Abstract
Chemokines are inflammatory molecules that act primarily as chemoattractants and as activators of leukocytes. Their role in antigen-specific immune responses is of importance, but their role in disease protection is unknown. Recently it has been suggested that chemokines modulate immunity along more classical Th1 and Th2 phenotypes. However, no data currently exist in an infectious challenge model system. We analyzed the modulatory effects of selected chemokines (interleukin-8 [IL-8], gamma interferon-inducible protein 10 [IP-10], RANTES, monocyte chemotactic protein 1 [MCP-1], and macrophage inflammatory protein 1α [MIP-1α]) on immune phenotype and protection against lethal challenge with herpes simplex virus type 2 (HSV-2). We observed that coinjection with IL-8 and RANTES plasmid DNAs dramatically enhanced antigen-specific Th1 type cellular immune responses and protection from lethal HSV-2 challenge. This enhanced protection appears to be mediated by CD4+ T cells, as determined by in vitro and in vivo T-cell subset deletion. Thus, IL-8 and RANTES cDNAs used as DNA vaccine adjuvants drive antigen-specific Th1 type CD4+ T-cell responses, which result in reduced HSV-2-derived morbidity, as well as reduced mortality. However, coinjection with DNAs expressing MCP-1, IP-10, and MIP-1α increased mortality in the challenged mice. Chemokine DNA coinjection also modulated its own production as well as the production of cytokines. These studies demonstrate that chemokines can dominate and drive immune responses with defined phenotypes, playing an important role in the generation of protective antigen-specific immunity.
The initiation of immune or inflammatory reactions is a complex process involving the coordinated expression of costimulatory molecules, adhesion molecules, cytokines, and chemokines. In particular, chemokines are important in the molecular regulation of trafficking of immune cells to the peripheral sites of host defenses. The chemokine superfamily consists of two subfamilies based upon the presence (α family) or absence (β family) of a single amino acid sequence separating two cysteine residues (1, 2, 30, 36, 47). These chemokines have been shown to induce direct migration of various immune cell types, including neutrophils, eosinophils, basophils, and monocytes (1, 2, 30, 36, 47). Recently, the α-chemokine family (CXC type), interleukin-8 (IL-8) and gamma interferon (IFN-γ)-inducible protein 10 (IP-10), and the β-chemokine family (CC type), RANTES (regulated on activation, normal T-cell expressed and secreted), monocyte chemotactic protein 1 (MCP-1), and macrophage inflammatory protein 1α (MIP-1α), have been shown to chemoattract T lymphocytes and alter cytokine production from T cells (4, 12, 17, 19, 48). In particular, RANTES chemoattracts unstimulated CD4+/CD45RO+ memory T cells and stimulated CD4+ and CD8+ T cells (24, 27, 37, 46). MIP-1α and MCP-1 also stimulate Th1 or Th2 type cytokine production from T cells (13, 46). Recent studies support the notions that chemokine receptors mark T-cell subsets and that chemokines may be involved in the generation of antigen-specific immune responses (14, 35).
In this study, we reasoned that we could utilize the DNA vaccine model to investigate whether chemokines could modulate immune responses and then impact protection from herpes simplex virus type 2 (HSV-2) challenge in a defined mouse model system. To investigate the modulation of immune responses and protective immunity, we codelivered a DNA expression construct encoding HSV-2 gD protein with plasmids encoding selected chemokines, specific-receptor-responsive chemokines (IL-8 and IP-10) and shared-receptor-responsive chemokines (RANTES, MCP-1, and MIP-1α). We then analyzed their modulatory effects on antigen-specific immune induction and protection from challenge. We observed that coinjection with IL-8 and RANTES enhanced antigen-specific Th1 type CD4+ T-cell immune responses and protection from HSV challenge. On the other hand, coinjection with IP-10, MCP-1, and MIP-1α had overall detrimental effects on protection status. These studies support the idea that chemokines can modulate important immune responses and disease progression in a manner reminiscent of cytokines. Significant immune modulation could be achieved through the use of codelivered chemokine cDNAs, impacting not just an immune response but also protection from disease. Furthermore, use of chemokine gene-delivered adjuvants, in particular IL-8 and RANTES, could be important in crafting more efficacious vaccines as immune therapies or contributors to immune therapies for HSV.
MATERIALS AND METHODS
Mice.
Female 4- to 6-week-old BALB/c mice were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.). They were cared for according to the guidelines of the National Institutes of Health (Bethesda, Md.) and the University of Pennsylvania IACUC (Philadelphia).
Reagents.
HSV-2 strain 186 (a kind gift from P. Schaffer, University of Pennsylvania, Philadelphia) was propagated in the Vero cell line. The DNA vaccine encoding HSV-2 gD protein, pAPL-gD2 (pgD), was previously described (31). The expression vectors pCDNA3-IL-8, pCDNA3-IP-10, pCDNA3-RANTES, pCDNA3-MCP-1, and pCDNA3-MIP-1α were previously constructed in our laboratory (14). Plasmid DNA was produced in bacteria and purified by double-banded CsCl preparations. Recombinant HSV-2 gD proteins, a generous gift from G. H. Cohen and R. J. Eisenberg, University of Pennsylvania, were used as recombinant antigens in these studies.
DNA inoculation of mice.
The quadriceps muscles of BALB/c mice were injected with gD DNA constructs formulated in 100 μl of phosphate-buffered saline and 0.25% bupivacaine-HCl (Sigma, St. Louis, Mo.) via a 28-gauge needle (Becton Dickinson, Franklin Lakes, N.J.). Samples of various chemokine and cytokine gene expression cassettes were mixed with pgD plasmid solution prior to injection.
ELISA.
An enzyme-linked immunosorbent assay (ELISA) was performed as previously described (40, 43). In particular, for the determination of relative levels of gD-specific immunoglobulin G (IgG) subclasses, anti-murine IgG1 and IgG2a conjugated with horseradish peroxidase (HRP) (Zymed, San Francisco, Calif.) were substituted for anti-murine IgG-HRP. To determine ELISA titers, pools comprising equal numbers of serum samples for each group were twofold serially diluted from 1:100 and reacted with gD protein. The titers were determined as the reciprocals of the highest serum dilutions showing optical density (OD) values twice as high as that of the negative control.
Th cell proliferation assay.
The T helper (Th) cell proliferation assay was performed as previously described (40, 41).
In vitro depletion of CD4+ and CD8+ T cells.
Splenocytes were reacted with anti-CD4 or anti-CD8 antibodies for 1 h at 4°C, followed by incubation with rabbit complements for 1 h at 37°C. Cell viability postdepletion was determined by trypan blue dye exclusion. Two cycles of antibodies plus complements resulted in depletion of more than 98% of each specific T-cell subpopulation as determined by fluorescence-activated cell sorter (FACS) analysis.
