Abstract
Background.
Anti-infective shortages are a pervasive problem in the United States. The objective of this study was to identify any associations between changes in prescribing of antibiotics that have a high risk for CDI during a piperacillin/tazobactam (PIP/TAZO) shortage and hospital-onset Clostridium difficile infection (HO-CDI) risk in 88 US medical centers.
Methods.
We analyzed electronically captured microbiology and antibiotic use data from a network of US hospitals from July 2014 through June 2016. The primary outcome was HO-CDI rate and the secondary outcome was changes in antibiotic usage. We fit a Poisson model to estimate the risk of HO-CDI associated with PIP/TAZO shortage that were associated with increased high-risk antibiotic use while controlling for hospital characteristics.
Results.
A total of 88 hospitals experienced PIP/TAZO shortage and 72 of them experienced a shift toward increased use of high-risk antibiotics during the shortage period. The adjusted relative risk (RR) of HO-CDI for hospitals experiencing a PIP/TAZO shortage was 1.03 (95% confidence interval [CI], .85–1.26; P = .73). The adjusted RR of HO-CDI for hospitals that both experienced a shortage and also showed a shift toward increased use of high-risk antibiotics was 1.30 (95% CI, 1.03–1.64; P < .05).
Conclusions.
Hospitals that experienced a PIP/TAZO shortage and responded to that shortage by shifting antibiotic usage toward antibiotics traditionally known to place patients at greater risk for CDI experienced greater HO-CDI rates; this highlights an important adverse effect of the PIP/TAZO shortage and the importance of antibiotic stewardship when mitigating drug shortages.
Keywords: Clostridium difficile, drug shortage, piperacillin/tazobactam
Anti-infective shortages have been an increasingly pervasive problem worldwide [1]. In a recent survey of 701 infectious diseases physicians in North America, 70% noted that they have had to modify anti-infective prescribing in the prior 2 years due to a drug shortage [2]. To date, it has been difficult to characterize the adverse effects of these anti-infective shortages on patient clinical outcomes at a population health level. At the same time, Clostridium difficile infection (CDI) is among the most common healthcare-associated infections in the United States and is associated with 15 000–29 000 deaths each year [3, 4]. The risk of CDI is increased with the use of high-risk antibiotics such as cephalosporins, fluoroquinolones, and clindamycin whereas some agents, including piperacillin/tazobactam (PIP/TAZO), have been found to be protective against CDI [5–8].
In December 2014, a PIP/TAZO shortage due to manufacturing problems with a major supplier affected many US hospitals [9]. Previous studies suggest that PIP/TAZO may be associated with a lower risk of CDI relative to other antibiotics in part due to its intrinsic activity against C. difficile [6, 10, 11]. Notably, 2 single-center studies suggested that a previous PIP/TAZO shortage and subsequent increases in cephalosporin use (ie ceftriaxone, cefotetan, cefotaxime) may have contributed to increased CDI rates in 2002 [5, 12]. Therefore, the objective of this study was to identify any associations between changes in antibiotic prescribing during the PIP/TAZO shortage and hospital-onset CDI (HO-CDI) risk in a large collection of US medical centers.
METHODS
Data
We used electronically captured detailed microbiological and pharmacy order data in MedMined, one of the clinical research databases, from Becton Dickinson and Company (Franklin Lakes, New Jersey) from 1 July 2014 through 30 June 2016 for the study. The electronic surveillance system and clinical research database have been previously described elsewhere [13–15]. The main data elements in the research database used for the current study included antibiotic days of therapy (DOT) per 1000 patient-days and C. difficile test results during the study period. The study protocol was approved by the New England Institutional Review Board (Wellesley, Massachusetts).
Definition of the Piperacillin/Tazobactam Shortage
The general shortage period was determined a priori to correspond to the national shortage period starting at the end of December 2014. We identified the shortage quarter that showed the greater percentage decrease in PIP/TAZO use among the first 2 quarters of 2015 as the “shortage period,” in comparison to the last 2 quarters of 2014 (pre–shortage period). A hospital was considered to experience a PIP/TAZO shortage if there was a statistically significant decrease in at least 1 of the first 2 quarters of 2015 compared to the last 2 quarters of 2014. The severity of PIP/TAZO shortage was classified for each hospital as either mild (≤33% decrease), moderate (34%–66% decrease), or severe (>66% decrease).
