Abstract
A 65-year-old Caucasian male was referred to an endodontic specialist practice in a private clinic in December 2019 for the management of an asymptomatic, radiolucent lesion located at the cervical level of the distal root of his right lower first molar, noticed during a routine periapical radiograph. After an accurate evaluation with cone-beam computed tomography (CBCT), the subgingival lesion was diagnosed as a supracrestal external cervical resorption (ECR), with a circumferential spread ⩽90°, confined to dentine without pulp involvement. The lesion was treated with the following sequence: (1) a full flap accessed the ECR, (2) the granulomatous tissue was removed from the root area, (3) the cavity was refreshed and filled with a well-refined and polished resin composite, (4) the flap was sutured at the cemento-enamel junction. A mandibular CBCT scan was performed before treatment, right after treatment, and 3 years postoperatively. Compared to the 3-year posttreatment CBCT scan, the immediate posttreatment one, revealed the absence of bone loss and an unexpected coronal bone remodeling with new bone formation over the treated lesion.
Keywords: Endodontics, dental restoration, bone regeneration, composite dental resin, root resorption, cone-beam computed tomography
Introduction
Root resorption can be defined as the loss of dental hard tissue (i.e., cementum, dentine) started on the external surface of the root as a consequence of odontoclastic action. 1 It is usually aggressive, causing a significant loss of tooth structure. 2 Root resorption usually occurs in the cervical region of the tooth, immediately below the epithelial attachment, 3 with a prevalence ranging from 0.02% to 2.3%.2,4 It has the potential to invade the root dentine in any direction and to varying extent. In advanced cases, external cervical resorption (ECR) can progress into the middle and apical thirds of the root or perforate into the root canal. There is no evidence of the precise etiology of ECR, 5 while recent studies support its multifactorial origin. The process is driven by osteoclast cells from the adjacent periodontium, which may invade and resorb the exposed root surface.5–7
Clinically, ECR may present as a cervical, irregular cavitation in the gingival contour and/or pinkish discoloration of the overlying enamel. However, the clinical findings can be variable depending on the severity and nature of the resorptive defect, tooth type, and stage of ECR. 5 In most cases, ECR is asymptomatic in the early stage, 7 and detection is often an incidental radiographic finding. 1 The affected tooth/teeth usually respond to pulp sensitivity tests, due to the presence of the pericanalar resorption-resistant sheet. 3 In advanced cases, the patient may present with pulpal and/or periapical symptoms. 8 Radiographically, ECR often presents as a radiolucency, and may be misdiagnosed as dental caries. Nevertheless, from a clinical perspective, the lesion presents has hard cavity, with profuse bleeding on probing. 8 Sometimes, the ECR may appear as an irregular, asymmetrical radiolucency where the root canal outline is detectable. Deposition of calcific tissue can result in a more radiopaque mottled appearance, which may represent a reparative stage in the development of the lesion.3,9
The management of ECR dependents on the accessibility and the restorability of the lesion. Furthermore, early diagnosis and appropriate management have been shown to improve the likelihood of tooth retention. 2 The treatment aims to inactivate the resorptive process by removing the resorptive tissue and sealing the defect on the root/tooth surface to prevent further clastic action.2,10 Several dental restorative materials have been proposed, such as amalgam, mineral trioxide aggregate, resin composite, glass ionomer cement, and resin-modified glass ionomer cement.11–14 Currently, there is not any material that ideally fulfills all the required characteristics, concerning adhesion, polishing and final finishing, biocompatibility, and esthetics. Moreover, the clinical site of restorations must be considered when managing the ECR, and indications and contraindications must be carefully evaluated. The management of ECR presented in this report underscores the potential for significant dental tissue preservation through timely and precise intervention. It highlights the broader clinical implications of such treatment strategies. This case demonstrates that teeth affected by ECR, which might otherwise be designated for monitoring or extraction, can be successfully treated and retained with appropriate diagnostic and treatment approaches. Thus, the insights from this case may encourage clinicians to adopt similar management strategies, potentially altering the standard care protocol for ECR and enhancing patient outcomes regarding tooth preservation and health.
The current case presentation aims to describe the treatment of a class 1 Heithersay, class 1Ad Patel et al. 15 subgingival ECR without pulp involvement, treated with composite material. This case is unique because of the new bone formation adjacent to the treated lesion, reported at the 3-year radiographic examination.
