Abstract
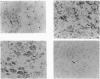
The activities and contents of the lysosomal cysteine proteinases cathepsins B, H and L were examined in xenografts of biopsied muscles transplanted from age-matched normal subjects and Duchenne-muscular-dystrophy (DMD) patients into nude mice. The activity of cathepsin B increased 9-fold and that of B-plus-L increased 24-fold in the first week after transplantation in normal muscle xenografts. By the third week, the activity of cathepsin B increased a total of 20-fold and B-plus-L increased to 36-fold the original level. The activity levels of cathepsin B, B-plus-L, H and D, and acid phosphatase in normal and DMD xenografts were not significantly different when compared 2 weeks after transplantation. However, the protein content of cathepsin B in DMD muscle xenografts was more than 3-fold that of normal xenografts at 2 weeks. The profile of cathepsin H activity in normal muscle xenografts was different than those of cathepsins B and B-plus-L. In the first week, the cathepsin H diminished sharply to about one-third of the biopsied muscle level and then, by 3 weeks after transplantation, it had increased slightly to about half the original level. The amount of endogenous cysteine-proteinase inhibitor changed in parallel with the activity of cathepsins B and B-plus-L. Cathepsins B and H, but not cathepsin L, were found immunohistochemically in regenerating muscle fibres of normal and DMD xenografts 2 weeks after transplantation. Staining of cathepsin B in DMD xenografts was slightly stronger than that in normal subjects. There was no immunostaining in degenerating or necrotic muscle fibres 2 weeks after transplantation. Western-blot analysis revealed that the cathepsin B band at 29 kDa was increased in normal xenografts 2 and 3 weeks after transplantation. Also, 2 weeks after transplantation the staining intensity of this band was slightly stronger in DMD xenografts than in normal xenografts. These results suggest that cathepsin B participates in the regeneration of transplanted muscle, both normal and DMD, and in the DMD muscle fibre-wasting processes, during regeneration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Eguchi C., Ishihara T., Nonaka I., Ozawa E., Sugita H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1988 Jun 30;333(6176):861–863. doi: 10.1038/333861a0. [DOI] [PubMed] [Google Scholar]
- Assfalg-Machleidt I., Jochum M., Klaubert W., Inthorn D., Machleidt W. Enzymatically active cathepsin B dissociating from its inhibitor complexes is elevated in blood plasma of patients with septic shock and some malignant tumors. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):263–269. [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Bonilla E., Samitt C. E., Miranda A. F., Hays A. P., Salviati G., DiMauro S., Kunkel L. M., Hoffman E. P., Rowland L. P. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988 Aug 12;54(4):447–452. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- Chauhan S. S., Goldstein L. J., Gottesman M. M. Expression of cathepsin L in human tumors. Cancer Res. 1991 Mar 1;51(5):1478–1481. [PubMed] [Google Scholar]
- Cullen M. J., Mastaglia F. L. Morphological changes in dystrophic muscle. Br Med Bull. 1980 May;36(2):145–122. doi: 10.1093/oxfordjournals.bmb.a071630. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. Lymphocyte stimulation: a rapid multiparameter analysis. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2881–2884. doi: 10.1073/pnas.73.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hudgson P., Pearce G. W., Walton J. N. Pre-clinical muscular dystrophy: histopathological changes observed on muscle biopsy. Brain. 1967 Sep;90(3):565–576. doi: 10.1093/brain/90.3.565. [DOI] [PubMed] [Google Scholar]