Definition of High-, Medium-, and Low-Risk Antibiotics for Acquisition of CDI
A priori categorization of classes of antibiotics and their associated CDI risk was done by literature review and obtaining consensus among 2 pharmacists (A. E. G. and V. G.) and 3 physicians (S. C. B., A. S., and R. S. J.) specializing in infectious diseases or gastroenterology [5, 16–19]. Antibiotics classified as having the highest risk for CDI included clindamycin; fluoroquinolones (ciprofloxacin, moxifloxacin, gatifloxacin, levofloxacin); carbapenems (ertapenem, meropenem, doripenem, imipenem); second/third/fourth-generation cephalosporins (cefotaxime, cefepime, ceftriaxone, ceftazidime, cefuroxime, cefotetan, cefoxitin); aztreonam; and ampicillin/sulbactam.
Outcome
A CDI case was defined as a positive result of C. difficile toxin or molecular assay of a stool specimen obtained from a patient without a positive assay in the previous 8 weeks. Of these CDI cases, HO-CDI was defined if the positive specimen was collected >3 calendar days after hospital admission.
Statistical Analysis
We first conducted univariate analysis using the Wilcoxon test on the difference between PIP/TAZO during the pre–shortage period vs shortage period and high-risk antibiotics use between the pre–shortage period and shortage period. We used DOT per 1000 days at risk (DAR), a commonly used analytic unit used by the Centers for Disease Control and Prevention and others, to evaluate drug use [20].
We modeled CDI rate differences before and during the shortage using Poisson regression with a difference in differences approach. The difference in differences approach regresses the outcome on indicators of the period, the group, and an interaction term between period and group. The 2 groups correspond to those hospitals that responded to the shortage with a shift toward antibiotics with a high-risk for CDI and those that did not shift. The interaction term represents the difference in differences estimator in CDI events pre- and post-PIP/TAZO shortage for those hospitals that responded with increased use of high-risk antibiotics vs those hospitals that did not shift toward use of high-risk antibiotics. Hospital teaching status, urban/rural status, and number of beds were controlled for. A post hoc subgroup analysis was conducted 1 year following the shortage period to characterize any recovery of antibiotic use to pre-shortage values. All analyses were conducted using SAS software version 9.2 (SAS Institute, Cary, North Carolina).
RESULTS
Piperacillin/Tazobactam Use Before Versus During the Shortage
Of 107 hospitals in the MedMined Clinical Research Database, 88 hospitals experienced a PIP/TAZO shortage. Among the 88 hospitals, 12 (14%) were teaching and 76 (86%) were nonteaching; 12 (14%) were rural, 76 (86%) were urban; and 6 (7%), 31 (35%), and 51 (58%) had <100, 100–300, and >300 beds, respectively. For hospitals experiencing a mild shortage (n = 39), the pooled pre–shortage period vs shortage period PIP/TAZO use was 72.1 vs 63.3 DOT/1000 DAR (P = .08) (Table 1). For hospitals experiencing a moderate shortage (n = 20), the corresponding pooled use was 83.5 vs 41.6 DOT/1000 DAR (P = .0001). For hospitals experiencing a severe shortage (n = 29), the corresponding pooled PIP/TAZO use was 79.1 vs 11.9 DOT/1000 DAR (P < .0001).
Table 1.
Descriptive Statistics
| Pre–Shortage Period |
Shortage Period |
|||||
|---|---|---|---|---|---|---|
| Shortage and Change | No. of Hospitals | Pooled | Median (1st, 3rd Quartiles) | Pooled | Median (1st, 3rd Quartiles) | P Value (Wilcoxon) |
|
| ||||||
| PIP/TAZO use (DOT/1000 DAR) | ||||||
| Mild shortage (≤33% reduction) | 39 | 72.1 | 68.0 (53.9, 86.1) | 63.3 | 58.4 (50.4, 78.3) | .0809 |
| Moderate shortage (34%–66% reduction) | 20 | 83.5 | 89.1 (674, 104.3) | 41.6 | 43.8 (33.8, 54.1) | <.0001 |
| Severe shortage (>66% reduction) | 29 | 79.1 | 80.8 (676, 100.7) | 11.9 | 8.1 (1.9, 19.3) | <.0001 |
| Moderate/severe combined | 49 | 80.8 | 81.4 (676, 100.7) | 23.6 | 19.6 (3.4, 38.7) | <.0001 |
| High-risk antibiotic use change (DOT/1000 DAR) | ||||||
| No increase (≤ 0%) | 16 | 237.0 | 239.5 (216.7, 294.7) | 224.8 | 222.3 (199.6, 273.3) | .2911 |
| Minor increase (1%–10%) | 21 | 239.0 | 274.1 (218.0, 303.2) | 250.9 | 289.7 (230.0, 321.0) | .0061 |
| Intermediate increase (11%–30%) | 35 | 271.2 | 2876 (222.0, 329.2) | 321.0 | 341.1 (268.4, 396.0) | <.0001 |
| Large increase (>30%) | 16 | 224.4 | 215.6 (1872, 291.2) | 309.0 | 319.1 (259.4, 3872) | <.0001 |
| All 3 increasing groups combined | 72 | 253.2 | 270.2 (212.2, 313.5) | 301.5 | 308.2 (252.1,385.4) | <.0001 |
Abbreviations: DAR, days at risk; DOT, days of therapy; PIP/TAZO, piperacillin/tazobactam.