Case presentation
A 65-year-old Caucasian male was referred to an endodontic specialist practice in a private clinic in December 2019 for the management of an asymptomatic, radiolucent lesion located at the cervical level of the distal root of the right lower first molar noticed during a routine periapical radiograph. The only symptom described was a periodic itching in the gum area. At the clinical examination of the area, periodontal pocket depth was between 2 and 3 mm, with no bleeding on probing and no furcation involvement. In addition, the tooth was positive for the electrical pulp vitality test. The patient’s dental history revealed no past orthodontic treatment or bleaching as possible predisposing factors. However, occlusal wear was present at the working cusps of the tooth, indicating a parafunctional activity. The patient has a good medical history of ASA1 without reported allergies. Using the tube shift method, a second periapical radiograph was taken to evaluate the relationship between the lesion and the tooth (Figure 1(a) and (b)). Finally, a diagnosis of ECR was made.
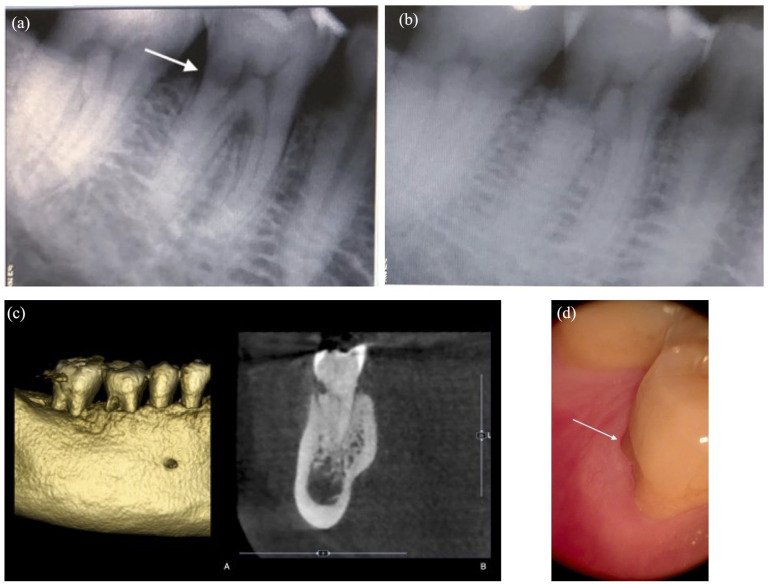
Figure 1.
Periapical radiographs were taken according to the horizontal tube shift method, which included eccentric (a) and orthoradial (b) projection. (b) Intraoral view of the right lower first molar. (c) Three- (A) and bidimensional (B) view of the lesion. (d) The ECR appears to be located over the buccal bone peak.
ECR: external cervical resorption.
The patient presented with a thick periodontal biotype, no history of tobacco use, good general health, and no contraindication to surgical treatment (Figure 1(c)). ECR is easily identified with the arrow.
A cone-beam computed tomography (CBCT) examination was requested to investigate the three-dimensional extent of the lesion to plan and discuss the treatment options with the patient (Figure 1(d)). At the CBCT examination, the ECR was classified as 1Ad according to Patel et al. 15 : 1 (height) at cemento-enamel junction (CEJ) level or coronal to the bone crest (supracrestal); a (circumferential spread) ⩽90°; and (proximity to the root canal) lesion confined to dentine.