In vivo depletion of CD4+ T cells.
One hundred microliters of anti-CD4 (clone GK1.5) ascites fluid (a kind gift from N. Chirmule of the University of Pennsylvania) was administered intraperitoneally (i.p.) as previously described (41). Antibody treatment resulted in more than 98% depletion of specific CD4+ T-cell subsets of representative animals over a 3-week period. Depleted mice were subsequently challenged with virus on day 0.
Th1 and Th2 type cytokines and chemokines.
A 1-ml aliquot containing 6 × 106 splenocytes was added to the wells of 24-well plates. Then 1 μg of HSV-2 gD protein/ml was added to each well. After 2 days of incubation at 37°C in 5% CO2, cell supernatants were secured and then used for detecting levels of IL-2, IL-4, IFN-γ, RANTES, and MCP-1 with commercial cytokine and chemokine kits (Biosource, Intl., Camarillo, Calif.; R&D Systems, Minneapolis, Minn.) by adding the extracellular fluids to the cytokine- or chemokine-specific ELISA plates.
i.vag. HSV-2 challenge.
Mice were challenged as previously described with some modifications (23, 26). Before inoculation with the virus, the intravaginal (i.vag.) area was swabbed with a cotton-tipped applicator (Hardwood Products Company, Guilford, Maine) soaked with 0.1 M NaOH solution and then cleaned with dry cotton applicators. Mice were then examined daily to evaluate pathological conditions and survival rates.
Statistical analysis.
Statistical analysis was done with the paired Student's t test or analysis of variance (ANOVA). Values for different immunization groups were compared. P values of <0.05 were considered significant.
RESULTS
Selection of chemokines.
Chemokines have been reported to bind to their own specific receptors for immune cell functions (1, 33). In particular, both CXCR1 and CXCR2 recognize IL-8, whereas CXCR3 interacts with IP-10. In contrast, four different chemokine receptors, CCR1, CCR3, CCR4, and CCR5, respond to RANTES. Similarly, CCR1, CCR4, and CCR5 recognize MIP-1α, while both CCR2 and CCR4 recognize MCP-1. In an effort to compare differential effects of these specific- or shared-receptor-responsive chemokines on the induction of antigen-specific immune responses as well as protective immunity, we selected the nonshared receptor-specific chemokines IL-8 and IP-10 as well as the shared-receptor-specific chemokines RANTES, MIP-1α and MCP-1.
Coadministration of chemokine plasmids influences systemic IgG production.
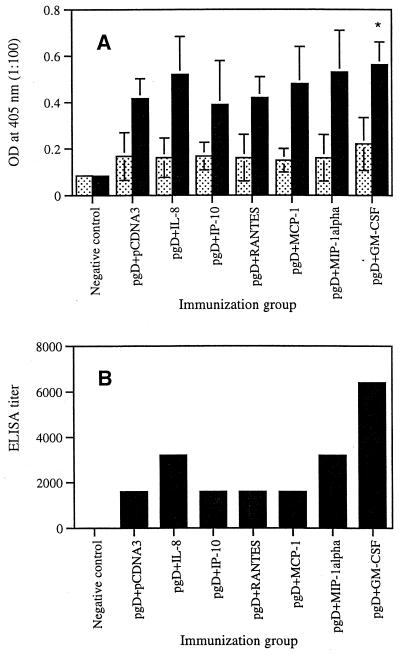
We first investigated the in vivo effects of selected chemokines on the induction of antigen-specific antibody responses. As shown in Fig. 1A, coinjection with IL-8 and MIP-1α cDNAs resulted in a slight increase in gD-specific IgG production compared to that with gD plus pCDNA3. However, coinjection with IP-10, RANTES, and MCP-1 showed gD-specific IgG production similar to that of gD plus pCDNA3. Granulocyte-macrophage colony-stimulating factor (GM-CSF) coinjection used as a control enhanced gD-specific antibody production significantly more than gD DNA vaccine alone. Figure 1B shows that ELISA titers of equally pooled sera collected 2 weeks after the second immunization were determined as 3,200 (for IL-8), 1,600 (for IP-10), 1,600 (for RANTES), 1,600 (for MCP-1), 3,200 (for MIP-1α), 6,400 (for GM-CSF), and 1,600 (for the gD DNA vaccine alone).
FIG. 1.
(A) Levels of systemic gD-specific IgG in mice coimmunized with chemokine cDNAs. Each group of mice (n = 10) was immunized with gD DNA vaccines (60 μg per mouse) plus chemokine genes (40 μg per mouse) or GM-CSF genes (40 μg per mouse) at 0 and 2 weeks. Mice were bled 2 weeks after each immunization, and each group's serum pool was diluted to 1:100 for reaction with gD. OD was measured at 405 nm. Values represent means (n = 10); error bars, standard deviations. Stippled bars, 2-week sera; solid bars, 4-week sera. (B) ELISA titers of 4-week sera. Equally pooled sera obtained 2 weeks following the second immunization were serially diluted from 1:100 for reaction with gD. The titers were determined as the reciprocal of the highest serum dilution showing an OD twice as high as that of negative controls. Asterisks indicate values that are statistically significant at a P value of <0.05 by Student's t test compared to that with pgD plus pCDNA3.
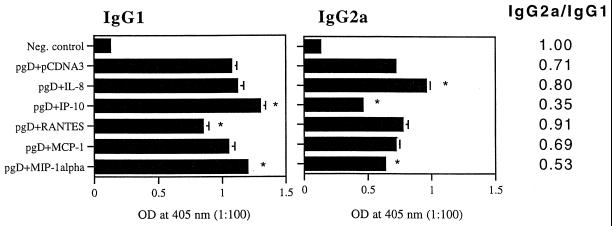
Coimmunization with chemokine plasmids shifts IgG subclasses to Th1 or Th2 isotypes.