Change in the Use of High-Risk Antibiotics
Of the 88 hospitals who experienced a PIP/TAZO shortage, 16 hospitals had no increase in high-risk antibiotic use during the PIP/TAZO shortage period; their pooled high-risk antibiotic use was 237.0 vs 224.8 DOT/1000 DAR (P = .29) (Table 1). The remaining 72 hospitals experienced different levels of increased high-risk antibiotic use (1%–10% [n = 21], 11%–30% [n = 35], and >30% [n = 16]); the high-risk antibiotic use was significantly greater in the shortage period for all 3 levels of high-risk antibiotic use (all P < .01). Changes in high-risk antibiotic use by antibiotic class are listed in Table 2. Most of the changes in antibiotic prescribing before and during the shortage among cephalosporins were due to increases in cefepime use (mean, 24.9 and 44.2 DOT/1000 DAR, respectively; P < .0001).
Table 2.
Use of Antibiotics With High Risk for Clostridium difficile infection Before and During the Piperacillin/Tazobactam Shortage
| Pre–Shortage Period |
Shortage Period |
||||
|---|---|---|---|---|---|
| Antibiotic | Pooled | Median (1st, 3rd Quartiles) | Pooled | Median (1st, 3rd Quartiles) | P Value |
|
| |||||
| β-lactams | |||||
| Ampicillin/sulbactam | 8.22 | 6.46 (2.96, 10.95) | 10.65 | 8.75 (4.28, 14,80) | .0008 |
| Aztreonam | 5.12 | 3.68 (1.62, 787) | 5.19 | 4.20 (1.80, 735) | .7727 |
| Carbapenems | 29.50 | 2773 (1753, 39.25) | 43.64 | 42.82 (19.41,5753) | <.0001 |
| 2nd/3rd/4th-generation Cephalosporins | 103.34 | 95.79 (73.32, 119.27) | 134.60 | 128.67 (94.91, 165.65) | <.0001 |
| Clindamycin | 18.98 | 18.17 (13.15, 21.84) | 20.41 | 18.86 (14.03, 24.87) | .0073 |
| Fluoroquinolones | 104.80 | 103.74 (74.42, 128.36) | 104.38 | 101.32 (75.38, 131.30) | .7956 |
Data are presented as days of therapy per 1000 days at risk.
CDI Rates by Piperacillin/Tazobactam Shortage and Change in High-Risk Antibiotic Use
The unadjusted pre–shortage period vs shortage period HO-CDI rates (HO-CDIs per 10 000 patient-days) by levels of PIP/TAZO shortages can be seen in Figure 1. Although the shortage period HO-CDI rates for moderate or severe shortage hospitals were slightly greater compared with the pre–shortage period, none of the P values were statistically significant.
Figure 1.
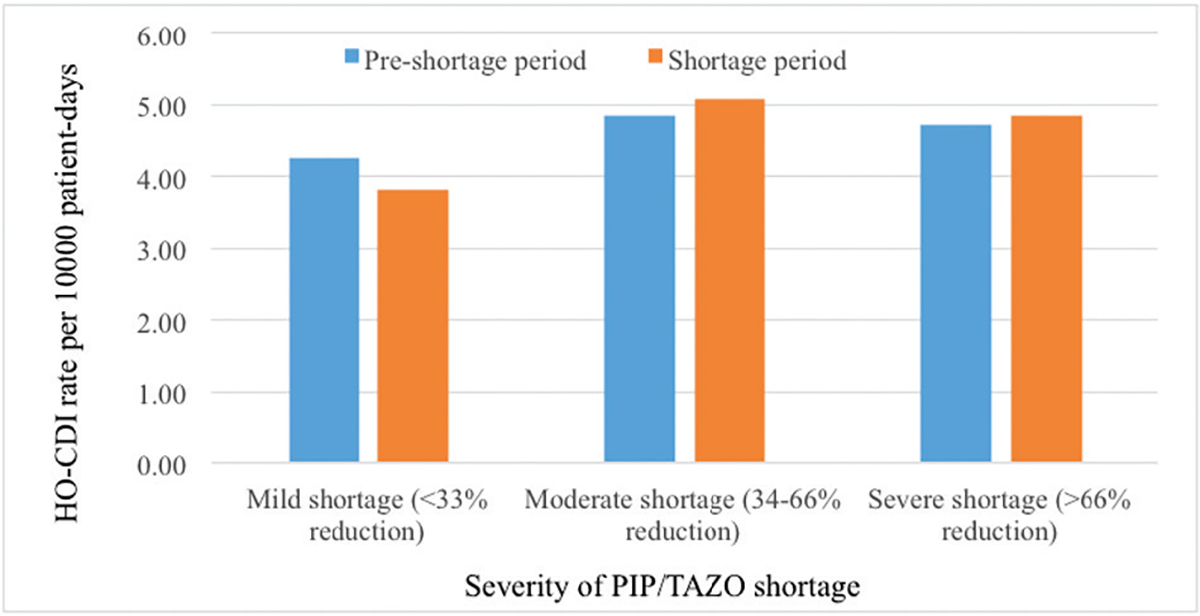
Unadjusted pre–shortage period vs shortage period hospital-onset Clostridium difficile infection rates by severity of piperacillin/tazobactam shortage. Abbreviations: CDI, Clostridium difficile infection; PIP/TAZO, piperacillin/tazobactam.