After an accurate evaluation of the clinical and radiographic data and after the patient’s consultation, a conservative approach was chosen based on external repair of ECR using resin composite, without endodontic therapy. The patient was informed about the nature of the intervention and signed a written consent for the surgical and conservative procedure and the use of all the radiologic and clinical data for publication. The principles embodied in the Helsinki Declaration of 2013 were strictly adhered to. All the procedures were performed by an expert clinician (FZ). Informed consent was obtained from the patient involved in the study. The patient’s written permission has been obtained to publish this article. 16
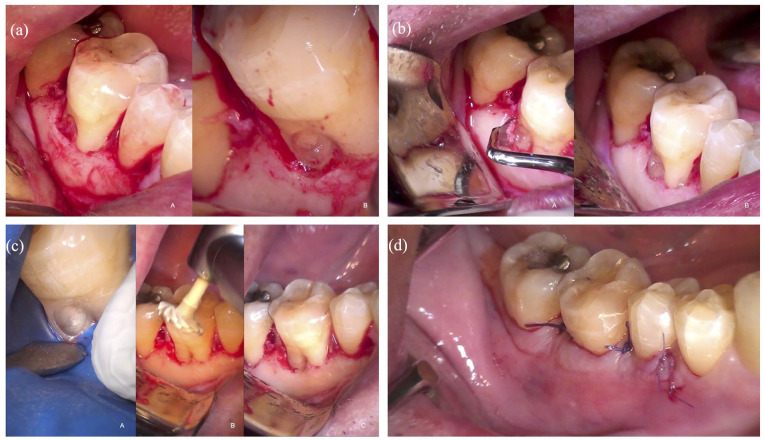
The patient received 2 g of amoxicillin 1 h before surgery (Zimox; Pfizer, Rome, Italy) and then 1 g twice daily for 8 days. The rationale for antibiotic prescription is due to an oral surgery maneuver with bone exposition. Local anesthesia was induced using articaine solution (4%) with epinephrine (1:100,000; Ubistein, 3M ESPE, Milan, Italy). By an expert oral surgeon (30+ years of experience), after local anesthesia, an intrasulcular incision was performed from the distal area of the second right lower molar to the mesial area of the second right lower premolar without releasing incisions. A mucoperiosteal flap was reflected to allow adequate access to the lesion (Figure 2(a-A) and (a-B)). The granulomatous tissue was removed from the root resorption area with a sharp excavator (HuFriedy EXC19; Hu-Friedy Italy Slr, Milan, Italy) under dental microscope examination (Leica M400; Mikros, Milan, Italy) (Figure 2(a)). To correct the inverse anatomy of the alveolar bone defect, minimizing the crater-like shape of the lesion, a bone remodeling was performed using hand instruments: Ochsenbein Periodontal Chisels (HuFriedy Och.1, Och.2; Hu-Friedy Italy Srl) and Rhodes Back-Action Periodontal chisel (HuFriedy C 36/37; Hu-Friedy Italy Srl) (Figure 2(b)). At this stage, a rubber dam was placed for the therapeutic approach. The reparative tissue was refreshed with a Muller burr (KOMET H1SML31.205.010; KOMET Italia Srl, Milan, Italy) and ultrasonic tip (SF 66; KOMET Italia Srl) to provide a dentin surface suitable for bonding to resin (Figure 2(c-A)).
Figure 2.
(a) Higher magnification of ECR lesion after mucoperiosteal flap reflection (A). After excavation, a shallow vestibular intrabony defect is evident at the bottom of the lesion (B). (b) Correction of the inverse anatomy of the marginal osseous defect using hand instrument (A, B). (c-A) Dentin surface suitable for bonding to resin, application of bonding material, (B) restoration polishing, (C) restoration refined and polished. (d) Flap sutured at the CEJ level.
ECR: external cervical resorption; CEJ: cemento-enamel junction.
A small cotton pledge embedded with 5% sodium hypochlorite was carefully applied to the resorptive site for 3–4 min, to promote coagulation necrosis of this tissue as it penetrates the smaller, more inaccessible recesses and resorptive channels which may not be identified and debrided by mechanical instrumentation alone. 16 It is important to consider that when NaOCl residues are not thoroughly removed from the dentin surface, they can oxidize the organic components, particularly collagen, essential for bonding resin-based materials. This oxidation impairs the ability of dentin bonding agents to effectively penetrate and interlock with the dentin matrix, leading to a compromised bond strength. The residual NaOCl can also directly interfere with the resin’s polymerization, affecting the restoration’s durability and integrity. Thus, ensuring the complete removal of NaOCl and proper neutralization of its effects is crucial for optimal resin adhesion and the longevity of restorative dental work. 17 A two-step self-etching adhesive was used (Clearfil SE BOND 2; Kuraray Noritake, Hattersheim, Germany) and the cavity was then filled with resin composite (Enamel Plus Hri; Micerium, Avegno (GE), Italy) (Figure 2(c)). The restoration was accurately refined and polished using diamond composite polishers (KOMET Italia Srl) (Figure 2(c-B) and (c-C)). The flap was sutured at the CEJ with 5-0 Vicyrl suture by completely covering the restored cavity (5-0 Vicryl, Ethicon; Johnson & Johnson, Pomezia, Italy) (Figure 2(d)).
Ketoprofen 80 mg (Oki; Dompé, Milan, Italy) was prescribed every 12 h when needed. The patient was instructed not to brush the surgical wound, to use chlorhexidine spray (Curasept SpA, Saronno, Italy) on the wound thrice daily, and to follow a soft food diet for 2 weeks. A complete sequence of the surgical and restorative procedures is shown (Figure 2).