IgG subclasses give an indication of the Th1 versus Th2 nature of the induced immune responses. It has been known that IgG1 and IgE are Th2-associated antibodies, whereas IgG2a is a Th1-associated isotype antibody (44). We analyzed the IgG subclasses induced by the coinjections. As shown in Fig. 2, IP-10 and MIP-1α coinjection enhanced IgG1 isotype production significantly over that with the gD DNA vaccine alone. In contrast, RANTES coinjection inhibited IgG1 isotype production significantly relative to that with the gD DNA vaccine alone. However, IL-8 and MCP-1 coinjection showed IgG1 isotype production similar to that with the gD DNA vaccine alone. In the case of IgG2a isotype production, IL-8 coinjection enhanced IgG2a production significantly over that with the gD DNA vaccine alone, whereas IP-10 and MIP-1α coinjection inhibited IgG2a production significantly relative to that with the gD DNA vaccine alone. In contrast, RANTES and MCP-1 coinjection showed minimal changes in IgG2a production compared to that with the gD DNA vaccine alone. The IgG2a/IgG1 ratios were calculated as 0.71 (gD DNA vaccine alone), 0.8 (IL-8 coinjection), 0.35 (IP-10 coinjection), 0.91 (RANTES coinjection), 0.69 (MCP-1 coinjection), and 0.53 (MIP-1α). Similar IgG2a/IgG1 ratios were obtained in three separate animal studies (data not shown). This analysis suggests that IL-8 and RANTES drive humoral immune responses towards a Th1 phenotype in vivo.
FIG. 2.
IgG1 versus IgG2a subclass levels in mice coimmunized with chemokine cDNAs. Each group of mice (n = 10) was immunized with gD DNA vaccines (60 μg per mouse) plus chemokine genes (40 μg per mouse) at 0 and 2 weeks. The mice were bled 2 weeks after the last immunization, and sera in each group were equally pooled and diluted to 1:100 for reaction with gD. Samples were assayed in triplicate. Values and error bars represent means (n = 3) and standard deviations, respectively. The IgG2a/IgG1 ratio was then calculated by dividing the mean OD of IgG2a by that of IgG1. Asterisks mark values that are statistically significant at a P value of <0.05 by Student's t test compared to that with the gD DNA vaccine alone.
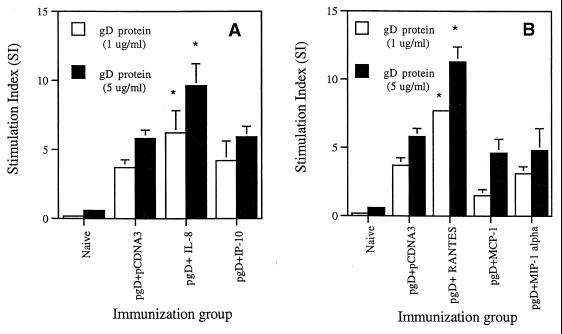
IL-8 and RANTES coinjections enhance Th cell proliferative responses.
Th cell proliferation is a standard parameter used to evaluate the potency of cell-mediated immunity. We measured Th cell proliferation responses following coimmunization with cytokine genes by stimulating splenocytes from immunized animals in vitro with gD proteins. As shown in Fig. 3, gD DNA vaccination alone resulted in gD-specific Th cell proliferative responses. We also observed the significant enhancement of Th cell proliferative responses over that with gD DNA vaccine alone by coinjection with IL-8 and RANTES cDNAs. In contrast, coimmunization with IP-10, MCP-1, and MIP-1α genes appeared to have minimal effects on the levels of Th cell proliferative responses. However, the coinjections showed no effects on phytohemagglutinin (PHA)-induced nonspecific Th cell proliferative responses (the stimulation index [SI] ranged from was 40 to 50). This finding supports a direct effect on memory T cells, not nonspecific immune induction. A lack of cytotoxic T-lymphocyte (CTL) responses against gD in BALB/c mice has been observed previously (6, 22). Furthermore, gD plasmid vaccination does not result in CTL responses in the BALB/c background (5, 6, 41). Therefore, to evaluate cellular effects in more detail, we next examined cytokine production profiles from chemokine-adjuvanted vaccines.
FIG. 3.
Th cell proliferation levels of splenocytes after in vitro gD stimulation in mice coimmunized with α-chemokine cDNAs (A) and β-chemokine cDNAs (B). Each group of mice (n = 2) was immunized with gD DNA vaccines (60 μg per mouse) plus chemokine genes (40 μg per mouse) at 0 and 2 weeks. Two weeks after the last DNA injection, two mice were sacrificed and spleen cells were pooled for the proliferation assay. Splenocytes were stimulated with 1 and 5 μg of gD-2 proteins per ml and 5 μg of PHA per ml as a positive control. After 3 days of stimulation, the cells were harvested and the counts per minute were determined. The PHA control sample showed a stimulation index of 40 to 50. Samples were assayed in triplicate. Values are means; error bars, standard deviations. Asterisks indicate values that are statistically significant at a P value of <0.05 by Student's t test compared to that with the gD DNA vaccine alone. The experiments were repeated 2 more times with similar results.
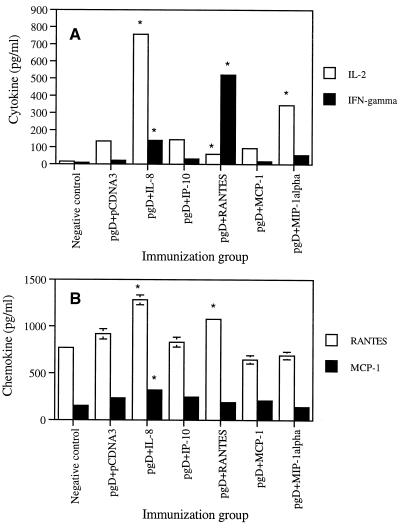
Chemokine coinjections influence production of Th1 type cytokines.
The functions of Th1 cytokines (IL-2 and IFN-γ) and Th2 cytokines (IL-4, IL-5, and IL-10) have been a mainstay in our understanding of the polarization of immune responses. Th1 immune responses are thought to drive induction of cellular immunity, whereas Th2 immune responses play an important role in humoral immunity. Based on the IgG phenotype results, we further evaluated the Th1-versus-Th2 issue by analyzing cytokine release directly. As shown in Fig. 4A, IL-2 production was dramatically increased, almost sevenfold, by coinjection with IL-8 cDNA. IL-2 was also induced by coinjection with the MIP-1α cassette. In particular, production of IFN-γ was most significantly enhanced by codelivery of RANTES (20-fold) and IL-8 (6-fold), further supporting the isotyping results and demonstrating that IL-8 and RANTES mediate Th1 type cellular immune responses in an antigen-dependent fashion. However, IL-4 production was not affected by these chemokine coinjections (data not shown). This illustrates that IL-8 and RANTES drive memory T-cell responses predominantly in a Th1 type fashion.
FIG. 4.
Cytokine (A) and chemokine (B) production levels of splenocytes after in vitro gD stimulation in mice coimmunized with chemokine cDNAs. Each group of mice (n = 2) was immunized with gD DNA vaccines (60 μg per mouse) plus chemokine genes (40 μg per mouse) at 0 and 2 weeks. Two weeks after the last DNA injection, two mice were sacrificed and spleen cells were pooled. Splenocytes were stimulated with 1 μg of gD proteins/ml for 2 days. Samples were assayed in triplicate. Values are means of released cytokine or chemokine concentrations; error bars, standard deviations. Asterisks indicate values that are statistically significant at a P value of <0.05 by Student's t test compared to that with the gD DNA vaccine alone. The experiments were repeated two more times with similar results.