The unadjusted pre–shortage period vs shortage period HO-CDI rates (CDIs per 10 000 patient days) by levels of increasing in high-risk antibiotic use during the shortage period can be seen in Figure 2. Hospitals without increasing high-risk antibiotic use showed lower HO-CDI in the shortage period. Hospitals experiencing increasing use of high-risk antibiotics showed greater HO-CDI rates in the shortage period.
Figure 2.
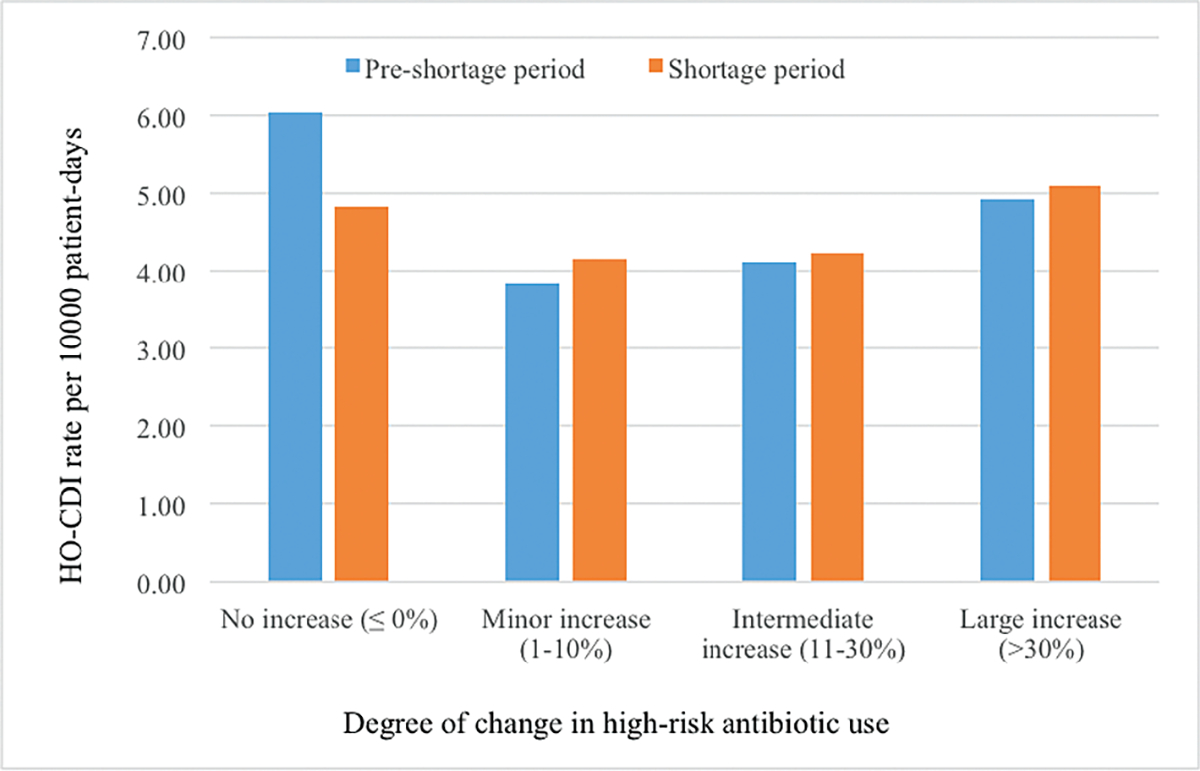
Unadjusted pre–shortage period vs shortage period hospital-onset Clostridium difficile infection rates by degree of change in high-risk antibiotic usage. Abbreviations: DAR, days at risk; DOT, days of therapy; CDI, Clostridium difficile infection.