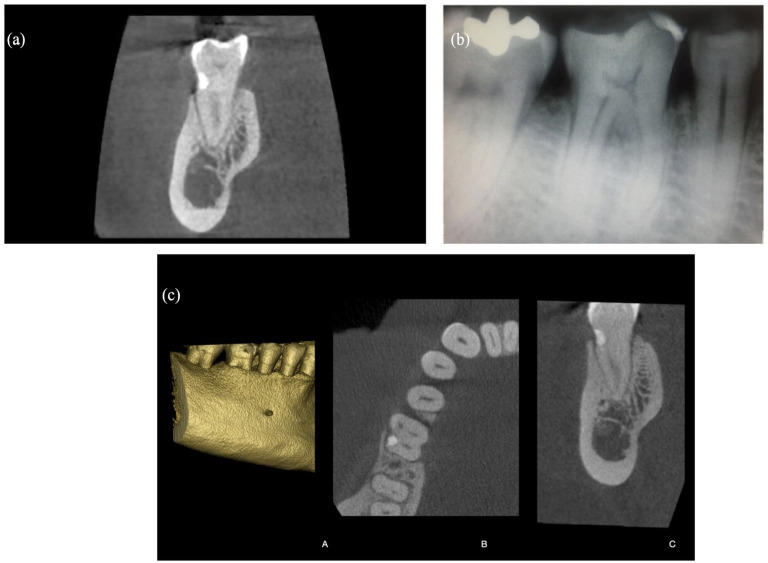
A postoperative CBCT scan was done to evaluate the relationship between the alveolar bone, the restored root, the pulp cavity, and the distal canal of the treated tooth (Figure 3(a)). During the 1-year follow-up examination, a clinical and radiographic follow-up was done. The treated tooth was vital, and the surrounding tissues were healthy (Figure 3(b)). The tooth was positive at the pulp sensitivity test during all follow-up periods.
Figure 3.
(a) Postoperative CBCT scan taken immediately after treatment. (b) Periapical radiograph at the 1-year follow-up. (c) CBCT Scan after 3 years of the therapy: paraxial view (A), axial view (B), and three-dimensional view (C) of the treated lesion. Bone remodeling occurred with new bone formation over the treated lesion.
CBCT: cone-beam computed tomography.
This maneuver has been performed with an optical 4.5× magnification system. Three years after treatment, the tooth is still vital. The patient underwent a new CBCT scan of the lower jaw for implant purposes in the third quadrant. Nevertheless, the CBCT revealed no bone loss and a coronal bone remodeling with new bone formation over the treated lesion (Figure 3c). Clinically, the gingival attachment appeared healthy with a probing depth of ⩽3 mm and no tooth mobility. Additional follow-up examinations were planned every year.
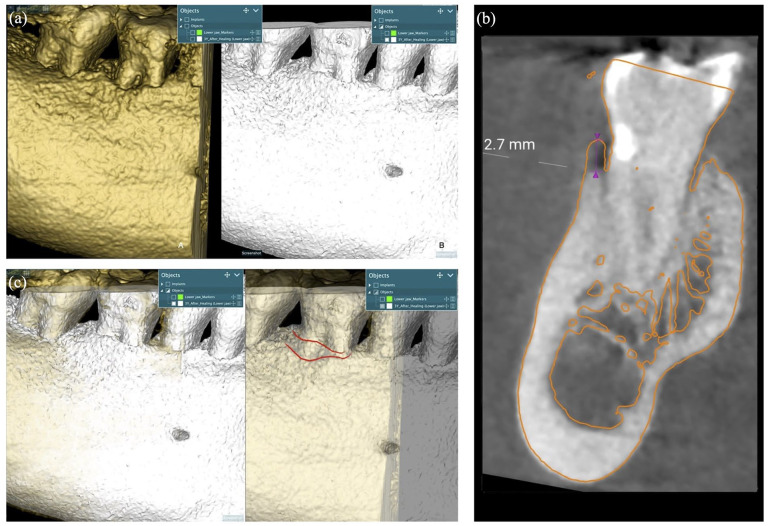
The 3-year CBCT scan was superimposed with the postoperative CBCT scan to evaluate the amount of bone remodeling over the treated lesion (Realguide software suite 5.1; 3DIEmme, Srl, Cantù (CO), Italy). The DICOM data were segmented to create STL files of the 3-year CBCT scan. Then, the generated 3D mesh object was matched point by point using the same regions of interest. The matching was then improved using a best-fit algorithm (Realguide software suite 5.1; 3DIEmme). Finally, bone augmentation was measured at the center of the lesion (Figure 4); the range amount varies from a minimum of 0.2 mm to a maximum of 1.2 mm.
