Abstract
Background
Running has been widely recognized as a beneficial activity for improving physical fitness, but it can also increase the risk of running-related injuries (RRIs). This study aims to assess the impact of long-term running on the structural and biochemical composition of the knee.
Methods
This study recruited a total of 32 participants, including 16 male recreational runners, aged 28–49 years, with a running experience of 2–7 years, and 16 matched sedentary controls. Magnetic resonance (MR) scans of T2* mapping and three-dimensional double-echo steady-state (3D-DESS) were performed on all participants. The volumes, thickness, and T2* values of joint articular cartilage were obtained via automatic segmentation software.
Results
Compared with the sedentary controls, runners exhibited significant increases in the volumes of both the femoral medial articular cartilage and the tibial medial articular cartilage. Additionally, there were significant increases in the thickness of several cartilage regions, including femoral medial cartilage, femoral medial articular cartilage, femoral medial thickness, femoral lateral cartilage, and tibial medial articular cartilage. Notably, the T2* values in the femoral lateral and tibial lateral cartilage of runners decreased significantly, while those in the patellar cartilage and medial tibial cartilage increased significantly. Runner pace was negatively correlated with the overall knee cartilage thickness (r=−0.556; P=0.02), femoral cartilage thickness (r=−0.533; P=0.03), and volume (r=−0.532; P=0.03) but positively correlated with the T2* value of the patellar cartilage (r=0.577; P=0.01).
Conclusions
Our study suggests that long-term mechanical stress from running may lead to increased thickness and volume in certain knee joint cartilage regions, possibly enhancing the functional adaptability of knee cartilage. The varying changes in T2* value in the tibial and fibular cartilage areas may indicate differing adaptability to pressure.
Keywords: Running, magnetic resonance imaging (MRI), cartilage, knee
Introduction
Running is currently one of the most popular sports and offers a wide range of health benefits. It has been shown to reduce the risk of various chronic diseases, including obesity, metabolic syndrome, diabetes, and cancer (1). Furthermore, running contributes to increased and sustained bone mineral content and bone density (2). Although running can be a cost-effective “good medicine” as it relates to improving health and extending life, running can also increase the risk of running-related injuries (RRIs).
Osteoarthritis (OA) is a disabling, chronic musculoskeletal disease with high prevalence in adults (3). Research suggests that early-stage OA may be a reversible process, with one of its defining features being cartilage degeneration (4). Quantitative magnetic resonance (MR) technology T2* mapping is one of the methods for the early identification of cartilage lesions (5). The T2* value can reflect and detect changes in water content and collagen fiber anisotropy in cartilage. The use of the three-dimensional double-echo steady-state (3D-DESS) sequence enhances the resolution for visualizing articular cartilage and offers a superior contrast between cartilage and joint synovial fluid, facilitating a more detailed morphological analysis of articular cartilage (6). MR Chondral Health application (Siemens Healthineers, Erlangen, Germany) is believed to provide accurate and reproducible automatic segmentation of knee joint cartilage and has been used in multiple studies (7-9).
Running is one of the most popular sports in the world. Nearly 50 million people in the United States participated in running and jogging in 2021 (https://www.statista.com/topics/1743/running-and-jogging/#topicOverview). In addition, China’s “2022 National Running Sports Health Report” shows that the number of active runners in China every month also exceeds 50,000 (https://health.gmw.cn/2022-12/15/content_36237123.htm). However, there is currently a lack of comprehensive comparative analyses of articular cartilage among this population of recreational runners. A recent meta-analysis (10) showed that the incidence of OA is 3.5% among recreational runners, 10.2% among sedentary individuals, and 13.3% among competitive runners. These findings suggest that recreational runners are at a lower risk of developing knee OA when compared to both sedentary individuals and competitive runners. Therefore, it is necessary to assess the state of knee joint cartilage among current recreational runners. This study used MR technology and automatic cartilage segmentation software to analyze the differences in cartilage morphology and biochemical composition between recreational runners and sedentary individuals. We considered two different results based on the joint load effect: (I) long-term regular running may reduce the T2* value of articular cartilage and increase the surface thickness and volume of cartilage; (II) conversely, long-term regular running may increase the T2* value of cartilage, and reduce the surface thickness and volume of cartilage. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1563/rc).
Methods
Study participants
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of The Affiliated Hospital of Hangzhou Normal University (No. 2020[E2]-HS-011). Informed consent was obtained from all individual participants. A total of 16 recreational runners were recruited from the Zhejiang University Entrepreneurs Outdoor Association, and 16 matched sedentary individuals were recruited from the community. Demographic and running related information were obtained from a questionnaire filled by the participants, including name, age, sex, height, weight, running experience, running days per week, daily running distance, monthly running distance, and running pace. Running pace is considered to be the amount of time it takes to cover a certain distance and is typically measured in minutes per mile or minutes per kilometer. Therefore, a smaller running pace value indicates a faster running speed, as it means the runner covers the distance in less time. To eliminate potential variations in cartilage data due to gender differences, all participants were male (11,12).
The inclusion criteria of the recreational runners were as follows: (I) no history of knee trauma or surgery, (II) consistent running experience spanning over 2 years, (III) a running distance of more than 80 km per month, and (IV) two or more regular weekly running sessions.
The inclusion criteria of sedentary controls were as follows: (I) no history of knee trauma or surgery, (II) no strong interest in sports, (III) daily work requiring long periods of sitting, and (IV) performing less than 150 minutes of moderate-intensity physical activity per week.
The exclusion criteria were as follows: (I) individuals with existing injuries or experiencing pain (greater than 3 on the visual analogue scale), (II) individuals with a history of knee trauma or surgery, and (III) individuals with contraindications to undergoing magnetic resonance imaging (MRI) scans.