Adjusted Risks of CDI for Piperacillin/Tazobactam Shortages and High-Risk Antibiotic Change
The adjusted relative risk (RR) of HO-CDI regardless of increased high-risk antibiotic use for moderate and severe shortages was similar. The combined moderate and severe shortage group had an RR of 1.03 (95% confidence interval [CI], .85–1.26; P = .73) compared to hospitals with mild PIP/TAZO shortages. However, for the group of hospitals that experienced a PIP/TAZO shortage and also showed a shortage period shift toward increasing use of high-risk antibiotics, the RR for HO-CDI was 1.30 (95% CI, 1.03–1.64; P < .05). With a rate of 4.5 CDI events per 10 000 patient-days among all hospitals experiencing a PIP/TAZO shortage, this would equate to 21 HO-CDI cases per a median hospital per annum. A 30% increase from this base rate would add 6 more HO-CDI cases per annum in a hospital experiencing both the shortage and responding with a shift toward higher-risk antibiotics.
Characterization of Piperacillin/Tazobactam Recovery, High-Risk Antibiotic Use, and Hospital-Onset Clostridium difficile Infection After the Shortage
We used data 1 year following the shortage period to examine degree of PIP/TAZO use recovery and high-risk antibiotic use recovery as a post hoc subgroup analysis. One of the original 72 hospitals that had a PIP/TAZO shortage could not provide data, and 6 hospitals showed no recovery at all in terms of PIP/TAZO usage. Of the 65 remaining hospitals, 27 (41%) showed complete recovery of PIP/TAZO use to preshortage levels. However, only 13 hospitals (18%) also showed complete recovery in terms of reduced use of high-risk antibiotics. Although the CDI rates and odds ratio from expanding the difference in difference method to a 3 time period variable are consistent with decreasing CDI rates in the recovery period, the small number of hospitals makes these estimates vulnerable to choice of the cutoffs for the definition of recovery.
DISCUSSION
In this study of 88 hospitals that experienced a PIP/TAZO shortage, we identified an association between the resulting increased use of high-risk antibiotics and HO-CDI. This is a noteworthy finding given that CDI is associated with at least 15 000 deaths and more than $1 billion in excess medical costs annually in the United States [4]. Of note, a local increase of 1.5 HO-CDI cases per quarter may easily be overlooked at an individual hospital, and it was only by aggregating multiple institutions that the effect was identified. Anti-infective shortages are increasingly common, and the adverse effects of drug shortages have not been well described at a population health level [21].
Two previous single-center studies suggested that a PIP/TAZO shortage, and subsequent increases in cephalosporin use, contributed to an increased CDI rate [5, 12]. Following a PIP/TAZO shortage beginning in December 2001, Wilcox et al observed a 370% overall increase in cefotaxime use in a geriatrics inpatient ward [5]. Subsequently, there was a 232% increase in overall CDI rate (P < .01). This study also demonstrated that CDI rates decreased by 52% (P = .008) during an earlier restriction of cefotaxime facilitated by active monitoring and prospective audit and feedback. At another medical center during the same PIP/TAZO shortage, Alston et al observed an increased rate of HO-CDI from 92 to 211 cases per 100 000 patient-days before and after the shortage, respectively (P < .001) [12]. Initially, cefotetan and ceftriaxone use increased in response to the shortage, followed by an increase in levofloxacin use. Mendez et al also evaluated the effects the same PIP/TAZO shortage at their institution and paradoxically found a 47% decrease in overall CDI. In multivariate analysis, PIP/TAZO use was not associated with CDI but reduced use of ceftriaxone was [22]. Collectively, these studies suggest the prior PIP/TAZO shortage may have impacted CDI rates; however, they are limited in their single-center nature. Furthermore, all the studies except for the study by Alston et al included community-onset CDI, which may be a suboptimal outcome measure as the antibiotic exposure is occurring on an inpatient basis.
