Breast Cancer Res Treat. 2019;178(3):683–691. doi: 10.1007/s10549-019-05418-2. [DOI] [PubMed] [Google Scholar]
- 27.Government of Canada SC Canadian cancer registry (CCR) [internet] 2023. https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3207
- 28.Statistics Canada Census forward sortation area boundary file [internet] 2024. https://www150.statcan.gc.ca/n1/en/catalogue/92-179-X
- 29.The Canadian Urban Environmental Health Research Consortium (CANUE) [internet] https://canue.ca/
- 30.The R Foundation R. The R project for statistical computing [internet] 2019. https://www.r-project.org/
- 31.Lagacé F., Noorah B.N., Conte S., et al. Assessing skin cancer risk factors, sun safety behaviors and melanoma concern in Atlantic Canada: a comprehensive survey study. Cancers. 2023;15(15):3753. doi: 10.3390/cancers15153753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill D., White V., Marks R., Theobald T., Borland R., Roy C. Melanoma prevention: behavioral and nonbehavioral factors in sunburn among an Australian urban population. Prev Med. 1992;21(5):654–669. doi: 10.1016/0091-7435(92)90072-p. [DOI] [PubMed] [Google Scholar]
- 33.City of Toronto Vitamin D. 2017. https://www.toronto.ca/community-people/health-wellness-care/health-programs-advice/eating-well-with-canadas-food-guide/vitamin-d/
- 34.Greenberg M.L., Barr R.D., DiMonte B., McLaughlin E., Greenberg C. Childhood cancer registries in Ontario, Canada: lessons learned from a comparison of two registries. Int J Cancer. 2003;105:88–91. doi: 10.1002/ijc.11004. [DOI] [PubMed] [Google Scholar]
- 35.Gupta S., Pole J.D. The validity of pediatric cancer diagnoses in a population-based general cancer registry in Ontario, Canada. BMC Cancer. 2016;16:885. doi: 10.1186/s12885-016-2931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall S., Schulze K., Groome P., Mackillop W., Holowaty E. Using cancer registry data for survival studies: the example of the Ontario Cancer Registry. J Clin Epidemiol. 2006;59:67–76. doi: 10.1016/j.jclinepi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Barnes P.W., Williamson C.E., Lucas R.M., et al. Ozone depletion, ultraviolet radiation, climate change and prospects for a sustainable future. Nat Sustain. 2019;2(7):569–579. [Google Scholar]