Chemokine coinjections influence production of β-chemokines.
To determine if chemokine coinjection would induce β-chemokine production in an antigen-dependent manner, we coimmunized and then analyzed release levels of β-chemokines from splenocytes after in vitro stimulation with recombinant gD antigen. As shown in Fig. 4B, IL-8 and RANTES cDNA coinjections enhanced RANTES production significantly over that with the gD DNA vaccine alone. In contrast, MCP-1 and MIP-1α cDNA coinjection showed decreased production levels of RANTES compared to that with the gD DNA vaccine alone or IP-10 cDNA coinjection. In the case of MCP-1 production, IL-8 cDNA coinjection enhanced MCP-1 production over that with the gD DNA vaccine alone. In contrast, RANTES and MIP-1α cDNA coinjection decreased MCP-1 production compared to that with the gD DNA vaccine alone. This suggests that chemokines influence their own production through their effector T cells.
IL-8 and RANTES coinjection enhances survival of i.vag. HSV-2 challenge.
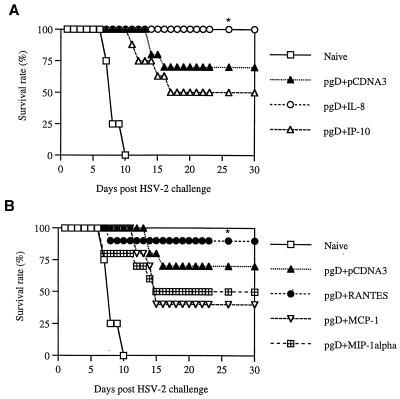
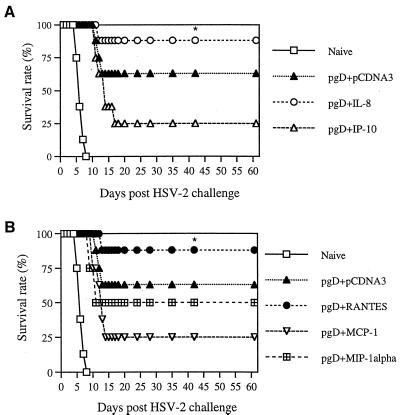
It is important that antigen-specific immune modulation influences a pathogen's replication. We analyzed the protective efficacy of chemokine coinjection in the murine HSV challenge model. The i.vag. challenge route was chosen because HSV-2 infects mucocutaneously (28). As shown in Fig. 5, mice were coimmunized intramuscularly (i.m.) with pgD (10 μg per mouse) and chemokine cDNAs (20 μg per-mouse) and then challenged i.vag. with low lethal doses (4 50% lethal doses [LD50]) of HSV-2. IL-8 and RANTES coinjection resulted in enhanced survival of lethal HSV-2 challenge, whereas the gD DNA vaccine alone showed a 70% survival rate. However, IP-10, MCP-1, and MIP-1α coinjection showed survival rates of 50, 40, and 50%, respectively. A similar finding was also obtained when mice were immunized twice with pgD (60 μg per mouse) plus chemokines (40 μg per mouse) and then challenged i.vag. with a very high lethal dose (200 LD50) of HSV-2 (Fig. 6). This illustrated that chemokines IL-8 and RANTES as vaccine adjuvants enhanced protection from HSV-2 infection through antigen-specific immune modulation.
FIG. 5.
Survival rates of mice immunized with gD DNA vaccines plus chemokine cDNAs after a low-lethal-dose challenge. Each group of mice (n = 10) was immunized with gD DNA vaccines (10 μg per mouse) plus α-chemokine (A) or β-chemokine (B) cDNAs (20 μg per mouse). Four weeks after the initial immunization, mice were challenged i.vag. with 4 LD50 of HSV-2 strain 186 (1.4 × 104 PFU). Asterisks indicate results that are statistically significant at a P value of <0.05 using ANOVA compared to those for mice coinjected with IP-10, MCP-1, or MIP-1α.
FIG. 6.
Survival rates of mice immunized with gD DNA vaccines plus chemokine cDNAs after a high-lethal-dose challenge. Each group of mice (n = 8) was immunized with gD DNA vaccines (60 μg per mouse) plus α-chemokine (A) or β-chemokine (B) cDNAs (40 μg per mouse) at 0 and 2 weeks. Three weeks after the second immunization, the mice were challenged i.vag. with 200 LD50 of HSV-2 strain 186 (7 × 105 PFU). Asterisks indicate results that are statistically significant at a P value of <0.05 using ANOVA compared to those for mice coinjected with IP-10 or MCP-1.
IL-8 and RANTES coadministration reduces morbidity following i.vag. HSV challenge.
Surviving mice challenged with 200 LD50 of HSV-2 were observed for herpetic lesions for 2 months postchallenge. The high LD50 was chosen to evaluate the disease status of animals after i.vag. HSV-2 challenge. Naive mice infected with HSV-2 started to show pathological signs, such as lethargy, abnormal gaits, and ruffling of fur, 2 to 3 days after virus infection. Naive mice started to die after 5 days of infection, and all were dead by 8 days after infection. As shown in Table 1, the groups coinjected with IL-8 and RANTES cDNAs had a lower number of mice exhibiting herpetic lesions in the vaginal area than the group immunized with pgD plus pCDNA3. Rather dramatically, not only did the pgD-plus-IL-8- and the pgD-plus-RANTES-immunized group have the fewest mice with herpetic lesions, but 100% of the mice recovered completely from the lesions at 42 and 60 days post-viral challenge, respectively. This study demonstrates two distinct advantages of such a vaccination scheme: one with regard to survival and one with regard to actual disease pathogenesis.
TABLE 1.
Number of mice showing herpetic lesions after HSV-2 infection
| Immunization group | No. of mice with herpetic lesions/no. survivinga (%) at the following day after HSV-2 challenge:
|
||||||
|---|---|---|---|---|---|---|---|
| 16 | 20 | 24 | 28 | 35 | 42 | 60 | |
| pgD + pCDNA | 5/5 (100) | 5/5 (100) | 4/5 (80) | 4/5 (80) | 3/5 (60) | 2/5 (40) | 2/5 (40) |
| pgD + IL-8 | 3/7 (43) | 2/7 (29) | 2/7 (29) | 1/7 (14) | 1/7 (14) | 0/7 (0) | 0/7 (0) |
| pgD + RANTES | 5/7 (71) | 4/7 (57) | 3/7 (43) | 3/7 (43) | 2/7 (29) | 1/7 (14) | 0/7 (0) |
Each group of mice (n = 8) was immunized with gD DNA vaccines (60 μg) plus chemokine cDNAs (40 μg) at 0 and 2 weeks. Three weeks after the last DNA injection, mice were challenged i.vag. with 200 LD50 of HSV-2 strain 186 (7 × 105 PFU). Surviving mice were checked every day after viral challenges to observe the pathological symptoms.