Figure 4.
Postoperative CBCT scan (a); CBCT scan after 3 years of the treatment (b). Superimposition of the 3-year CBCT scan with the postoperative CBCT (a); amount of bone remodeling over the treated lesion (b). (c) Vertical bone augmentation remodeling was measured at the center of the lesion after matching improvement using a best-fit algorithm.
CBCT: cone-beam computed tomography.
Discussion
In the present case report, aggressive ECR was treated with a surgical approach and filled with composite material without endodontic treatment. After 3 years, the bone was remodeled over the composite material, which makes the healing process exceptional. A CBCT scan in the lower arch for implant purposes clearly showed this.
Adequate and predictable management of ECR depends on the early and accurate assessment of the true nature of the lesion. Several studies have shown that ECR may have an unusual and complex pattern of invasion, which can make evaluation challenging. 18 Complex patterns of ECR invasion, portal of entry, small channels, and their interconnections with the periodontal ligament should also be evaluated.19,20 For the latter, periapical radiographs may not reflect the true nature of the resorptive process, compared to CBCT scan.8,19,21 Instead, CBCT is a reliable diagnostic tool for assessing ECR. This gives a three-dimensional insight into the lesion and the ability to determine the most suitable treatment plan.17,20 In the present study, the ECR was classified as class 1Ad according to Patel et al. 15 Early diagnosis and management contribute to the successful treatment results.
Moreover, no other articles reported bone remodeling over the composite material to the authors’ knowledge. Heithersay, in 1999, 14 reviewed the outcome of 101 surgically treated ECR cases and reported a 100%, 77.8%, and 12.5% success rate for classes 1 and 2 (combined), 3 and 4, respectively. 21 These results indicate that extensive and potentially less accessible lesions may have a poor prognosis.
It is well known that the degree of roughness of restorations is also associated with plaque accumulation and gingival inflammation. 22 If the resorptive defect is near or in communication with the sulcus, the restorative material will probably be in contact with saliva and bacteria in the oral cavity. When communicating with the oral environment, the cavity should be filled with a composite resin or glass-ionomer cement restoration. In the present clinical case, a well-finished and polished composite resin was used to restore the residual cavity. It is also important to accurately choose the suitable material to restore the resorptive defect. Subgingival restorations may cause direct trauma to the periodontal tissues or facilitate subgingival plaque accumulation with consequent inflammatory alterations and/or recession of adjacent gingival tissue.23–25 Parma-Benfenali et al., 26 also observed inflammation and bone resorption along the restoration surface following cavity preparation and amalgam restoration placement at the bone margin level. This response suggests a possible attempt of the organism to reestablish the dimension of the supracrestal attached tissues. Additional authors observed bone resorption around teeth prosthetically prepared or root planed to the crest of bone, allowing the formation of the new supracrestal attached tissues.27–29 The literature presents a similar case by Okamoto et al. 30 ; the article details a compelling case study on repairing an extensive ECR lesion utilizing intentional replantation with a crown rotation technique. Intentional replantation with crown rotation saved a severely affected tooth due to ECR. A successful comprehensive plan included orthodontic extrusion, surgical extraction, tooth rotation, and replantation. Epithelial attachment refers to the complex biological interface between the epithelial tissue and the tooth surface, typically at or below the gingival line. This attachment is crucial for maintaining periodontal health as it is a barrier against bacterial invasion and helps stabilize the gingiva around the teeth. The integrity of the epithelial attachment is pivotal in preventing periodontal diseases and is a key focus in therapeutic interventions to preserve or restore periodontal health. Subgingival reconstruction, on the other hand, involves various dental procedures that aim to restore the structure and function of teeth and their supporting tissues below the gum line. This can include managing subgingival caries, placing restorations like fillings or crowns that extend beneath the gum tissue, and surgical interventions such as flap surgery to access and treat root surfaces affected by resorption or periodontal disease. The goal of subgingival reconstruction is to restore the tooth to its normal function and aesthetics while ensuring the reestablishment of a healthy and stable periodontal environment, which includes the reformation of a functional epithelial attachment to the tooth structure. These procedures require meticulous care to prevent damage to the periodontal tissues and ensure the compatibility of restorative materials with the biological tissues.29,30 The studies reviewed explored various aspects of external root resorption (ERR) detection, management, and etiology using advanced imaging modalities and clinical interventions. Studies31,33,34 underscored the superior diagnostic accuracy of CBCT over digital periapical radiography for identifying ERR lesions, particularly in complex cases and nonendodontically treated teeth. CBCT also proved valuable in assessing root morphology changes following orthodontic treatments. 32 In addition, studies highlighted the prevalence 35 and clinical implications of multiple ERR in systemic sclerosis patients, suggesting new insights into its manifestations. Diagnostic advancements, such as optimizing voxel size in CBCT scans, 36 were shown to enhance detection efficacy while minimizing radiation exposure. Management strategies discussed included effective interventions37,38 and the role of regenerative endodontic procedures in halting resorption progression. 48 The review emphasized the importance of accurate diagnosis39,40-42 and tailored treatment approaches using biocompatible materials5,43-51 to preserve tooth structure and function.