Multiple studies have evaluated the relative risk of various antibiotics for C. difficile colonization and symptomatic disease. Two meta-analyses found clindamycin, cephalosporins, and carbapenems to be among the highest-risk agents for CDI while both analyses found penicillins and penicillin combinations to have the lowest risk [18, 19]. PIP/TAZO’s relatively low risk for CDI noted in previously mentioned studies may be due to its intrinsic activity against C. difficile and its ability to decrease sporulation [23]. PIP/TAZO can prevent de novo colonization with C. difficile given its ability to achieve inhibitory concentrations in the gastrointestinal tract [8]. A study from Dubberke et al that screened 235 patients at admission and discharge for C. difficile showed that β-lactam/β-lactamase inhibitor or metronidazole exposure was associated with a loss of C. difficile colonization during hospitalization (P = .04 and P = .03, respectively), whereas cephalosporin use was associated with acquisition of C. difficile (P = .03) [24]. Notably, although carbapenems often have activity against C. difficile, these remain a relatively strong risk factor for CDI; potentially this is due to their inability to achieve adequate C. difficile inhibitory activity in the gastrointestinal tract, unlike PIP/TAZO [25].
As expected, this study revealed that many centers that experienced a PIP/TAZO shortage shifted prescribing to alternative antipseudomonal β-lactams. Importantly, some centers shifted prescribing primarily to carbapenems whereas others shifted to cefepime. Carbapenems are more broad-spectrum relative to PIP/TAZO and cefepime, given that they are typically active against extended-spectrum β-lactamase–producing Enterobacteriaceae. This shift to carbapenems from PIP/TAZO is disconcerting given that carbapenem use is associated with carbapenem resistance development and that severe infections secondary to carbapenem-resistant Enterobacteriaceae are associated with an excess absolute mortality rate of approximately 27% [26–29]. The reason some institutions shifted use to cefepime while others shifted to carbapenems is not known. Intuitively, differences in drug activity may drive prescribing. However, in a 2012–2013 survey of 3082 Pseudomonas aeruginosa isolates from 71 US medical centers, 79%, 84%, and 82% of isolates were susceptible to PIP/TAZO, cefepime, and meropenem, respectively [30]. Additionally, among 13 820 Enterobacteriaceae isolates, 93%, 95%, and 99% were susceptible to PIP/TAZO, cefepime, and meropenem, respectively. This shows that cefepime has similar or better activity than PIP/TAZO for P. aeruginosa and Enterobacteriaceae and therefore is probably an ideal agent (with or without metronidazole) to substitute for PIP/TAZO. The potentially inappropriate use of carbapenems during the PIP/TAZO shortage highlights the vital role of antimicrobial stewardship in mitigating anti-infective shortages. Antimicrobial stewardship programs can provide tailored education to clinicians regarding preferred alternative agents with similar activity and reserve any remaining stock of the agent on shortage for selected patients [31].
Antimicrobial shortages continue to be a problem in the United States. Between 2001 and 2013, 148 antibacterials were on shortage, with PIP/TAZO being on shortage 5 times for a total of 1858 days [21]. And the rate of drug shortages is increasing: From July 2007 to December 2013, 0.35 additional drugs were on shortage each month. In a 2012 survey of hospital pharmacy staff conducted by the Institute for Safe Medication Practices, the most common adverse effect of drug shortages reported was inadequate treatment of disease due to the drug of choice being unavailable [32]. Examples of this for anti-infectives include shortages of penicillin for the treatment of neurosyphilis or shortages of amikacin for the treatment of multidrug-resistant gram-negative bacteria [33, 34]. The use of less effective alternative agents, increases in medical errors, and potential for increased CDI highlight the need to mitigate drug shortages locally through antimicrobial stewardship programs, but this problem also needs to be addressed nationally. Increased communication between manufacturers, regulatory bodies, and practitioners of impending drug shortages can potentially decrease untoward effects. In July 2012, the Food and Drug Administration Safety and Innovation Act was enacted to provide the Food and Drug Administration additional authority to assist manufacturers in preventing impending shortages [35]. Given that manufacturing and regulatory issues are common causes for drug shortages, it is hoped this intervention will decrease the rate of drug shortages; however, drug shortages still continue as evidenced by the PIP/TAZO and other shortages [9].
This study has some limitations. HO-CDI was not based on symptoms; however, most patients who have a C. difficile assay drawn will be symptomatic with diarrhea. Another limitation is that we used pharmacy orders and not antimicrobial administration to evaluate change in antimicrobial usage. Because the same process was used to measure antimicrobial use, we believe that this would not change the directionality of the changes. It is also possible that some hospitals may have changed testing procedures during the study, which may have impacted HO-CDI rates. The changes in HO-CDI between the pre–shortage period and shortage period may have been due in part to seasonality; to address this we selected a recovery period for subgroup analysis that included the same seasonal months as the shortage period. However, the number of hospitals that fully recovered after the shortage was too small to conclusively demonstrate a subsequent fall in HO-CDI rates although the point estimates decreased. Finally, our assessment of hospitals experiencing a PIP/TAZO shortage was based on drug use data and not on direct confirmation from the hospitals that they had experienced a shortage; however, as 82% of hospitals in the database experienced decreased use of PIP/TAZO, it is more likely these changes were a result of the national shortage and not a local stewardship intervention aimed at decreasing PIP/TAZO use. Strengths of this study include the use of microbiologic and antibiotic use data from a large collection of US hospitals and use of a standard definition for HO-CDI.