CD4+ T cells mediate enhancement of antigen-specific Th1 type cellular responses by IL-8 or RANTES coinjection in vitro.
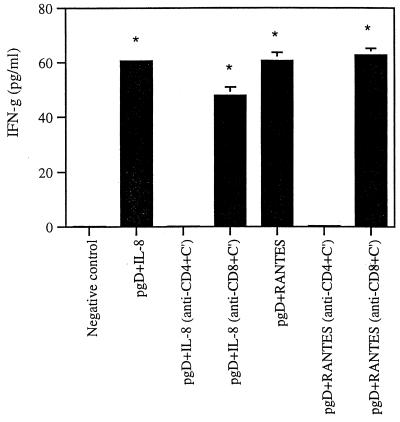
Next we sought to evaluate whether CD4+ or CD8+ T cells are responsible for the enhanced cellular responses induced by IL-8 or RANTES coinjection. Following vaccination we depleted CD4+ or CD8+ T cells in vitro from splenocytes of immunized mice and then tested the effects of specific cell populations on IFN-γ production. As shown in Fig. 7, when CD4+ T cells were depleted, IFN-γ production was decreased to a background level, whereas CD8+ T cell depletion resulted in the same enhancement of IFN-γ production as that seen in whole splenocytes from animals coinjected with IL-8 or RANTES. This supports the idea that CD4+ T cells are responsible for enhanced Th1 type cellular responses through coinjection of IL-8 or RANTES cDNAs.
FIG. 7.
IFN-γ production levels after in vitro CD4+ or CD8+ T-cell subset depletion in mice coimmunized with IL-8 or RANTES cDNAs. Each group of mice (n = 4) was immunized once with gD DNA vaccines (30 μg per mouse) and IL-8 or RANTES cDNAs (20 μg of each per mouse). Three weeks after the initial DNA injection, two mice were sacrificed and spleen cells were pooled. CD4+ or CD8+ T cells were depleted in vitro from splenocytes, followed by 3 days of stimulation with 1 μg of gD protein/ml. Cell supernatants were assayed in triplicate. Asterisks indicate results that are statistically significant at a P value of <0.05 using Student's t test compared to that for the CD4+ T-cell depletion group. The experiments were repeated two more times with similar results.
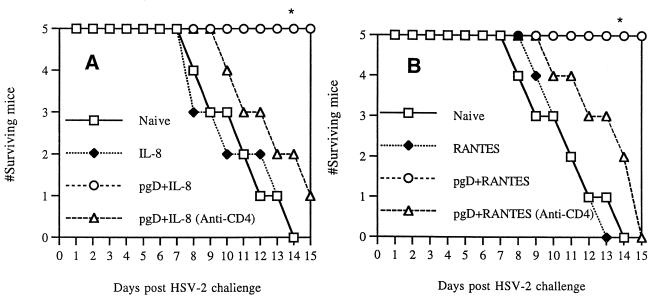
CD4+ T cells are involved in enhanced protection by IL-8 or RANTES coinjection.
We next focused on possible roles of CD4+ T cells in inducing IL-8- or RANTES-enhanced protective immunity against viral infection. It has also been reported that Th1 type CD4+ T cells, but not CD8+ T cells, are responsible for protecting animals from HSV challenge (16, 20, 26). As shown in Fig. 8, all animals immunized with pgD plus IL-8 or pgD plus RANTES survived lethal HSV challenge. However, coinjected animals treated with anti-CD4 antibodies, like naïve control animals, failed to survive lethal challenge. In particular, IL-8 or RANTES control plasmid-injected animals showed survival rates similar to those of naïve control mice. These data confirm that IL-8 or RANTES can enhance survival through effects on CD4+ T cells in vivo.
FIG. 8.
In vivo depletion of CD4+ T cells and protective immunity against HSV challenge. Each group of inbred BALB/c mice (n = 5) was immunized with 20 μg of IL-8 cDNAs (A) or RANTES cDNAs (B) and/or gD DNA vaccine (30 μg). After 3 weeks following the initial DNA immunization, one group of mice was administered 100 μl of anti-CD4 (clone GK1.5) ascites fluid i.p. on days −3, 0, and 3 of viral challenge. Mice were subsequently challenged i.vag. with 4 LD50 of HSV-2 (strain 186) and then checked for 15 days to determine survival rates. Asterisks indicate results that are statistically significant at a P value of <0.05 using ANOVA compared to that for naive control mice. The experiments were repeated with similar results.
DISCUSSION
HSV is the causative agent of a spectrum of human diseases, such as cold sores, ocular infections, encephalitis, and genital infections (28). HSV can establish viral latency with frequent recurrences in the host (34). During viral infection, neutralizing antibody inactivates viral particles but is unable to control intracellular HSV infection (29). Rather, cell-mediated immunity is a major effector function which kills HSV-infected cells (20, 39). The ability of B-cell-suppressed mice to control primary HSV infection (11) or the ability of adoptively transferred T cells to prevent subsequent viral infection (39) further suggests that cell-mediated immunity might be directly related to the inhibition of viral infection and its spread. It has also been well documented that Th1 type CD4+ T cells play a more crucial role for protection from HSV-2 challenge (16, 20, 26). For example, when CD4+ T cells were depleted in vivo, protective immunity against HSV was lost. Moreover, Th1 type CD4+ T cells generate a large amount of IFN-γ (26). IFN-γ upregulates major histocompatibility complex (MHC) expression on HSV-infected cells to allow better recognition by cytotoxic CD4+ T cells (25) and CD8+ CTLs (49), and has direct anti-HSV effects (10). Recently, direct antiviral effects of cytokines including IFN-γ and tumor necrosis factor alpha (TNF-α) have been reported in hepatitis B virus and lymphocytic choriomeningitis virus infection models (7, 8), supporting the possibility that IFN-γ might have its own anti-HSV control mechanism. We recently reported that codelivery with Th1 type cytokine cDNAs enhanced survival after lethal HSV-2 challenge, while codelivery with Th2 type cytokine cDNAs worsened the disease status (40). Similarly, protection enhanced by codelivery with a prototypic Th1 type cytokine IL-12 cDNA was mediated by Th1 type CD4+ T cells in an HSV challenge model (41), underscoring the importance of Th1 type T-cell-mediated protective immunity against HSV infection.