Effective management of the lesion is also crucial. Good visualization and accessibility to the lesion represent another key factor.1,19 Coronal remodeling of bone is a possible healing modality over a well-finished and polished resin composite filling. The present study used an operative dental microscope to approach and treat the lesion. Although the use of the microscope is increasing and the benefits of optical magnification have been recognized, its general use in dental practice still needs to be improved. 52
Conclusions
Early diagnosis and proper treatment are essential to ensure a longer prognosis of the restored teeth. A CBCT scan should be recommended for diagnosis and assessment of resorptive lesions. The management is focused on completely removing and restoring the resorptive tissue. This knowledge may influence clinicians to treat and save teeth with ECR lesions that they otherwise would have monitored or recommended for extraction. Despite the limitations of a case report and the anecdotal value of the related information, this case shows that coronal remodeling of bone is a possible healing modality over a well-finished and polished resin composite filling.
Footnotes
Author contributions: F.Z. and G.P.: Conceptualization; G.C.: Data Curation; A.H., L.F., and G.C.: Writing—Original draft; M.C., M.T., and L.F.: Writing—Review and editing. All authors read and approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Informed consent was obtained from all subjects involved in the study. Written, educated permission has been obtained from the patient to publish this article.
ORCID iD: Artak Heboyan  https://orcid.org/0000-0001-8329-3205
https://orcid.org/0000-0001-8329-3205
References
- 1. Patel S, Kanagasingam S, Pitt Ford T. External cervical resorption: a review. J Endod 2009; 35(5): 616–625. [DOI] [PubMed] [Google Scholar]
- 2. Heithersay GS. Invasive cervical resorption: an analysis of potential predisposing factors. Quintessence Int 1999; 30(2): 83–95. [PubMed] [Google Scholar]
- 3. Mavridou AM, Hauben E, Wevers M, et al. Understanding external cervical resorption in vital teeth. J Endod 2016; 42(12): 1737–1751. [DOI] [PubMed] [Google Scholar]
- 4. Irinakis E, Aleksejuniene J, Shen Y, et al. External cervical resorption: a retrospective case-control study. J Endod 2020; 46(10): 1420–1427. [DOI] [PubMed] [Google Scholar]
- 5. Patel S, Mavridou AM, Lambrechts P, et al. External cervical resorption-part 1: histopathology, distribution and presentation. Int Endod J 2018; 51(11): 1205–1223. [DOI] [PubMed] [Google Scholar]
- 6. Gold SI, Hasselgren G. Peripheral inflammatory root resorption. A review of the literature with case reports. J Clin Periodontol 1992; 19(8): 523–534. [DOI] [PubMed] [Google Scholar]
- 7. Li XY, Zou XY, Yue L. Pathogenesis and classification of tooth resorption. Chinese J Stomatol 2022; 57(11): 1177–118. [DOI] [PubMed] [Google Scholar]
- 8. Iqbal MK. Clinical and scanning electron microscopic features of invasive cervical resorption in a maxillary molar. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 103(6): e49–e54. [DOI] [PubMed] [Google Scholar]
- 9. Patel S, Dawood A, Wilson R, et al. The detection and management of root resorption lesions using intraoral radiography and cone beam computed tomography—an in vivo investigation. Int Endod J 2009; 42(9): 831–838. [DOI] [PubMed] [Google Scholar]
- 10. Evans RI. Invasive cervical resorption—a periodontist’s perspective. Ann R Australas Coll Dent Surg 2000; 15: 327–330. [PubMed] [Google Scholar]
- 11. White C, Jr., Bryant N. Combined therapy of mineral trioxide aggregate and guided tissue regeneration in treating external root resorption and an associated osseous defect. J Periodontol 2002; 73(12): 1517–1521. [DOI] [PubMed] [Google Scholar]
