Abstract
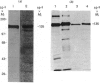
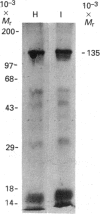
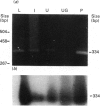
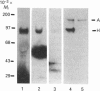
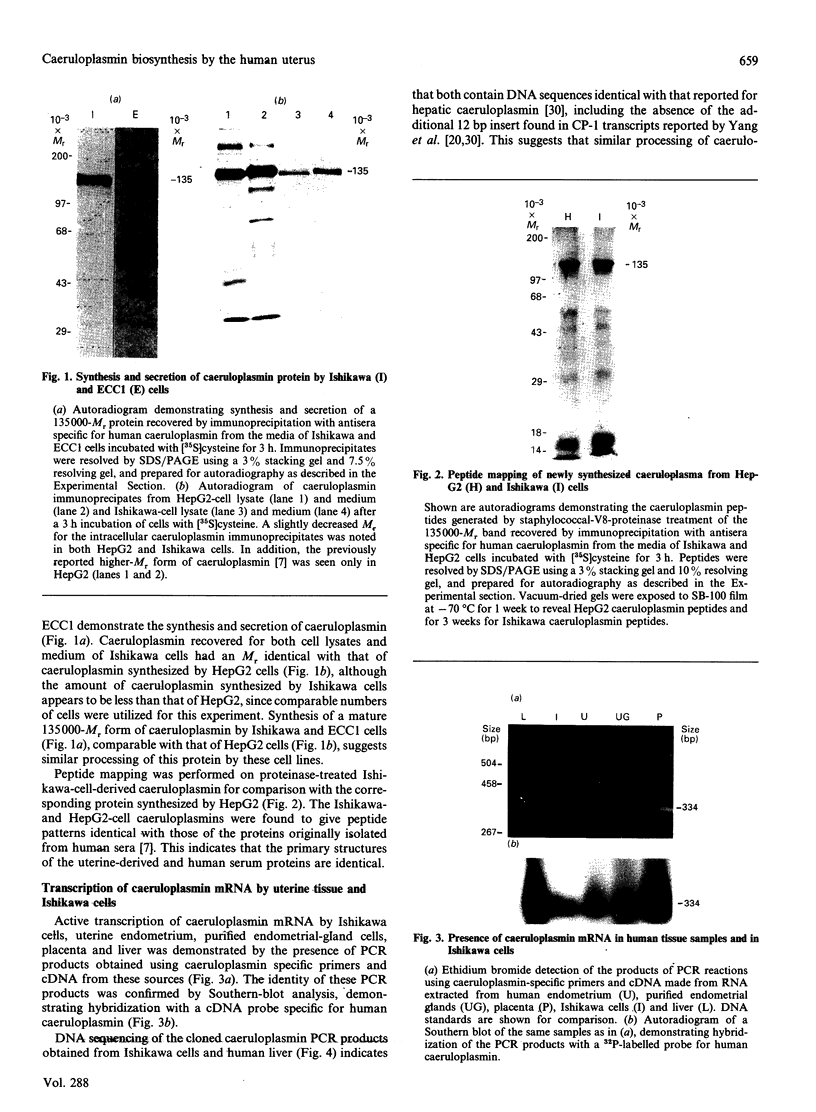
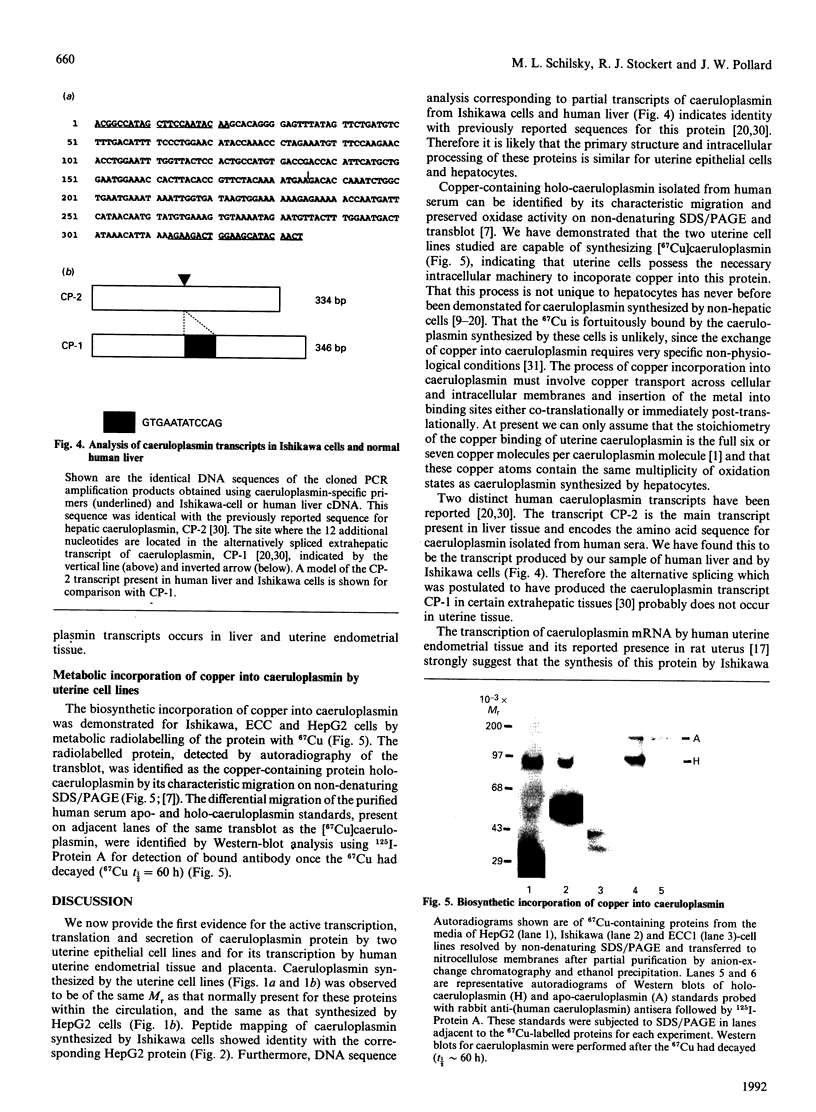
The blue copper protein caeruloplasmin is synthesized mainly by hepatocytes. An alternative transcript for caeruloplasmin produced in certain extrahepatic tissues, CP-1, contains an additional 12 nucleotides encoding 4 amino acids not present in the hepatic transcript, CP-2 [Yang, Friedrichs, Cupples, Bonifacio, S Sanford, Horton & Bowman (1990) J. Biol. Chem. 265, 10780-10785]. We have demonstrated transcription of caeruloplasmin mRNA by a well-differentiated human uterine epithelial adenocarcinoma cell line, Ishikawa, and by human uterine endometrium and purified endometrial glands. Identical CP-2 nucleotide sequences were obtained for partial caeruloplasmin transcripts from human liver and Ishikawa cells, indicating that CP-2 transcripts are produced by uterine epithelial lining cells. The synthesis of caeruloplasmin protein was demonstrated for Ishikawa cells and another uterine adenocarcinoma cell line, ECC1. Peptide-mapping analysis indicated that caeruloplasmin secreted by Ishikawa cells was structurally identical with the protein synthesized by the human hepatoblastoma cell line HepG2. The secretion of a 135,000-M(r) caeruloplasmin by Ishikawa and ECC1 cells, comparable with that of the human hepatoblastoma cell line, HepG2, indicated similar processing of uterine and hepatic caeruloplasmin. Incorporation of 67Cu into caeruloplasmin was demonstrated for Ishikawa and ECC1 cells, suggesting that the human uterus produces a bioactive form of caeruloplasmin and possesses the necessary metal transporters and intracellular machinery for copper incorporation into this protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldred A. R., Grimes A., Schreiber G., Mercer J. F. Rat ceruloplasmin. Molecular cloning and gene expression in liver, choroid plexus, yolk sac, placenta, and testis. J Biol Chem. 1987 Feb 25;262(6):2875–2878. [PubMed] [Google Scholar]