In animal models, immunization with some HSV glycoproteins or DNA constructs expressing specific viral components provides complete or partial protection against viral challenge (3, 15, 18, 20, 21, 32). Several HSV proteins have been analyzed as potential immunization targets. Immunization with cDNA encoding the gC, ICP-27, or gD protein has been shown to induce antigen-specific immune responses and protection against in vivo challenge with HSV in animals (3, 15, 20, 21). Recently, clinical trials using a subunit vaccine failed to protect from recurrent HSV infection (45). This might be due to the fact that this subunit vaccine induces Th2 type cellular responses, which are not correlated with protection from HSV-2-derived morbidity (42). Thus, additional insight is needed to design a more effective approach for this pathogen, and studies should focus on morbidity as well as mortality.
We investigated the in vivo effects of selected chemokines on the induction of protective immunity against HSV-2 infection by coinjecting them as plasmid cassettes along with gD DNA vaccine constructs. We observed that the groups coimmunized with IL-8 and MIP-1α chemokine genes had slightly higher IgG responses than the gD-immunized group, an effect similar to that with GM-CSF as a vaccine adjuvant. Furthermore, modulation of antigen-specific IgG isotype responses has been achieved by using chemokines as molecular adjuvants. We have observed that IL-8 significantly increased the production of gD-specific IgG2a, while RANTES alone inhibited IgG1 production, compared to gD DNA vaccine alone or coinjection with MCP-1. However, coinjection with IP-10 and MIP-1α genes induced more favorable production of IgG1, compared to IgG2a. Thus, these results extend prior findings in the human immunodeficiency virus (HIV) model (14) that the shift in humoral immune responses to either Th1 or Th2 could be modulated by chemokines, again supporting the idea that chemokines can modulate cytokine production in vivo.
In vitro immune parameters, such as Th cell proliferative and CTL responses, have been used to evaluate the potency of cell-mediated immunity. We observed that only plasmid coinjection with IL-8 and RANTES induced higher Th cell proliferation than the plasmid vaccine alone. IL-8 coimmunization also resulted in significantly increased production of IL-2 and IFN-γ over that with the gD DNA vaccine alone, further supporting the isotyping results and demonstrating that IL-8 mediates Th1 type cellular immune responses in an antigen-dependent fashion. IL-8 coinjection also enhanced MCP-1 and RANTES production in an antigen-specific fashion, indicating that IL-8 can modulate β-chemokine production in vivo. We also observed that RANTES coinjection resulted in increased production of IFN-γ and RANTES, but decreased production of IL-2 and MCP-1. This indicates that RANTES modulates antigen-specific immune responses differently from IL-8 in the HSV model.
In HSV challenge studies, gD vaccination alone showed a 70% survival rate at the challenge inoculum of 4 LD50 of HSV-2. By coinjection of chemokine IL-8 and RANTES cDNAs, better survival rates (90 to 100%) were achieved. In contrast, codelivery of chemokine genes (IP-10, MCP-1, or MIP-1α) reduced the rate of survival of challenged mice to 40 to 50%, more than a 20 to 30% reduction in overall survival from that with the gD vaccine alone. At the challenge inoculum of 200 LD50 of HSV-2, gD vaccination alone showed 63% survival rates. By coinjection of chemokine IL-8 and RANTES cDNAs, better survival rates (88%) and less-severe herpetic lesion formation were achieved. In contrast, codelivery of chemokine genes (IP-10 and MCP-1) reduced the rate of survival of challenged mice to 25%, more than a 50% reduction in overall survival from that with the gD vaccine alone. Similarly, MIP-1α coinjection also negatively influenced the survival rate of vaccinated animals. This indicates that coinjection with IL-8 and RANTES chemokine-expressing plasmids enhances protection from lethal HSV challenge, while coinjection with IP-10, MCP-1, and MIP-1α makes animals more susceptible to the effects of viral infection. In the case of morbidity, we also observed that coinjection with IL-8 and RANTES resulted in a reduction in the number of mice with herpetic lesions, compared to that with the pgD vaccine alone. We previously reported that coinjection with a Th1 type cytokine gene enhances the rate of protection from lethal HSV challenge, while Th2 type cytokine coinjection increases the susceptibility of animals to viral infection (40). In pathogenesis studies, the importance of a Th1-like cytokine response for resistance to other pathogenic infections has been reported (7, 9, 38). In particular, Th1 type CD4+ T-cell responses mediate protective immunity against HSV infections (16, 20, 26). We hypothesized that chemokine adjuvanting plasmid vaccines act either directly or indirectly on CD4+ T cells, thus stimulating greater immunity against HSV challenge. To test this hypothesis, in vitro and in vivo subset depletion studies were performed. Our T-cell subset depletion studies confirmed that IL-8 or RANTES coinjection enhanced Th1 type CD4+ T cells, resulting in enhanced protection from HSV challenge. Thus, it seems likely that Th1 and/or Th2 type immune responses are being driven by these chemokines, resulting in an impact on protection from HSV infectious challenge based on the quality of the immune responses.
Chemokines have been known to recognize different types of receptors for immune cell functions (33). In particular, RANTES recognizes four different chemokine receptors, CCR1, CCR3, CCR4, and CCR5, whereas MIP-1α recognizes three different chemokine receptors, CCR1, CCR4, and CCR5. In contrast, MCP-1 recognizes CCR2 and CCR4. In our studies, RANTES had stronger immune-stimulatory effects and protective immunity against HSV-2, compared to MIP-1α and MCP-1. This indicates that selective interaction of RANTES with one of these receptors could be important for inducing greater protective immunity against HSV-2. Similarly, the IL-8 receptors, CXCR1 and CXCR2, could play an important role in triggering and enhancing Th1 type immune responses. RANTES has also been reported to chemoattract both antigen-producing cells (APCs) and memory T cells (37). Changing the environment that is responsible for APC activity could impact on the Th1 versus Th2 nature of the response. However, the IL-2 and IFN-γ production data support a direct effect on T cells. Similarly, the IL-8 response could again take place through attraction and activation of APCs or through direct effects on lymphocyte subsets, or both. However, it is more likely that IL-8 and RANTES play more important roles in acting directly on T cells, as a combination study using IL-8 and RANTES plasmid adjuvants resulted in inhibition of antigen-specific T-cell responses (data not shown). Further study of the direct immune biology is important and will give insight into the mechanism of immune stimulation by these potent adjuvants.