MRI procedures
All participants were scanned in the supine position. The knee joint was bent naturally within a coil fixed with sandbags to reduce motion artifacts. The maximum flexion angle for the knee joint was limited to 15°. Additionally, all scans were conducted in the evening (7:00 PM to 10:00 PM) to eliminate potential influences from diurnal variations and daily physical activities on the assessment of knee cartilage. All participants were instructed not to perform any form of exercise for at least 3 days before the MRI scan. Furthermore, to minimize the potential impact of recent physical activities on knee cartilage, they were asked to lie down and rest for an additional 30 minutes prior to the scan. All participants worked during the day and rested at night.
MRI was performed using a 1.5 T MR scanner (MAGNETOM Aera, Siemens Healthineers, Erlangen, Germany) equipped with a 15-channel knee coil. The automated day optimizing throughput (DOT) engine was used to customize the MRI examination for each participants, ensuring as consistent positioning as feasible for each scan. The scanning sequence include (I) a sagittal T1-weighted sequence (T1W) [repetition time (TR) =700.00 ms, time to echo (TE) =12.00 ms, flip angle =150°, field of view (FOV) =160.00 mm × 160.00 mm, slice thickness =3.00 mm, gap =0.60 mm, bandwidth =150 Hz/Px, scan time =1 min 27 s], (II) a sagittal proton density-weighted fat-saturated turbo spin echo sequence (PDw FS TSE) (TR =3,300.00 ms, TE =38.00 ms, FOV =160.00 mm × 160.00 mm, slice thickness =3.00 mm, gap =0.60 mm, bandwidth =193 Hz/Px, scan time =1 min 23 s), (III) a 3D-DESS sequence (TR =19.88 ms, TE =7.34 ms, FOV =160.00 mm × 160.00 mm, slice thickness =0.64 mm, gap =0.12 mm, bandwidth =199 Hz/Px, scan time =6 min), and (IV) sagittal T2* mapping (TR =905.00 ms, TE =4.35/11.57/18.89/26.21/33.53 ms, FOV =160.00 mm × 160.00 mm, slice thickness =3.00 mm, gap =0.60 mm, bandwidth =260 Hz/Px, and scan time =2 min 18 s). The total scan time was 11 min and 8 s.
Image analysis
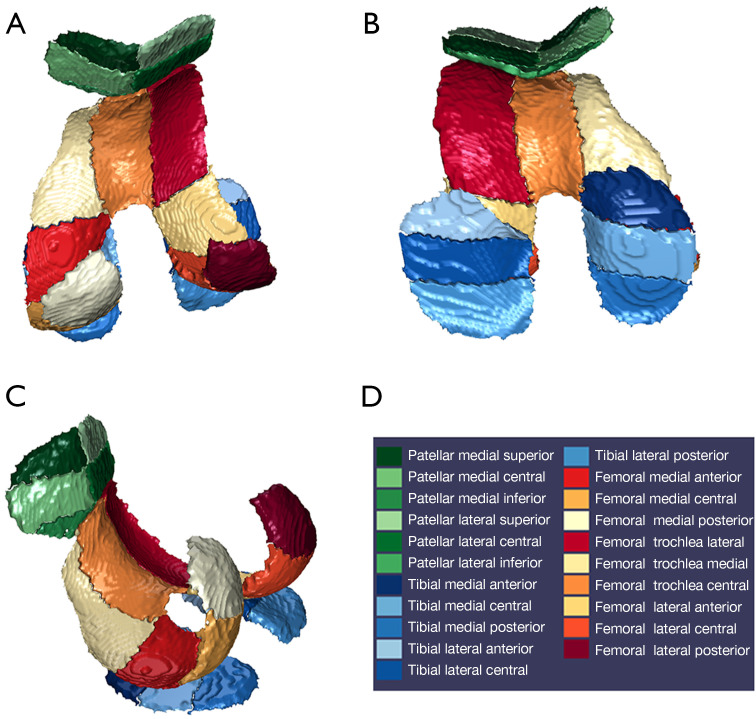
Based on 3D-DESS and T2* mapping images, we used automated segmentation software (Siemens Chondral Health 3.1.0, Siemens Healthineers, Erlangen, Germany) to segment the knee joint cartilage into 21 subregions. The software automatically computed the volume, thickness, and T2* value of each of these cartilage areas. Following the completion of the automatic cartilage segmentation process, necessary manual adjustments were made to avoid synovial fluid from affecting the accuracy of the results (see Table 1, Figure 1). The postprocessing of images was completed by a physician with over 5 years of expertise in musculoskeletal imaging diagnosis. Notably, the doctor did not know the specific grouping of the participants during the postprocessing phase.
Table 1. Twenty-one subregions of the automatic segmentation of cartilage.
| Variables | Subregion |
|---|---|
| Femur | Femoral medial posterior |
| Femoral trochlear medial | |
| Femoral lateral posterior | |
| Femoral medial central | |
| Femoral trochlear central | |
| Femoral lateral central | |
| Femoral medial anterior | |
| Femoral trochlear lateral | |
| Femoral lateral anterior | |
| Patella | Patellar lateral inferior |
| Patellar medial inferior | |
| Patellar lateral central | |
| Patellar medial central | |
| Patellar lateral superior | |
| Patellar medial superior | |
| Tibia | Tibial lateral posterior |
| Tibial medial posterior | |
| Tibial lateral central | |
| Tibial medial central | |
| Tibial lateral anterior | |
| Tibial medial anterior |
Figure 1.