- Igarashi M., Hollander V. P. Acid phosphatase from rat liver. Purification, crystallization, and properties. J Biol Chem. 1968 Dec 10;243(23):6084–6089. [PubMed] [Google Scholar]
- Ishiura S., Nonaka I., Fujita T., Sugita H. Effect of cycloheximide administration on bupivacaine-induced acute muscle degradation. J Biochem. 1983 Nov;94(5):1631–1636. [PubMed] [Google Scholar]
- Jimi T., Satoh Y., Takeda A., Shibuya S., Wakayama Y., Sugita K. Strong immunoreactivity of cathepsin L at the site of rimmed vacuoles in diseased muscles. Brain. 1992 Feb;115(Pt 1):249–260. doi: 10.1093/brain/115.1.249. [DOI] [PubMed] [Google Scholar]
- Kar N. C., Pearson C. M. A calcium-activated neutral protease in normal and dystrophic human muscle. Clin Chim Acta. 1976 Dec 1;73(2):293–297. doi: 10.1016/0009-8981(76)90175-3. [DOI] [PubMed] [Google Scholar]
- Kar N. C., Pearson C. M. Early elevation of cathepsin B1 in human muscle disease. Biochem Med. 1977 Aug;18(1):126–129. doi: 10.1016/0006-2944(77)90059-x. [DOI] [PubMed] [Google Scholar]
- Kar N. C., Pearson C. M. Muscular dystrophy and activation of proteinases. Muscle Nerve. 1978 Jul-Aug;1(4):308–313. doi: 10.1002/mus.880010407. [DOI] [PubMed] [Google Scholar]
- Katunuma N., Kominami E. Abnormal expression of lysosomal cysteine proteinases in muscle wasting diseases. Rev Physiol Biochem Pharmacol. 1987;108:1–20. doi: 10.1007/BFb0034070. [DOI] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Kominami E., Bando Y., Ii K., Hizawa K., Katunuma N. Increases in cathepsins B and L and thiol proteinase inhibitor in muscle of dystrophic hamsters. Their localization in invading phagocytes. J Biochem. 1984 Dec;96(6):1841–1848. doi: 10.1093/oxfordjournals.jbchem.a135018. [DOI] [PubMed] [Google Scholar]
- Kominami E., Bando Y., Wakamatsu N., Katunuma N. Different tissue distributions of two types of thiol proteinase inhibitors from rat liver and epidermis. J Biochem. 1984 Nov;96(5):1437–1442. doi: 10.1093/oxfordjournals.jbchem.a134972. [DOI] [PubMed] [Google Scholar]
- Kominami E., Kunio I., Katunuma N. Activation of the intramyofibral autophagic-lysosomal system in muscular dystrophy. Am J Pathol. 1987 Jun;127(3):461–466. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mason R. W., Green G. D., Barrett A. J. Human liver cathepsin L. Biochem J. 1985 Feb 15;226(1):233–241. doi: 10.1042/bj2260233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastaglia F. L., Kakulas B. A. Regeneration in Duchenne muscular dystrophy: a histological and histochemical study. Brain. 1969;92(4):809–818. doi: 10.1093/brain/92.4.809. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- PEARSON C. M. Histopathological features of muscle in the preclinical stages of muscular dystrophy. Brain. 1962 Mar;85:109–120. doi: 10.1093/brain/85.1.109. [DOI] [PubMed] [Google Scholar]
- Pennington R. J., Robinson J. E. Cathepsin activity in normal and dystrophic human muscle. Enzymol Biol Clin (Basel) 1968;9(3):175–182. doi: 10.1159/000458253. [DOI] [PubMed] [Google Scholar]
- Sano M., Wada Y., Ii K., Kominami E., Katunuma N., Tsukagoshi H. Immunolocalization of cathepsins B, H and L in skeletal muscle of X-linked muscular dystrophy (mdx) mouse. Acta Neuropathol. 1988;75(3):217–225. doi: 10.1007/BF00690529. [DOI] [PubMed] [Google Scholar]