In conclusion, a national shortage of PIP/TAZO, and subsequent increases in high-risk antibiotic use, was associated with an increased HO-CDI rate. Urgent action is required to mitigate drug shortages nationally as well as to further describe the adverse effects of these drug shortages with additional multicenter studies. These data can help guide optimal stewardship interventions in response to drug shortages.
Acknowledgments.
The authors thank Xiaowu Sun, PhD, and Stephen Kurtz, MS, for their statistical support and John Murray, MPH, for creation of the dataset for this study.
Footnotes
Potential conflicts of interest. V. G., Y. P. T., and R. S. J. are all current employees of Becton, Dickinson and Company. A. E. G. has received speaking honoraria from Becton, Dickinson and Company. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Griffith MM, Gross AE, Sutton SH, et al. The impact of anti-infective drug shortages on hospitals in the United States: trends and causes. Clin Infect Dis 2012; 54:684–91. [DOI] [PubMed] [Google Scholar]
- 2.Infectious Diseases Society of America Emerging Infections Network. Report for query: “antimicrobial drug shortages 2016.” Available at: http://www.int-med.uiowa.edu/Research/EIN/FinalReport_DrugShortages2016.pdf. Accessed 21 August 2016.
- 3.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed 21 August 2016.
- 5.Wilcox MH, Freeman J, Fawley W, et al. Long-term surveillance of cefotaxime and piperacillin-tazobactam prescribing and incidence of Clostridium difficile diarrhoea. J Antimicrob Chemother 2004; 54:168–72. [DOI] [PubMed] [Google Scholar]
- 6.Valiquette L, Cossette B, Garant MP, Diab H, Pépin J. Impact of a reduction in the use of high-risk antibiotics on the course of an epidemic of Clostridium difficile-associated disease caused by the hypervirulent NAP1/027 strain. Clin Infect Dis 2007; 45(suppl 2):S112–21. [DOI] [PubMed] [Google Scholar]
- 7.Baines SD, Freeman J, Wilcox MH. Effects of piperacillin/tazobactam on Clostridium difficile growth and toxin production in a human gut model. J Antimicrob Chemother 2005; 55:974–82. [DOI] [PubMed] [Google Scholar]
- 8.Kundrapu S, Sunkesula VC, Jury LA, et al. Do piperacillin/tazobactam and other antibiotics with inhibitory activity against Clostridium difficile reduce the risk for acquisition of C. difficile colonization? BMC Infect Dis 2016; 16:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Society of Health-System Pharmacists. Drug shortages. Available at: http://www.ashp.org/menu/DrugShortages. Accessed 21 August 2016.
- 10.Pultz NJ, Donskey CJ. Effect of antibiotic treatment on growth of and toxin production by Clostridium difficile in the cecal contents of mice. Antimicrob Agents Chemother 2005; 49:3529–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Settle CD, Wilcox MH, Fawley WN, Corrado OJ, Hawkey PM. Prospective study of the risk of Clostridium difficile diarrhoea in elderly patients following treatment with cefotaxime or piperacillin-tazobactam. Aliment Pharmacol Ther 1998; 12:1217–23. [DOI] [PubMed] [Google Scholar]
- 12.Alston WK, Ahern JW. Increase in the rate of nosocomial Clostridium difficile-associated diarrhoea during shortages of piperacillin-tazobactam and piperacillin. J Antimicrob Chemother 2004; 53:549–50. [DOI] [PubMed] [Google Scholar]
- 13.Brossette SE, Hacek DM, Gavin PJ, et al. A laboratory-based, hospital-wide, electronic marker for nosocomial infection: the future of infection control surveillance? Am J Clin Pathol 2006; 125:34–9. [PubMed] [Google Scholar]
- 14.Ridgway JP, Sun X, Tabak YP, Johannes RS, Robicsek A. Performance characteristics and associated outcomes for an automated surveillance tool for bloodstream infection. Am J Infect Control 2016; 44:567–71. [DOI] [PubMed] [Google Scholar]
- 15.Tabak YP, Zilberberg MD, Johannes RS, Sun X, McDonald LC. Attributable burden of hospital-onset Clostridium difficile infection: a propensity score matching study. Infect Control Hosp Epidemiol 2013; 34:588–96. [DOI] [PubMed] [Google Scholar]
- 16.Thomas C, Stevenson M, Riley TV. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review. J Antimicrob Chemother 2003; 51:1339–50. [DOI] [PubMed] [Google Scholar]
- 17.Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect 1998; 40:1–15. [DOI] [PubMed] [Google Scholar]
- 18.Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother 2014; 69:881–91. [DOI] [PubMed] [Google Scholar]
- 19.Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother 2013; 57:2326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Antimicrobial use and resistance (AUR) module. Available at: http://www.cdc.gov/nhsn/PDFs/pscManual/11pscAURcurrent.pdf. Accessed 21 August 2016.