Cartilage automatically segmented using MR Chondral Health: (A) cranial view; (B) caudal view; (C) side view; (D) the color codes of the 21 subregions. MR, magnetic resonance.
Statistical analysis
SPSS 23.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Measurement data are presented as the mean ± standard deviation (SD). The independent samples t-test was used to compare the knee joint cartilage thickness, volume, and T2* value between the running group and the control group. Pearson correlation coefficient was used to evaluate the correlation between daily running conditions and the average thickness, volume, and T2* value of knee cartilage. Statistical significance was set at P<0.05.
Results
All participants (16 recreational runners and 16 sedentary controls) completed the MRI examinations. The demographic data of all participants are detailed in Table 2.
Table 2. The characteristics of the participants [mean ± SD (range)].
| Variables | Recreational runners (N=16) | Sedentary controls (N=16) | F | P value |
|---|---|---|---|---|
| Age (years) | 37.19±6.89 (28–49) | 35.94±6.01 (28–49) | 0.254 | 0.61 |
| Height (cm) | 170.25±4.55 (160–179) | 169.44±6.54 (156–182) | 0.92 | 0.34 |
| Weight (kg) | 65.13±5.85 (56–75) | 67.31±7.37 (55–83) | 0.648 | 0.42 |
| BMI (kg/m2) | 22.49±2.02 (18.73–25.35) | 23.44±2.11 (20.06–28.72) | 0.218 | 0.64 |
| Running experience (years) | 3.50±1.28 (2–7) | – | – | – |
| Running days per week (days) | 4.16±1.23 (2–6) | – | – | – |
| Daily running distance (km) | 8.94±2.43 (4–14) | – | – | – |
| Monthly running distance (km) | 165.00±65.80 (80–315) | – | – | – |
| Pace (min/km) | 5.25±0.54 (4.15–6.00) | – | – | – |
SD, standard deviation; BMI, body mass index.
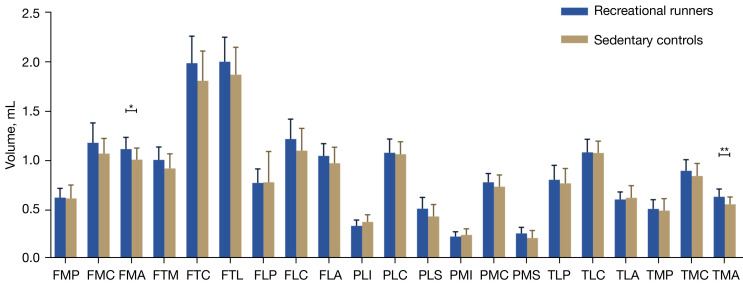
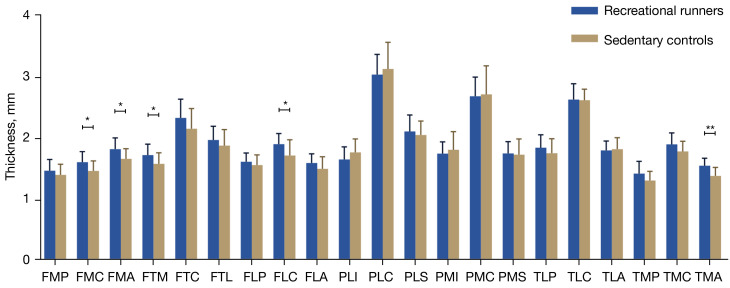
Compared with the sedentary controls, the runners exhibited significant increases in the volumes of both the femoral medial anterior (FMA) (10.6%) and tibial medial anterior (TMA) (13.6%). Additionally, there were significant increases in the thickness of several cartilage regions, including the femoral medial central (FMC) (9.5%), FMA (9.6%), femoral trochlear medial (FTM) (8.9%), femoral lateral central (FLC) (10.8%), and TMA (12.0%). No significant differences were found between the two groups in the volume or thickness of the remaining areas (Tables 3,4; Figures 2,3).
Table 3. Comparison of knee cartilage subregion volume between recreational runners and sedentary controls (mean ± SD).
| Group | Recreational runners (mL) | Sedentary controls (mL) | t value | P value |
|---|---|---|---|---|
| Femoral medial posterior | 1.22±0.20 | 1.10±0.23 | 0.211 | 0.83 |
| Femoral medial central | 1.05±0.12 | 0.98±0.16 | 1.726 | 0.09 |
| Femoral medial anterior | 0.34±0.06 | 0.38±0.07 | 2.579 | 0.01 |
| Femoral trochlear medial | 1.08±0.14 | 1.07±0.13 | 1.725 | 0.09 |
| Femoral trochlear central | 0.52±0.12 | 0.44±0.12 | 1.754 | 0.09 |
| Femoral trochlear lateral | 0.23±0.05 | 0.25±0.06 | 1.416 | 0.16 |
| Femoral lateral posterior | 0.78±0.09 | 0.74±0.12 | −0.073 | 0.94 |
| Femoral lateral central | 0.27±0.06 | 0.22±0.07 | 1.581 | 0.12 |
| Femoral lateral anterior | 0.81±0.15 | 0.77±0.15 | 1.446 | 0.15 |
| Patellar lateral inferior | 1.09±0.13 | 1.08±0.12 | −1.769 | 0.08 |
| Patellar lateral central | 0.61±0.08 | 0.63±0.12 | 0.304 | 0.76 |
| Patellar lateral superior | 0.51±0.09 | 0.50±0.12 | 1.851 | 0.07 |
| Patellar medial inferior | 0.90±0.11 | 0.85±0.13 | −0.913 | 0.36 |
| Patellar medial central | 0.64±0.08 | 0.56±0.08 | 1.149 | 0.26 |
| Patellar medial superior | 1.22±0.20 | 1.10±0.23 | 1.88 | 0.07 |
| Tibial lateral posterior | 1.05±0.12 | 0.98±0.16 | 0.704 | 0.48 |
| Tibial lateral central | 0.34±0.06 | 0.38±0.07 | 0.14 | 0.89 |
| Tibial lateral anterior | 1.08±0.14 | 1.07±0.13 | −0.453 | 0.65 |
| Tibial medial posterior | 0.52±0.12 | 0.44±0.12 | 0.442 | 0.66 |
| Tibial medial central | 0.23±0.05 | 0.25±0.06 | 1.24 | 0.22 |
| Tibial medial anterior | 0.78±0.09 | 0.74±0.12 | 2.787 | 0.009 |
SD, standard deviation.