- 12. Aiuto R, Fumei G, Lipani E, et al. Conservative therapy of external invasive cervical resorption with adhesive systems: a 6-year follow-up case report and literature review. Case Rep Dent 2022; 2022: 9620629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vinothkumar TS, Tamilselvi R, Kandaswamy D. Reverse sandwich restoration for managing invasive cervical resorption: a case report. J Endod 2011; 37(5): 706–710. [DOI] [PubMed] [Google Scholar]
- 14. Heithersay GS. Treatment of invasive cervical resorption: an analysis of results using topical application of trichloroacetic acid, curettage, and restoration. Quintessence Int 1999; 30(2): 96–110. [PubMed] [Google Scholar]
- 15. Patel S, Foschi F, Mannocci F, et al. External cervical resorption: a three-dimensional classification. Int Endod J 2018; 51(2): 206–214. [DOI] [PubMed] [Google Scholar]
- 16. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310(20): 2191–2194. [DOI] [PubMed] [Google Scholar]
- 17. Yuan Y, Intajak P, Islam R, et al. Effect of sodium hypochlorite on bonding performance of universal adhesives to pulp chamber dentin. J Dent Sci 2023; 18(3): 1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vaz de Souza D, Schirru E, Mannocci F, et al. External cervical resorption: a comparison of the diagnostic efficacy using 2 different cone-beam computed tomographic units and periapical radiographs. J Endod 2017; 43(1): 121–125. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz RS, Robbins JW, Rindler E. Management of invasive cervical resorption: observations from three private practices and a report of three cases. J Endod 2010; 36(10): 1721–1730. [DOI] [PubMed] [Google Scholar]
- 20. Gunst V, Mavridou A, Huybrechts B, et al. External cervical resorption: an analysis using cone beam and microfocus computed tomography and scanning electron microscopy. Int Endod J 2013; 46(9): 877–887. [DOI] [PubMed] [Google Scholar]
- 21. Estevez R, Aranguren J, Escorial A, et al. Invasive cervical resorption Class III in a maxillary central incisor: diagnosis and follow-up using cone-beam computed tomography. J Endod 2010; 36(12): 2012–2014. [DOI] [PubMed] [Google Scholar]
- 22. Waerhaug J. Effect of rough surfaces upon gingival tissue. J Dent Res 1956; 35(2): 323–325. [DOI] [PubMed] [Google Scholar]
- 23. Trope M. Root resorption of dental and traumatic origin: classification based on etiology. Pract Periodontics Aesthet Dent 1998; 10(4): 515–522. [PubMed] [Google Scholar]
- 24. Heithersay GS. Management of tooth resorption. Aust Dent J 2007; 52(1 Suppl): S105–S121. [DOI] [PubMed] [Google Scholar]
- 25. Sirajuddin S, Narasappa KM, Gundapaneni V, et al. Iatrogenic damage to periodontium by restorative treatment procedures: an overview. Open Dent J 2015; 9: 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parma-Benfenali S, Fugazzoto PA, Ruben MP. The effect of restorative margins on the postsurgical development and nature of the periodontium. Part I. Int J Periodontics Restorative Dent 1985; 5(6): 30–51. [PubMed] [Google Scholar]
- 27. Heboyan A, Avetisyan A, Karobari MI, et al. Tooth root resorption: a review. Sci Prog 2022; 105(3): 368504221109217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carnevale G, Sterrantino SF, Di Febo G. Soft and hard tissue wound healing following tooth preparation to the alveolar crest. Int J Periodontics Restorative Dent 1983; 3(6): 36–53. [PubMed] [Google Scholar]
- 29. Wyatt CCL. The effect of prosthodontic treatment on alveolar bone loss: a literature review. J Prosthet Dent 1998; 80(3): 362–366. [DOI] [PubMed] [Google Scholar]
- 30. Okamoto M, Asahi Y, Duncan HF, et al. Repair of an extensive external cervical resorption lesion using intentional replantation with crown rotation. Case Rep Dent 2023; 2023: 2103999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parrales-Bravo C, Friedrichsdorf SP, Costa C, et al. Does endodontics influence radiological detection of external root resorption? An in vitro study. BMC Oral Health 2023; 23: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pereira ABN, Almeida R, Artese F, et al. External root resorption evaluated by CBCT 3D models superimposition. Dental Press J Orthod 2022; 