- Schwartz W. N., Barrett A. J. Human cathepsin H. Biochem J. 1980 Nov 1;191(2):487–497. doi: 10.1042/bj1910487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane B. F., Moin K., Krepela E., Rozhin J. Cathepsin B and its endogenous inhibitors: the role in tumor malignancy. Cancer Metastasis Rev. 1990 Dec;9(4):333–352. doi: 10.1007/BF00049523. [DOI] [PubMed] [Google Scholar]
- Sohar I., Laszlo A., Gaal K., Mechler F. Cysteine and metalloproteinase activities in serum of Duchenne muscular dystrophic genotypes. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):277–279. [PubMed] [Google Scholar]
- Takagi A., Nonaka I. Duchenne muscular dystrophy: unusual activation of single fibers in vitro. Muscle Nerve. 1981 Jan-Feb;4(1):10–15. doi: 10.1002/mus.880040104. [DOI] [PubMed] [Google Scholar]
- Takeda A., Nakamura Y., Aoki Y. Enzyme-linked immunosorbent assay for the detection of cathepsin-kininogen complexes in human plasma. J Immunol Methods. 1992 Mar 4;147(2):217–223. doi: 10.1016/s0022-1759(12)80011-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama Y., Watanabe T., Watanabe M., Ishii Y., Matsuba H., Waguri S., Kominami E. Immunocytochemical localization of cathepsins B, H, L, and T4 in follicular cells of rat thyroid gland. J Histochem Cytochem. 1989 May;37(5):691–696. doi: 10.1177/37.5.2703704. [DOI] [PubMed] [Google Scholar]
- Wakayama Y., Jimi T., Misugi N., Kumagai T., Miyake S., Shibuya S., Miike T. Dystrophin immunostaining and freeze-fracture studies of muscles of patients with early stage amyotrophic lateral sclerosis and Duchenne muscular dystrophy. J Neurol Sci. 1989 Jun;91(1-2):191–205. doi: 10.1016/0022-510x(89)90087-7. [DOI] [PubMed] [Google Scholar]
- Wakayama Y., Jimi T., Takeda A., Misugi N., Kumagai T., Miyake S., Shibuya S. Immunoreactivity of antibodies raised against synthetic peptide fragments predicted from mid portions of dystrophin cDNA. J Neurol Sci. 1990 Jul;97(2-3):241–250. doi: 10.1016/0022-510x(90)90222-9. [DOI] [PubMed] [Google Scholar]
- Wakayama Y., Matsuzaki H., Nakai Y. Golgi apparatus: distinct structure of acid phosphatase localization in regenerating human skeletal muscle fiber. Dev Neurosci. 1983;6(3):152–160. doi: 10.1159/000112342. [DOI] [PubMed] [Google Scholar]
- Wakayama Y., Ohbu S. Light- and electron-microscopic studies of transplanted human dystrophic muscles to nude mice. J Neurol Sci. 1982 Jul;55(1):59–77. doi: 10.1016/0022-510x(82)90170-8. [DOI] [PubMed] [Google Scholar]
- Wakayama Y., Schotland D. L., Bonilla E., Orecchio E. Quantitative ultrastructural study of muscle satellite cells in Duchenne dystrophy. Neurology. 1979 Mar;29(3):401–407. doi: 10.1212/wnl.29.3.401. [DOI] [PubMed] [Google Scholar]
- Wakayama Y., Schotland D. L., Bonilla E. Transplantation of human skeletal muscle to nude mice: a sequential morphologic study. Neurology. 1980 Jul;30(7 Pt 1):740–748. doi: 10.1212/wnl.30.7.740. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Higashi T., Watanabe A., Osawa T., Sato Y., Kimura Y., Tominaga S., Hashimoto N., Yoshida Y., Morimoto S. Cathepsin B and L activities in gastric cancer tissue: correlation with histological findings. Biochem Med Metab Biol. 1989 Aug;42(1):21–29. doi: 10.1016/0885-4505(89)90037-6. [DOI] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E. E., Bulman D. E., Karpati G., Burghes A. H., Belfall B., Klamut H. J., Talbot J., Hodges R. S., Ray P. N., Worton R. G. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988 Jun 2;333(6172):466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]