Table 4. Comparison of knee cartilage subregion thickness between recreational runners and sedentary controls (mean ± SD).
| Group | Recreational runners (mm) | Sedentary controls (mm) | t value | P value |
|---|---|---|---|---|
| Femoral medial posterior | 1.47±0.18 | 1.41±0.18 | 1.076 | 0.29 |
| Femoral medial central | 1.61±0.17 | 1.47±0.16 | 2.362 | 0.02 |
| Femoral medial anterior | 1.83±0.18 | 1.67±0.17 | 2.593 | 0.01 |
| Femoral trochlear medial | 1.73±0.18 | 1.59±0.18 | 2.219 | 0.03 |
| Femoral trochlear central | 2.34±0.30 | 2.16±0.33 | 1.561 | 0.12 |
| Femoral trochlear lateral | 1.98±0.22 | 1.89±0.26 | 1.052 | 0.30 |
| Femoral lateral posterior | 1.62±0.14 | 1.57±0.17 | 0.937 | 0.35 |
| Femoral lateral central | 1.91±0.17 | 1.72±0.26 | 2.397 | 0.02 |
| Femoral lateral anterior | 1.60±0.15 | 1.50±0.20 | 1.562 | 0.12 |
| Patellar lateral inferior | 1.66±0.20 | 1.77±0.22 | −1.531 | 0.13 |
| Patellar lateral central | 3.04±0.33 | 3.13±0.43 | −0.678 | 0.50 |
| Patellar lateral superior | 2.11±0.27 | 2.06±0.23 | 0.639 | 0.52 |
| Patellar medial inferior | 1.75±0.19 | 1.82±0.30 | −0.713 | 0.48 |
| Patellar medial central | 2.69±0.31 | 2.72±0.47 | −0.205 | 0.83 |
| Patellar medial superior | 1.76±0.19 | 1.74±0.26 | 0.271 | 0.78 |
| Tibial lateral posterior | 1.85±0.21 | 1.76±0.24 | 1.175 | 0.24 |
| Tibial lateral central | 2.63±0.26 | 2.62±0.18 | 0.12 | 0.90 |
| Tibial lateral anterior | 1.81±0.15 | 1.83±0.19 | −0.311 | 0.75 |
| Tibial medial posterior | 1.43±0.20 | 1.32±0.15 | 1.763 | 0.08 |
| Tibial medial central | 1.91±0.19 | 1.79±0.16 | 1.833 | 0.07 |
| Tibial medial anterior | 1.56±0.12 | 1.39±0.14 | 3.564 | 0.001 |
SD, standard deviation.
Figure 2.
Comparison of knee cartilage volumes in 21 subregions between recreational runners and sedentary participants. *, P<0.05; **, P<0.01. FMP, femoral medial posterior; FMC, femoral medial central; FMA, femoral medial anterior; FTM, femoral trochlear medial; FTC, femoral trochlear central; FTL, femoral trochlear lateral; FLP, femoral lateral posterior; FLC, femoral lateral central; FLA, femoral lateral anterior; PLI, patellar lateral inferior; PLC, patellar lateral central; PLS, patellar lateral superior; PMI, patellar medial inferior; PMC, patellar medial central; PMS, patellar medial superior; TLP, tibial lateral posterior; TLC, tibial lateral central; TLA, tibial lateral anterior; TMP, tibial medial posterior; TMC, tibial medial central; TMA, tibial medial anterior.
Figure 3.
Comparison of knee cartilage thickness in the 21 subregions between the recreational runners and sedentary participants. *, P<0.05; **, P<0.01. FMP, femoral medial posterior; FMC, femoral medial central; FMA, femoral medial anterior; FTM, femoral trochlear medial; FTC, femoral trochlear central; FTL, femoral trochlear lateral; FLP, femoral lateral posterior; FLC, femoral lateral central; FLA, femoral lateral anterior; PLI, patellar lateral inferior; PLC, patellar lateral central; PLS, patellar lateral superior; PMI, patellar medial inferior; PMC, patellar medial central; PMS, patellar medial superior; TLP, tibial lateral posterior; TLC, tibial lateral central; TLA, tibial lateral anterior; TMP, tibial medial posterior; TMC, tibial medial central; TMA, tibial medial anterior.