27(2): e2219315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi J. Risk factors for external root resorption of maxillary second molars associated with third molars. Imaging Sci Dent 2022; 52(3): 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sayyad Soufdoost R, Jamali Ghomi A, Labbaf H. Endodontic management of a tooth with apical overfilling and perforating external root resorption: a case report. Clin Case Rep 2020; 8(12): 3278–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Memida T, Matsuda S, Kajiya M, et al. Multiple external root resorption of teeth as a new manifestation of systemic sclerosis—a cross-sectional study in Japan. J Clin Med 2019; 8(10): 1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nikneshan S, Valizadeh S, Javanmard A, et al. Effect of voxel size on detection of external root resorption defects using cone beam computed tomography. Iran J Radiol 2016; 13(3): 34985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahangari Z, Nasser M, Mahdian M, et al. Interventions for the management of external root resorption. Cochrane Database Syst Rev 2015; 2015(11): CD008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kheirieh S, Fazlyab M, Torabzadeh H, et al. Extraoral retrograde root canal filling of an orthodontic-induced external root resorption using CEM cement. Iran Endod J 2014; 9(2): 149–152. [PMC free article] [PubMed] [Google Scholar]
- 39. Hegde N, Hegde MN. Internal and external root resorption management: a report of two cases. Int J Clin Pediatr Dent 2013; 6(1): 44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bolhari B, Meraji N, Nosrat A. Extensive idiopathic external root resorption in first maxillary molar: a case report. Iran Endod J 2013; 8(2): 72–74. [PMC free article] [PubMed] [Google Scholar]
- 41. Dalili Z, Taramsari M, Mousavi Mehr SZ, et al. Diagnostic value of two modes of cone-beam computed tomography in evaluation of simulated external root resorption: an in vitro study. Imaging Sci Dent 2012; 42(1): 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jung YH, Cho BH. External root resorption after orthodontic treatment: a study of contributing factors. Imaging Sci Dent 2011; 41(1): 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sreeja R, Minal C, Madhuri T, et al. A scanning electron microscopic study of the patterns of external root resorption under different conditions. J Appl Oral Sci 2009; 17(5): 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abbott PV. Prevention and management of external inflammatory resorption following trauma to teeth. Aust Dent J 2016; 61(Suppl 1): 82–94. [DOI] [PubMed] [Google Scholar]
- 45. Iandolo A, Pisano M, Abdellatif D, et al. Effectiveness of different irrigation techniques on post space smear layer removal: SEM evaluation. Prosthesis 2023; 5(2): 539–549. [Google Scholar]
- 46. Marchese M, Denise PIK, Ferrari Cagidiaco E, et al. Endodontic irrigants and their activation efficacy on cleansing post-space root canal walls. Prosthesis 2021; 3(4): 406–414. [Google Scholar]
- 47. Li Q, Deacon AD, Coleman NJ. Iodoform-blended portland cement for dentistry. Prosthesis 2020; 2(4): 277–296. [Google Scholar]
- 48. Yoshpe M, Einy S, Ruparel N, et al. Regenerative endodontics: a potential solution for external root resorption (case series). J Endod 2020; 46(2): 192–199. [DOI] [PubMed] [Google Scholar]
- 49. Warnsinck CJ, Shemesh H. [External cervical root resorption]. Ned Tijdschr Tandheelkd 2018; 125(2): 109–115. [DOI] [PubMed] [Google Scholar]
- 50. Heithersay GS. External root resorption. Ann R Australas Coll Dent Surg 1994; 12: 46–59. [PubMed] [Google Scholar]
- 51. Rotondi O, Waldon P, Kim SG. The Disease process, diagnosis and treatment of invasive cervical resorption: a review. Dent J 2020; 8(3): 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bud MG, Pop OD, Cîmpean S. Benefits of using magnification in dental specialties—a narrative review. Med Pharm Rep 2023; 96(3): 254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]