- Chu F. F., Olden K. The expression of ceruloplasmin, an angiogenic glycoprotein, by mouse embryonic fibroblasts. Biochem Biophys Res Commun. 1985 Jan 16;126(1):15–24. doi: 10.1016/0006-291x(85)90565-0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W. Peptide mapping in one dimension by limited proteolysis of sodium dodecyl sulfate-solubilized proteins. Methods Enzymol. 1983;96:222–229. doi: 10.1016/s0076-6879(83)96020-2. [DOI] [PubMed] [Google Scholar]
- Fleming R. E., Gitlin J. D. Primary structure of rat ceruloplasmin and analysis of tissue-specific gene expression during development. J Biol Chem. 1990 May 5;265(13):7701–7707. [PubMed] [Google Scholar]
- Fleming R. E., Whitman I. P., Gitlin J. D. Induction of ceruloplasmin gene expression in rat lung during inflammation and hyperoxia. Am J Physiol. 1991 Feb;260(2 Pt 1):L68–L74. doi: 10.1152/ajplung.1991.260.2.L68. [DOI] [PubMed] [Google Scholar]
- Frieden E. Caeruloplasmin: a multi-functional metalloprotein of vertebrate plasma. Ciba Found Symp. 1980;79:93–124. doi: 10.1002/9780470720622.ch6. [DOI] [PubMed] [Google Scholar]
- Gitlin J. D., Gitlin J. I., Gitlin D. Localizing of C-reactive protein in synovium of patients with rheumatoid arthritis. Arthritis Rheum. 1977 Nov-Dec;20(8):1491–1499. doi: 10.1002/art.1780200808. [DOI] [PubMed] [Google Scholar]
- Gitlin J. D. Transcriptional regulation of ceruloplasmin gene expression during inflammation. J Biol Chem. 1988 May 5;263(13):6281–6287. [PubMed] [Google Scholar]
- Holinka C. F., Hata H., Kuramoto H., Gurpide E. Responses to estradiol in a human endometrial adenocarcinoma cell line (Ishikawa). J Steroid Biochem. 1986 Jan;24(1):85–89. doi: 10.1016/0022-4731(86)90036-1. [DOI] [PubMed] [Google Scholar]
- Jaeger J. L., Shimizu N., Gitlin J. D. Tissue-specific ceruloplasmin gene expression in the mammary gland. Biochem J. 1991 Dec 15;280(Pt 3):671–677. doi: 10.1042/bj2800671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MORELL A. G., SCHEINBERG I. H. Preparation of an apoprotein from ceruloplasmin by reversible dissociation of copper. Science. 1958 Mar 14;127(3298):588–590. doi: 10.1126/science.127.3298.588-a. [DOI] [PubMed] [Google Scholar]