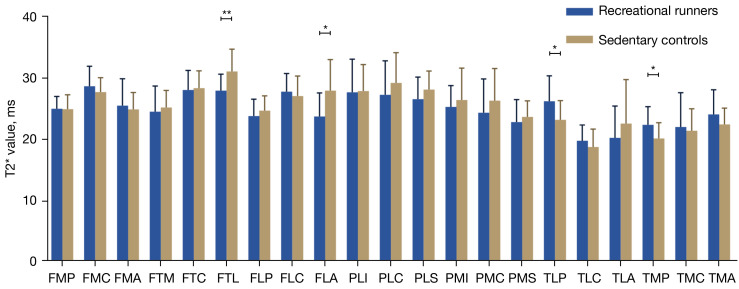
However, there were two differences in the T2* values of the knee cartilage among runners compared to matched sedentary people. The T2* value of runners’ knee cartilage was significantly lower than that of sedentary individuals in the femoral trochlear lateral (FTL) (−10.0%) and femoral lateral anterior (FLA) (−15.0%). Conversely, the T2* values of runners were significantly higher than those of the control group in the tibial lateral posterior (TLP) (13.0%) and tibial medial posterior (TMP) (11.2%). No significant differences were found between the two groups for the T2* values in the other areas (Table 5, Figure 4).
Table 5. Comparison of knee cartilage subregion T2* value between recreational runners and sedentary controls (mean ± SD).
| Group | Recreational runners (ms) | Sedentary controls (ms) | Y value | P value |
|---|---|---|---|---|
| Femoral medial posterior | 25.00±2.02 | 24.95±2.34 | 0.07 | 0.94 |
| Femoral medial central | 28.67±3.25 | 27.71±2.34 | 0.966 | 0.34 |
| Femoral medial anterior | 25.51±4.39 | 24.90±2.74 | 0.469 | 0.64 |
| Femoral trochlear medial | 24.54±4.18 | 25.21±2.80 | −0.532 | 0.59 |
| Femoral trochlear central | 28.06±3.20 | 28.36±2.82 | −0.283 | 0.77 |
| Femoral trochlear lateral | 27.98±2.68 | 31.09±3.62 | −2.766 | 0.01 |
| Femoral lateral posterior | 23.84±2.73 | 24.72±2.40 | −0.967 | 0.34 |
| Femoral lateral central | 27.78±2.95 | 27.07±3.27 | 0.647 | 0.52 |
| Femoral lateral anterior | 23.77±3.80 | 27.98±5.02 | −2.669 | 0.01 |
| Patellar lateral inferior | 27.68±5.39 | 27.88±4.32 | −0.114 | 0.91 |
| Patellar lateral central | 27.29±5.53 | 29.20±4.91 | −1.033 | 0.31 |
| Patellar lateral superior | 26.58±3.59 | 28.13±3.01 | −1.325 | 0.19 |
| Patellar medial inferior | 25.30±3.48 | 26.45±5.18 | −0.736 | 0.46 |
| Patellar medial central | 24.36±5.51 | 26.33±5.21 | −1.04 | 0.30 |
| Patellar medial superior | 22.84±3.67 | 23.68±2.61 | −0.747 | 0.46 |
| Tibial lateral posterior | 26.24±4.12 | 23.23±3.12 | 2.335 | 0.02 |
| Tibial lateral central | 19.79±2.56 | 18.80±2.91 | 1.028 | 0.31 |
| Tibial lateral anterior | 20.28±5.17 | 22.61±7.16 | −1.053 | 0.30 |
| Tibial medial posterior | 22.41±2.94 | 20.15±2.60 | 2.301 | 0.02 |
| Tibial medial central | 22.04±5.60 | 21.41±3.60 | 0.375 | 0.71 |
| Tibial medial anterior | 24.09±4.00 | 22.47±2.64 | 1.353 | 0.18 |
SD, standard deviation.
Figure 4.
Comparison of knee cartilage T2* values in 21 subregions between recreational runners and sedentary participants. *, P<0.05; **, P<0.01. FMP, femoral medial posterior; FMC, femoral medial central; FMA, femoral medial anterior; FTM, femoral trochlear medial; FTC, femoral trochlear central; FTL, femoral trochlear lateral; FLP, femoral lateral posterior; FLC, femoral lateral central; FLA, femoral lateral anterior; PLI, patellar lateral inferior; PLC, patellar lateral central; PLS, patellar lateral superior; PMI, patellar medial inferior; PMC, patellar medial central; PMS, patellar medial superior; TLP, tibial lateral posterior; TLC, tibial lateral central; TLA, tibial lateral anterior; TMP, tibial medial posterior; TMC, tibial medial central; TMA, tibial medial anterior.
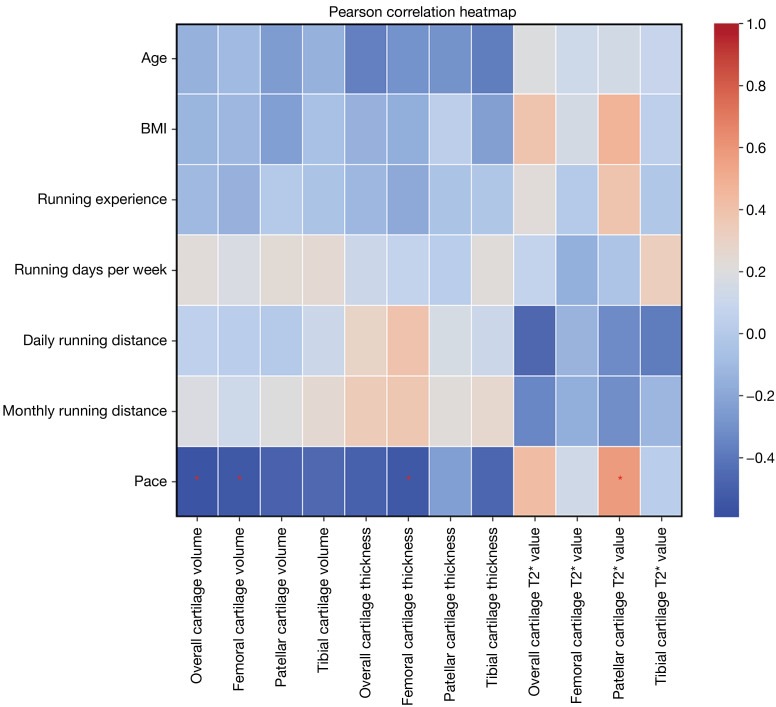
In addition, the running pace was negatively correlated with overall knee cartilage thickness (r=−0.556; P=0.02), femoral cartilage thickness (r=−0.533; P=0.03), and volume (r=−0.532; P=0.03) but positively correlated with the T2* value of the patellar cartilage (r=0.577; P=0.01) The smaller the pace number is, the faster the pace. Running experience, running days per week, daily running distance, and monthly running distance had no significant correlation with knee joint cartilage thickness, volume, or T2* value (Figure 5).