In conclusion, the data presented here demonstrate that chemokines can modulate immune responses towards a Th1 and/or Th2 phenotype in an antigen-dependent fashion and thus modulate protection from lethal challenge in vivo (Table 2). Such activities have been previously associated particularly with cytokines. These data imply that chemokines may have as central a role as cytokines in the induction of antigen-specific CD4+ T-cell immunity. This finding broadens our weapons for vaccination as well as therapy for infectious diseases. Furthermore, the use of chemokines to modulate immune responses for the beneficial manipulation of cancer therapies could also be considered.
TABLE 2.
Summary of the effects of α- and β-chemokine coinjection on IgG levels, the ratio of IgG2a to IgG1, T helper cell proliferation responses, mortality, and morbidity
| Chemokine type | Effecta on:
|
||||
|---|---|---|---|---|---|
| IgG level | IgG2a/ IgG1 ratio | Th cell response | Mortality | Morbidity | |
| CXC type | |||||
| IL-8 | ↑ | ↑ | ↑ | ↓ | ↓ |
| IP-10 | — | ↓ | — | ↑ | ND |
| CC type | |||||
| RANTES | — | ↑ | ↑ | ↓ | ↓ |
| MCP-1 | — | — | — | ↑ | ND |
| MIP-1α | ↑ | ↓ | — | ↑ | ND |
↑ and ↓, increase and decrease in the response, respectively, compared to that without cytokine coinjections; —, no change; ND, not determined.
ACKNOWLEDGMENTS
We thank G. Cohen and R. Eisenberg for providing HSV-2 gD (306t). We also thank P. Schaffer for providing a stock of HSV-2 for this study. Also, Naren Chirmule kindly provided anti-CD4 ascites fluids for this study. J.-I. Sin thanks Weibin Xu for advice on statistical analysis.
REFERENCES
- 1.Baggiolini M, Beatrice D, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines: CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 3.Bourne N, Stanberry L R, Bernstein D I, Lew D. DNA immunization against experimental genital herpes simplex virus infection. J Infect Dis. 1996;173:800–807. doi: 10.1093/infdis/173.4.800. [DOI] [PubMed] [Google Scholar]
- 4.Carr M W, Roth S J, Luther E, Rose S S, Springer T A. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz P E, Khalil P L, Dryden T D, Chiou H C, Fink P S, Berberich S J, Bigley N J. A novel immunization method to induce cytotoxic T-lymphocyte responses (CTL) against plasmid-encoded herpes simplex virus type-1 glycoprotein D. Vaccine. 1999;17:1091–1099. doi: 10.1016/s0264-410x(98)00326-0. [DOI] [PubMed] [Google Scholar]
- 6.Ghiasi H, Cai S, Slanina S, Nesburn A B, Wechsler S L. Vaccination of mice with herpes simplex virus type 1 glycoprotein D DNA produces low levels of protection against lethal HSV-1 challenge. Antivir Res. 1995;28:147–157. doi: 10.1016/0166-3542(95)00045-n. [DOI] [PubMed] [Google Scholar]
- 7.Guidotti L G, Borrow P, Brown A, McClary H, Koch R, Chisari F V. Noncytopathic clearance of lymphocyte choriomeningitis virus from the hepatocyte. J Exp Med. 1999;189:1555–1564. doi: 10.1084/jem.189.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guidotti L G, Rochford R, Chung J, Shapiro M, Purcell R, Chisari F V. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 9.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho M. Interferon as an agent against herpes simplex virus. J Investig Dermatol. 1990;95:158S–160S. doi: 10.1111/1523-1747.ep12875164. [DOI] [PubMed] [Google Scholar]
- 11.Kapoor A K, Nash A A, Wildy P. Pathogenesis of herpes simplex virus in B cell-suppressed mice: the relative roles of cell mediated and humoral immunity. J Gen Virol. 1982;61:127–131. doi: 10.1099/0022-1317-61-1-127. [DOI] [PubMed] [Google Scholar]
- 12.Karpus W J, Lukacs N W, Kennedy K J, Smith W S, Hurst S D, Barrett T A. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- 13.Karpus W J, Kennedy K J, Kunkel S L, Lukacs N W. Monocyte chemotactic protein 1 regulates oral tolerance induction by inhibition of T helper cell 1-related cytokines. J Exp Med. 1998;187:733–741. doi: 10.1084/jem.187.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J J, Nottingham L K, Sin J I, Tsai A, Morrison L, Dang K, Hu Y, Kazahaya K, Bennett M, Dentchev T, Wilson D M, Chalian A A, Boyer J D, Agadjanyan M G, Weiner D B. CD8 positive T-cells influence antigen-specific immune responses through the expression of chemokines. J Clin Investig. 1998;102:1112–1124. doi: 10.1172/JCI3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kriesel J D, Spruance S L, Daynes R A, Araneo B A. Nucleic acid vaccine encoding gD2 protects mice from herpes simplex virus type 2 disease. J Infect Dis. 1996;173:536–541. doi: 10.1093/infdis/173.3.536. [DOI] [PubMed] [Google Scholar]
- 16.Kuklin N A, Daheshia M, Chun S, Rouse B T. Role of mucosal immunity in herpes simplex virus infection. J Immunol. 1998;160:5998–6003. [PubMed] [Google Scholar]
- 17.Larsen C G, Anderson A O, Appella E, Oppenheim J J, Matsushima K. The neutrophil activating factor (NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243:1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- 18.Long D, Madara T J, de Leon M P, Cohen G H, Montgomery P C, Eisenberg R J. Glycoprotein D protects mice against lethal challenge with herpes simplex virus types 1 and 2. Infect Immun. 1984;43:761–764. doi: 10.1128/iai.43.2.761-764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukacs N W, Chensue S W, Karpus W J, Lincoln P, Keefer C, Strieter R M, Kunkel S L. C-C chemokines differentially alter interleukin-4 production from lymphocytes. Am J Pathol. 1997;150:1861–1868. [PMC free article] [PubMed] [Google Scholar]
- 20.Manickan E, Rouse R J D, Yu Z, Wire W S, Rouse B T. Genetic immunization against herpes simplex virus. Protection is mediated by CD4+ T lymphocytes. J Immunol. 1995;155:259–265. [PubMed] [Google Scholar]
- 21.Manickan E, Yu Z, Rouse R J D, Wire W S, Rouse B T. Induction of protective immunity against herpes simplex virus with DNA encoding the immediate early protein ICP 27. Viral Immunol. 1995;8:53–61. doi: 10.1089/vim.1995.8.53. [DOI] [PubMed] [Google Scholar]