- Marklund S. L. Ceruloplasmin, extracellular-superoxide dismutase, and scavenging of superoxide anion radicals. J Free Radic Biol Med. 1986;2(4):255–260. doi: 10.1016/s0748-5514(86)80007-1. [DOI] [PubMed] [Google Scholar]
- Morell A. G., Van den Hamer C. J., Scheinberg I. H. Physical and chemical studies on ceruloplasmin. VI. Preparation of human ceruloplasmin crystals. J Biol Chem. 1969 Jul 10;244(13):3494–3496. [PubMed] [Google Scholar]
- Neifakh S. A., Monakhov N. K., Shaposhnikov A. M., Zubzhitski Y. N. Localization of ceruloplasmin biosynthesis in human and monkey liver cells and its copper regulation. Experientia. 1969 Apr 15;25(4):337–344. doi: 10.1007/BF01899902. [DOI] [PubMed] [Google Scholar]
- Ortel T. L., Takahashi N., Putnam F. W. Structural model of human ceruloplasmin based on internal triplication, hydrophilic/hydrophobic character, and secondary structure of domains. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4761–4765. doi: 10.1073/pnas.81.15.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampfer S., Tabibzadeh S., Chuan F. C., Pollard J. W. Expression of colony-stimulating factor-1 (CSF-1) messenger RNA in human endometrial glands during the menstrual cycle: molecular cloning of a novel transcript that predicts a cell surface form of CSF-1. Mol Endocrinol. 1991 Dec;5(12):1931–1938. doi: 10.1210/mend-5-12-1931. [DOI] [PubMed] [Google Scholar]
- Sato M., Schilsky M. L., Stockert R. J., Morell A. G., Sternlieb I. Detection of multiple forms of human ceruloplasmin. A novel Mr 200,000 form. J Biol Chem. 1990 Feb 15;265(5):2533–2537. [PubMed] [Google Scholar]
- Shvartsman A. L., Voronina O. V., Gaitskhoki V. S., Patkin E. L. Ekspressiia gena tseruloplazmina v organakh mlekopitaiushchikh po dannym gibridizatsionnogo analiza s komplementarnymi DNK-zondami. Mol Biol (Mosk) 1990 May-Jun;24(3):657–662. [PubMed] [Google Scholar]