Figure 5.
The heatmap of correlation matrix for running dataset. In this heatmap, warm colors represent positive correlations, while cool colors represent negative correlations. The chart has been truncated to display essential data. *, P<0.05. BMI, body mass index.
Discussion
This study discovered significant differences in knee cartilage between recreational runners and sedentary individuals. Long-term running may be associated with increased thickness and volume in certain cartilage regions as well as alterations in the biochemical composition. Furthermore, these changes seemed to be more pronounced in faster runners.
We observed a significant increase in the thickness and volume of knee joint cartilage among recreational runners compared to sedentary individuals. This finding is consistent with previous research (13-15). Babayeva et al. (13) found that femoral cartilage thickness was greater in elite athletes than in sedentary individuals, with physical activity level (PA) identified as an independent factor influencing cartilage thickness. Moshtagh et al. (16) indicated that moderate-intensity running could lead to the increase and hypertrophy of chondrocytes in rats. Petrigna et al. (17) reported that running can intervene in the progression of OA and consolidate articular cartilage tissue. Liu et al. (9) found that daily training for up to 1 year could increase the volume of knee cartilage in college students, although it did not affect cartilage thickness. In contrast, the immediate changes in cartilage thickness and volume following a single running are reduced or unchanged (12,18,19) and often return to baseline within 1–24 hours (20,21). Although changes in cartilage thickness and volume caused by a single run are reversible, long-term running may enhance knee joint functional adaptability by increasing cartilage thickness and volume.
T2* mapping is a valuable imaging technique that not only captures variations in collagen fiber anisotropy and water content within cartilage but also provides additional information into local field inhomogeneities. This additional capability makes it potentially more sensitive to detecting changes in tissue composition (22). Tsai et al. (22) demonstrated the feasibility of using 1.5 T MR T2* measurements to detect early changes in cartilage degeneration. As individuals age or OA progresses, there are alterations in the collagen network, an increase in water content within the joint cartilage, and an associated rise in the T2* value. Recent research suggests that changes in the knee cartilage composition caused by a single run are mild and transient (23). It is safe for knee cartilage to repeatedly withstand the mechanical stimulation of running after a certain period of rest. Furthermore, after running, the acute change in T2* value of cartilage may be related to the running distance (8,24). The T2* value of cartilage tends to decrease after short-distance running, increase after long-distance running, and return to baseline levels within a few months (25). Schütz et al. (24) found that the T2* value of knee joint cartilage in runners of a 4,486 km transcontinental multistage ultra-marathon increased significantly within the first 1,100 km, but a decreasing trend in cartilage T2* value was observed after 3,500 km of running. In addition, they found that the change in T2* value of femoral cartilage after running was significantly higher than that of tibial cartilage, which may be related to joint load and glycosaminoglycan content of cartilage. In our study, we observed that the T2* values in the femoral and tibial cartilage areas of the running group exhibited contrasting tendencies when compared to the control group. We hypothesize that these differences may be attributed to the distinct abilities of various cartilage regions to respond to mechanical loading. However, this still needs to be substantiated by mechanical experiments. In addition, our findings indicated that areas with variations in cartilage thickness and volume did not align with areas displaying differences in T2* values. This suggests that changes in cartilage composition do not always correlate with changes in cartilage thickness (26) and vice versa.
Furthermore, our findings indicate that the smaller a runner’s pace value is (the faster running speed), the greater thickness and volume of the cartilage and the lower the local T2* values. Running experience, running days per week, daily running distance, and monthly running distance were not significantly correlated with the thickness, volume, or T2* value of knee joint cartilage. This suggests that within a certain range, running at a faster pace may be more beneficial to cartilage health. While the pace range within our runner’s group was limited (4.15–6.00 min/km), we still believe that this trend may follow an inverted U-shaped curve, with health benefits suddenly decreasing when running faster than a certain value (27). McAlindon et al. (28) found that high-intensity physical activity is an important risk factor for knee OA in older adults. A meta-analysis by Bricca et al. (26) concluded that moderate exercise can facilitate the nourishment of cells within the cartilage matrix by the synovial fluid, which increases cartilage thickness. On the other hand, high-intensity exercise may overload the joint, leading to pathological changes. Further research could investigate the differences in cartilage morphology and T2* values between professional and recreational runners.
Moreover, it is critical to examine the correlation between long-term running and knee cartilage health. Both animal experiments and human experiments suggest that regular moderate exercise does not increase the risk of knee OA and can maintain the metabolic homeostasis of cartilage tissue (17,29). Our study evaluated differences in knee cartilage between recreational runners and healthy sedentary individuals. Similar to previous studies (23,30), we believe that running can be used as one of the preventive measures for cartilage lesions.