- 22.Martin S, Moss B, Berman P W, Laskey L A, Rouse B T. Mechanisms of antiviral immunity induced by a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: cytotoxic T cells. J Virol. 1987;61:726–734. doi: 10.1128/jvi.61.3.726-734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDermott M R, Smiley J R, Leslie P, Brais J, Rudzroga H E, Bienenstock J. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J Virol. 1984;51:747–753. doi: 10.1128/jvi.51.3.747-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meurer R, Van Riper G, Feeney W, Cunningham P, Hora D, Jr, Springer M S, MacIntyre D E, Rosen H. Formation of eosinophilic and monocytic intradermal inflammatory sites in the dog by injection of human RANTES but not human monocyte chemoattractant protein 1, human macrophage inflammatory protein 1 alpha, or human interleukin 8. J Exp Med. 1993;178:1913–1921. doi: 10.1084/jem.178.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikloska A, Cunningham A L. Herpes simplex virus type 1 glycoproteins gB, gC, and gD are major targets for CD4 T-lymphocyte cytotoxicity in HLA-DR expressing human epidermal keratinocytes. J Gen Virol. 1998;79:353–361. doi: 10.1099/0022-1317-79-2-353. [DOI] [PubMed] [Google Scholar]
- 26.Milligan G N, Bernstein D I. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology. 1995;212:481–489. doi: 10.1006/viro.1995.1506. [DOI] [PubMed] [Google Scholar]
- 27.Murphy W J, Taub D D, Anver M, Conlon K, Oppenheim J J, Kelvin D J, Longo D L. Human RANTES induces the migration of human T lymphocytes into the peripheral tissues of mice with severe combined immune deficiency. Eur J Immunol. 1994;24:1823–1827. doi: 10.1002/eji.1830240815. [DOI] [PubMed] [Google Scholar]
- 28.Nahmias A J, Dannenbarger J, Wickliffe C, Muther J. Clinical aspects of infection with herpes simplex virus 1 and 2. New York, N.Y: Elsevier; 1980. [Google Scholar]
- 29.Notkins A L. Immune mechanisms by which the spread of viral infections is stopped. Cell Immunol. 1974;11:478–483. doi: 10.1016/0008-8749(74)90045-8. [DOI] [PubMed] [Google Scholar]
- 30.Oppenheim J J, Zachariae C O, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 31.Pachuk C J, Arnold R, Herold K, Ciccarelli R B, Higgins T J. Humoral and cellular immune responses to herpes simplex virus-2 glycoprotein D generated by facilitated DNA immunization of mice. Curr Top Microbiol Immunol. 1998;226:79–89. doi: 10.1007/978-3-642-80475-5_6. [DOI] [PubMed] [Google Scholar]
- 32.Peng T, Ponce-de-Leon M, Jiang H, Dubin G, Lubinski J M, Eisenberg R J, Cohen G H. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J Virol. 1998;72:65–72. doi: 10.1128/jvi.72.1.65-72.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Premack B A, Schall T J. Chemokine receptors: gateways to inflammation and infection. Nat Med. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 34.Price R W, Walz M A, Wohlenberg C, Notkins A L. Latent infection of sensory ganglia with herpes simplex virus: efficacy of immunization. Science. 1975;188:938–940. doi: 10.1126/science.166432. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto F, Lenig D, Mackay C R, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schall T J. Biology of the RANTES/SIS cytokine family. Cytokine. 1991;3:165–183. doi: 10.1016/1043-4666(91)90013-4. [DOI] [PubMed] [Google Scholar]
- 37.Schall T J, Bacon K, Toy K J, Goeddel D V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 38.Scott P, Natovitz P, Coffman R L, Pearce E, Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sethi K K, Omata Y, Schneweis K E. Protection of mice from lethal herpes simplex virus type 1 infection by adoptive transfer of cloned virus-specific and H-2-restricted cytotoxic T lymphocytes. J Gen Virol. 1983;64:443–447. doi: 10.1099/0022-1317-64-2-443. [DOI] [PubMed] [Google Scholar]
- 40.Sin J I, Kim J J, Boyer J D, Higgins T J, Ciccarelli R B, Weiner D B. In vivo modulation of vaccine-induced immune responses toward a Th1 phenotype increases potency and vaccine effectiveness in a herpes simplex virus type 2 mouse model. J Virol. 1999;73:501–509. doi: 10.1128/jvi.73.1.501-509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sin J I, Kim J J, Arnold R L, Shroff K E, McCallus D, Pachuk C, McElhiney S P, Wolf M W, Pompa-de Bruin S J, Higgins T J, Ciccarelli R B, Weiner D B. Interleukin-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1 type CD4+ T cell-mediated protective immunity against HSV-2 challenge. J Immunol. 1999;162:2912–2921. [PubMed] [Google Scholar]
- 42.Sin J I, Ayyavoo V, Boyer J, Kim J, Ciccarelli R B, Weiner D B. Protective immune correlates can segregate by vaccine type in a murine herpes model system. Int Immunol. 1999;11:1763–1773. doi: 10.1093/intimm/11.11.1763. [DOI] [PubMed] [Google Scholar]
- 43.Sin J I, Sung J H, Suh Y S, Lee A H, Chung J H, Sung Y C. Protective immunity against heterologous challenge with encephalomyocarditis virus by VP1 DNA vaccination: effect of co-injection with a granulocyte-macrophage colony-stimulating factor gene. Vaccine. 1997;15:1827–1833. doi: 10.1016/s0264-410x(97)88856-1. [DOI] [PubMed] [Google Scholar]
- 44.Snapper C M, Paul W E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 45.Straus S E, Wald A, Kost R G, McKenzie R, Langenberg A G, Hohman P, Lekstrom J, Cox E, Nakamura M, Sekulovich R, Izu A, Dekker C, Corey L. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoproteins D and B: results of a placebo-controlled vaccine trial. J Infect Dis. 1997;176:1129–1134. doi: 10.1086/514103. [DOI] [PubMed] [Google Scholar]
- 46.Taub D D, Turcovski-Corrales S M, Key M L, Longo D L, Murphy W J. Chemokines and T lymphocyte activation. I. Beta chemokines costimulate human T lymphocyte activation in vitro. J Immunol. 1996;156:2095–2103. [PubMed] [Google Scholar]
- 47.Taub D D, Oppenheim J J. Chemokines, inflammation and the immune system. Ther Immunol. 1994;1:229–246. [PubMed] [Google Scholar]
- 48.Taub D D, Conlon K, Lloyd A R, Oppenheim J J, Kelvin D J. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1α and MIP-1β. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 49.Tigges M A, Leng S, Johnson D C, Burke R L. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with IFN-gamma or when virion host shutoff functions are disabled. J Immunol. 1996;156:3901–3910. [PubMed] [Google Scholar]