- 21.Quadri F, Mazer-Amirshahi M, Fox ER, et al. Antibacterial drug shortages from 2001 to 2013: implications for clinical practice. Clin Infect Dis 2015; 60:1737–42. [DOI] [PubMed] [Google Scholar]
- 22.Mendez MN, Gibbs L, Jacobs RA, McCulloch CE, Winston L, Guglielmo BJ. Impact of a piperacillin-tazobactam shortage on antimicrobial prescribing and the rate of vancomycin-resistant enterococci and Clostridium difficile infections. Pharmacotherapy 2006; 26:61–7. [DOI] [PubMed] [Google Scholar]
- 23.Garneau JR, Valiquette L, Fortier LC. Prevention of Clostridium difficile spore formation by sub-inhibitory concentrations of tigecycline and piperacillin/tazobactam. BMC Infect Dis 2014; 14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubberke ER, Reske KA, Seiler S, Hink T, Kwon JH, Burnham CA. Risk factors for acquisition and loss of Clostridium difficile colonization in hospitalized patients. Antimicrob Agents Chemother 2015; 59:4533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Büchler AC, Rampini SK, Stelling S, et al. Antibiotic susceptibility of Clostridium difficile is similar worldwide over two decades despite widespread use of broad-spectrum antibiotics: an analysis done at the University Hospital of Zurich. BMC Infect Dis 2014; 14:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Routsi C, Pratikaki M, Platsouka E, et al. Risk factors for carbapenem-resistant gram-negative bacteremia in intensive care unit patients. Intensive Care Med 2013; 39:1253–61. [DOI] [PubMed] [Google Scholar]
- 27.Daikos GL, Vryonis E, Psichogiou M, et al. Risk factors for bloodstream infection with Klebsiella pneumoniae producing VIM-1 metallo-beta-lactamase. J Antimicrob Chemother 2010; 65:784–8. [DOI] [PubMed] [Google Scholar]
- 28.Tumbarello M, Trecarichi EM, Tumietto F, et al. Predictive models for identification of hospitalized patients harboring KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2014; 58:3514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauck C, Cober E, Richter SS, et al. ; Antibacterial Resistance Leadership Group. Spectrum of excess mortality due to carbapenem-resistant Klebsiella pneumoniae infections. Clin Microbiol Infect 2016; 22:513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sader HS, Castanheira M, Flamm RK, Mendes RE, Farrell DJ, Jones RN. Ceftazidime/avibactam tested against gram-negative bacteria from intensive care unit (ICU) and non-ICU patients, including those with ventilator-associated pneumonia. Int J Antimicrob Agents 2015; 46:53–9. [DOI] [PubMed] [Google Scholar]
- 31.Griffith MM, Patel JA, Sutton SH, et al. Prospective approach to managing antimicrobial drug shortages. Infect Control Hosp Epidemiol 2012; 33:745–52. [DOI] [PubMed] [Google Scholar]
- 32.Institute for Safe Medication Practices. A shortage of everything except errors: harm associated with drug shortages. Available at: https://www.ismp.org/newsletters/acutecare/showarticle.aspx?id=20. Accessed 21 August 2016.
- 33.Griffith MM, Pentoney Z, Scheetz MH. Antimicrobial drug shortages: a crisis amidst the epidemic and the need for antimicrobial stewardship efforts to lessen the effects. Pharmacotherapy 2012; 32:665–7. [DOI] [PubMed] [Google Scholar]
- 34.Dowell ME, Ross PG, Musher DM, Cate TR, Baughn RE. Response of latent syphilis or neurosyphilis to ceftriaxone therapy in persons infected with human immunodeficiency virus. Am J Med 1992; 93:481–8. [DOI] [PubMed] [Google Scholar]
- 35.United States Government Accountability Office. Drug shortages: threat to public health persists, despite actions to help maintain product availability. Available at: http://www.gao.gov/assets/670/660771.pdf. Accessed 21 August 2016.