Our study also has certain limitations that should be mentioned. First, this study employed a cross-sectional design, and thus it is difficult to determine the causal relationship between running and cartilage health. Further longitudinal research, such as that involving the long-term (10–20 years) tracking of runners’ knee cartilage health, is needed. Second, our study analyzed a small sample size, and only male runners were included. Therefore, the findings of this study may not be directly applicable to female runners. To add further credibility to our findings, we plan to increase the sample size and include female runners in the future.
Conclusions
We found that in certain regions, the knee cartilage thickness and volume of mass in recreational runners are greater than those of sedentary individuals. This could be attributed to the long-term mechanical stress associated with running, which appears to stimulate cartilage metabolism and enhance the functional adaptability of knee joint cartilage. Compared with sedentary individuals, recreational runners had lower T2* values in the femoral cartilage area and higher T2* values in the tibial cartilage area. These differences might be linked to the varying adaptability of different cartilage regions to mechanical stress. Furthermore, changes in cartilage composition do not necessarily cause changes in cartilage thickness. Within a certain range, running at a faster pace appears to be associated with better knee joint health.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank the members of the Zhejiang University Entrepreneurs Outdoor Association (Zhejiang, China) for participating in this project.
Funding: This work was supported by the Basic Public Welfare Research Program of Zhejiang Province (No. GF21H180014), the Medical and Health Science & Technology Project of Hangzhou City (Nos. A20200360 and 20211231Y029), the Hangzhou Biomedicine and Health Industry Development Support Special Program (No. 2021WJCY053), the Zhejiang Medical and Health Technology Project (No. 2022KY962), and the Key Medical Disciplines of Hangzhou (No. YDYX).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of The Affiliated Hospital of Hangzhou Normal University (No. 2020[E2]-HS-011). Informed consent was obtained from all individual participants.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1563/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1563/coif). T.C.C. is an employee of Siemens Healthineers and provided technical MRI guidance for this paper. The other authors have no conflicts of interest to declare.
References
- 1.Ceyssens L, Vanelderen R, Barton C, Malliaras P, Dingenen B. Biomechanical Risk Factors Associated with Running-Related Injuries: A Systematic Review. Sports Med 2019;49:1095-115. 10.1007/s40279-019-01110-z [DOI] [PubMed] [Google Scholar]
- 2.Gao J, Fang J, Gong H, Gao B. Morphological and Microstructural Alterations of the Articular Cartilage and Bones during Treadmill Exercises with Different Additional Weight-Bearing Levels. J Healthc Eng 2017;2017:8696921. 10.1155/2017/8696921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amoako AO, Pujalte GG. Osteoarthritis in young, active, and athletic individuals. Clin Med Insights Arthritis Musculoskelet Disord 2014;7:27-32. 10.4137/CMAMD.S14386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu CR, Williams AA, Coyle CH, Bowers ME. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther 2012;14:212. 10.1186/ar3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maas KJ, Warncke M, Behzadi C, Welsch GH, Schoen G, Kaul MG, Adam G, Bannas P, Henes FO. Correlation of T2* relaxation times of the retropatellar cartilage with tibial tuberosity-trochlea groove distance in professional soccer players. Sci Rep 2020;10:15355. 10.1038/s41598-020-72299-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyu J, Zhang Y, Zhu W, Li D, Lin W, Chen K, Xia J. Correlation between the subchondral bone marrow lesions and cartilage repair tissue after matrix-associated autologous chondrocyte implantation in the knee: a cross-sectional study. Acta Radiol 2021;62:1072-9. 10.1177/0284185120969955 [DOI] [PubMed] [Google Scholar]
- 7.Juras V, Szomolanyi P, Janáčová V, Trattnig S. Initial Experience with Automatic Knee Cartilage Segmentation using MR Chondral Health. MAGNETOM Flash 2021;79:15-22. [Google Scholar]
- 8.Zhang P, Yu B, Zhang R, Chen X, Shao S, Zeng Y, Cui J, Zhao J. Longitudinal study of the morphological and T2* changes of knee cartilages of marathon runners using prototype software for automatic cartilage segmentation. Br J Radiol 2021;94:20200833. 10.1259/bjr.20200833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Liu H, Zhen Z, Zheng Y, Zhou X, Raithel E, Du J, Hu Y, Chen W, Hu X. Analysis of Knee Joint Injury Caused by Physical Training of Freshmen Students Based on 3T MRI and Automatic Cartilage Segmentation Technology: A Prospective Study. Front Endocrinol (Lausanne) 2022;13:839112. 10.3389/fendo.2022.839112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alentorn-Geli E, Samuelsson K, Musahl V, Green CL, Bhandari M, Karlsson J. The Association of Recreational and Competitive Running With Hip and Knee Osteoarthritis: A Systematic Review and Meta-analysis. J Orthop Sports Phys Ther 2017;47:373-90. 10.2519/jospt.2017.7137 [DOI] [PubMed] [Google Scholar]