- Skinner M. K., Griswold M. D. Sertoli cells synthesize and secrete a ceruloplasmin-like protein. Biol Reprod. 1983 Jun;28(5):1225–1229. doi: 10.1095/biolreprod28.5.1225. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S. Human endometrium: an active site of cytokine production and action. Endocr Rev. 1991 Aug;12(3):272–290. doi: 10.1210/edrv-12-3-272. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S., Kaffka K. L., Kilian P. L., Satyaswaroop P. G. Human endometrial epithelial cell lines for studying steroid and cytokine actions. In Vitro Cell Dev Biol. 1990 Dec;26(12):1173–1179. doi: 10.1007/BF02623695. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Ortel T. L., Putnam F. W. Single-chain structure of human ceruloplasmin: the complete amino acid sequence of the whole molecule. Proc Natl Acad Sci U S A. 1984 Jan;81(2):390–394. doi: 10.1073/pnas.81.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T., Schreiber G., Jaworowski A. Developmental patterns of gene expression of secreted proteins in brain and choroid plexus. Dev Biol. 1989 Jul;134(1):38–47. doi: 10.1016/0012-1606(89)90076-6. [DOI] [PubMed] [Google Scholar]
- Thomas T., Schreiber G. The expression of genes coding for positive acute-phase proteins in the reproductive tract of the female rat. High levels of ceruloplasmin mRNA in the uterus. FEBS Lett. 1989 Jan 30;243(2):381–384. doi: 10.1016/0014-5793(89)80166-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. W., Musto N. A., Mather J. P., Bardin C. W. Sertoli cells secrete both testis-specific and serum proteins. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7565–7569. doi: 10.1073/pnas.78.12.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F. M., Friedrichs W. E., Cupples R. L., Bonifacio M. J., Sanford J. A., Horton W. A., Bowman B. H. Human ceruloplasmin. Tissue-specific expression of transcripts produced by alternative splicing. J Biol Chem. 1990 Jun 25;265(18):10780–10785. [PubMed] [Google Scholar]
- Yang F., Naylor S. L., Lum J. B., Cutshaw S., McCombs J. L., Naberhaus K. H., McGill J. R., Adrian G. S., Moore C. M., Barnett D. R. Characterization, mapping, and expression of the human ceruloplasmin gene. Proc Natl Acad Sci U S A. 1986 May;83(10):3257–3261. doi: 10.1073/pnas.83.10.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]