- 11.Faber SC, Eckstein F, Lukasz S, Mühlbauer R, Hohe J, Englmeier KH, Reiser M. Gender differences in knee joint cartilage thickness, volume and articular surface areas: assessment with quantitative three-dimensional MR imaging. Skeletal Radiol 2001;30:144-50. 10.1007/s002560000320 [DOI] [PubMed] [Google Scholar]
- 12.Brenneman Wilson EC, Gatti AA, Maly MR. A new technique to evaluate the impact of running on knee cartilage deformation by region. MAGMA 2021;34:593-603. 10.1007/s10334-020-00896-8 [DOI] [PubMed] [Google Scholar]
- 13.Babayeva N, Dönmez G, Özçakar L, Torgutalp ŞŞ, Karaçoban L, Gedik E, Korkusuz F, Doral MN. Mean femoral cartilage thickness is higher in athletes as compared with sedentary individuals. Knee Surg Sports Traumatol Arthrosc 2021;29:1206-14. 10.1007/s00167-020-06146-7 [DOI] [PubMed] [Google Scholar]
- 14.Abdelghani KB, Slouma M, Souabni L, Kassab S, Chekili S, Laatar A, Zakraoui L. AB0988 effect of football on knee cartilage thickness: an ultrasonographic assessment. Ann Rheum Dis 2014;73:1127. 10.1136/annrheumdis-2014-eular.5362 [DOI] [Google Scholar]
- 15.Mühlbauer R, Lukasz TS, Faber TS, Stammberger T, Eckstein F. Comparison of knee joint cartilage thickness in triathletes and physically inactive volunteers based on magnetic resonance imaging and three-dimensional analysis. Am J Sports Med 2000;28:541-6. 10.1177/03635465000280041601 [DOI] [PubMed] [Google Scholar]
- 16.Moshtagh PR, Korthagen NM, Plomp SG, Pouran B, Sanchez C, Henrotin Y, Zadpoor A, Weinans H. Effect of moderate increasing exercise on the mechanical balance of the knee joint in young rats. Osteoarthritis Cartilage 2017;25:S66-7. 10.1016/j.joca.2017.02.120 [DOI] [Google Scholar]
- 17.Petrigna L, Roggio F, Trovato B, Zanghì M, Guglielmino C, Musumeci G. How Physical Activity Affects Knee Cartilage and a Standard Intervention Procedure for an Exercise Program: A Systematic Review. Healthcare (Basel) 2022;10:1821. 10.3390/healthcare10101821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan MCM, O'Donovan J, Charlton JM, Roy JS, Hunt MA, Esculier JF. The Influence of Running on Lower Limb Cartilage: A Systematic Review and Meta-analysis. Sports Med 2022;52:55-74. 10.1007/s40279-021-01533-7 [DOI] [PubMed] [Google Scholar]
- 19.Bratke G, Bruggemann GP, Willwacher S, Mählich D, Trudeau MB, Rohr E, Weir G, Maintz D, Hamill J. Does footwear affect articular cartilage volume change after a prolonged run? Scand J Med Sci Sports 2020;30:332-8. 10.1111/sms.13576 [DOI] [PubMed] [Google Scholar]
- 20.Heckelman LN, Riofrio AD, Vinson EN, Collins AT, Gwynn OR, Utturkar GM, Goode AP, Spritzer CE, DeFrate LE. Dose and Recovery Response of Patellofemoral Cartilage Deformations to Running. Orthop J Sports Med 2020;8:2325967120967512. 10.1177/2325967120967512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kessler MA, Glaser C, Tittel S, Reiser M, Imhoff AB. Recovery of the menisci and articular cartilage of runners after cessation of exercise: additional aspects of in vivo investigation based on 3-dimensional magnetic resonance imaging. Am J Sports Med 2008;36:966-70. 10.1177/0363546507313093 [DOI] [PubMed] [Google Scholar]
- 22.Tsai PH, Wong CC, Chan WP, Lu TW. The value of MR T2* measurements in normal and osteoarthritic knee cartilage: effects of age, sex, and location. Eur Radiol 2019;29:4514-22. 10.1007/s00330-018-5826-z [DOI] [PubMed] [Google Scholar]
- 23.Coburn SL, Crossley KM, Kemp JL, Warden SJ, West TJ, Bruder AM, Mentiplay BF, Culvenor AG. Is running good or bad for your knees? A systematic review and meta-analysis of cartilage morphology and composition changes in the tibiofemoral and patellofemoral joints. Osteoarthritis Cartilage 2023;31:144-57. 10.1016/j.joca.2022.09.013 [DOI] [PubMed] [Google Scholar]
- 24.Schütz U, Ehrhardt M, Göd S, Billich C, Beer M, Trattnig S. A mobile MRI field study of the biochemical cartilage reaction of the knee joint during a 4,486 km transcontinental multistage ultra-marathon using T2* mapping. Sci Rep 2020;10:8157. 10.1038/s41598-020-64994-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shu D, Chen F, Guo W, Ding J, Dai S. Acute changes in knee cartilage and meniscus following long-distance running in habituate runners: a systematic review on studies using quantitative magnetic resonance imaging. Skeletal Radiol 2022;51:1333-45. 10.1007/s00256-021-03943-0 [DOI] [PubMed] [Google Scholar]
- 26.Bricca A, Juhl CB, Grodzinsky AJ, Roos EM. Impact of a daily exercise dose on knee joint cartilage - a systematic review and meta-analysis of randomized controlled trials in healthy animals. Osteoarthritis Cartilage 2017;25:1223-37. 10.1016/j.joca.2017.03.009 [DOI] [PubMed] [Google Scholar]
- 27.Rios JL, Boldt KR, Mather JW, Seerattan RA, Hart DA, Herzog W. Quantifying the Effects of Different Treadmill Training Speeds and Durations on the Health of Rat Knee Joints. Sports Med Open 2018;4:15. 10.1186/s40798-018-0127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: the Framingham study. Am J Med 1999;106:151-7. 10.1016/S0002-9343(98)00413-6 [DOI] [PubMed] [Google Scholar]
- 29.Gahunia HK, Pritzker KP. Effect of exercise on articular cartilage. Orthop Clin North Am 2012;43:187-99, v. 10.1016/j.ocl.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 30.Huang L, Zhang Y, Li Q. Investigating the causal relationship between physical activity and incident knee osteoarthritis: a two-sample Mendelian randomization study. Sci Rep 2024;14:1663. 10.1038/s41598-024-52175-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as