Abstract
Background:
Laparoscopic Roux-en-Y gastric bypass (LRYGB) provides sustained weight loss. However, short-term studies have suggested that African Americans (AAs) are not as successful as Caucasians (CAs) after LRYGB.
Objective:
The present study was designed to test the hypothesis that at longer term follow-up AAs are just as successful as CAs after LRYGB.
Setting:
University hospital, United States.
Methods:
A nested case-control study designed to examine the effect of race as covariate in the long-term success of women undergoing LRYGB. The study matched 3 controls per case subject, and the final numbers for analyses were 78 case patients (AA) and 204 control patients (CA). Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using conditional logistic regression analysis.
Results:
The 2 cohorts (N = 282) were well matched for age (AA 40.3 ± 9.1 years versus CA 41.1 ± 8.9 years), preoperative body mass index (AA 50.6 ± 7.5 kg/m2 versus CA 50.2 ± 7.1 kg/m2), prevalence of type 2 diabetes (T2D) (AA 20.5% versus CA 21.1%), hypertension (AA 69.1% versus CA 52%), and sleep apnea (AA 35.9% versus CA 34.8%). In the AA group, the long-term curve for percentage of excess weight loss (EWL) was significantly (P < .001) lower than the CA group at any time-point. In the present model, diagnosis of T2D in the AA group (OR = 6.1 E8) significantly (P = .002) predicted adequate EWL at 3 years, after controlling for relevant confounders.
Conclusion:
Race significantly affected the long-term EWL at 3 years for patients undergoing LRYGB at the authors’ institution. Future research should be directed at detemiining potential genetic reasons for these differences, including genes associated with T2D.
Keywords: Roux-en-Y gastric bypass, Type 2 diabetes, Weight loss, African American
Laparoscopic Roux-en-Y gastric bypass (LRYGB) is a treatment for morbid obesity associated with long-term excess weight loss (EWL) and amelioration of co-morbid conditions, such as type 2 diabetes (T2D), hypertension, hyperlipidemia, and obstructive sleep apnea [1]. Proven efficacy combined with a low rate of perioperative morbidity and mortality account for the marked increase in the volume of LRYGBs performed in the United States each year since its first report in the literature in 1993 [2]. Several studies with long-term follow-up have shown >50% sustained EWL [3]. Short-term studies have also suggested racial differences in response to bariatric surgery [4]. Specifically, African Americans (AAs) might have a lower EWL compared with Caucasians (CAs) after similar bariatric procedures [5–8]. At all age groups, AA women tend to be more obese and are more resistant to weight loss compared with CA women [9,10]. The mechanism for this phenomenon is unknown, but it is thought likely secondary to a combination of biological, behavioral, and psychological factors. The biological and genetic mechanisms behind this outcome disparity are still not well studied. A study from Buffington and Marema found that AA females with morbid obesity have a greater amount of adipose tissue than CA women and lose significantly less body fat after obesity surgery [6]. Madan et al. hypothesized that inflammatory cytokines produced by the adipose tissue, which play a role in obesity, were potentially different among races [11]. Their analysis did not reveal an ethnic difference.
Most studies aimed at evaluating racial differences in outcome after bariatric surgery are characterized by short-term follow-up. The aim of this study was to test the hypothesis that at long-term follow-up, AA women are equally as successful as CA women after LRYGB and to ascertain predictors of successful postoperative weight loss.
Methods
Patients
A retrospective analysis of the prospectively maintained institutional bariatric database was conducted. The database is maintained under institutional review board (IRB) approval and with patient consent. Women undergoing LRYGB at the authors’ institution with at least a 3-year complete follow-up were included in this study. Revisional LRYGB cases and patients with <3 years of follow-up were excluded. Demographic information, preoperative co-morbidities, and pre- and postoperative anthropometric variables were analyzed. Percentage excess weight loss (EWL) at each yearly interval was calculated.
A nested case-control study was designed to examine the effect of race as a covariate in the long-term success of women undergoing LRYGB. AA patients (case patients) were matched according to age, preoperative body mass index (BMI), and prevalence of co-morbidities with a subset of CA patients (controls patients). The matching ratio was of 3 controls for every case subject.
The AA cohort was stratified into 2 subgroups using a cutoff point for EWL at 3 years termed as adequate EWL. Adequate EWL cutoff point was set at mean – 1 SD of the entire study’s cohort as previously described [12].
Statistical analysis
Logistic regression analysis was used in univariate and multivariate modes to identify patients’ demographic characteristics and co-morbidity data that were predictive of adequate EWL in the AA group. Preoperative factors with a P value <.1 were entered into the binary logistic regression using the conditional stepwise method. The logistic regression analysis output was expressed as an odds ratio and computed with a 95% confidence interval. The SPSS statistical software program (version 19; SPSS, Chicago, IL, USA) was used for all analyses. All tests were 2-tailed. P values < .05 were considered to indicate statistical significance. The results are expressed as mean ± SD.
Results
The study enrolled a total of 282 patients. The AA group consisted of 78 patients and the CA group of 204 patients. The follow-up rate was 91% at 1 year, 80% at 2 years, and 100% at 3 years. As shown in Table 1, AA and CA groups were well matched for age, preoperative BMI, prevalence of type 2 diabetes, hypertension, sleep apnea, and insurance status. Excess weight loss at 3 years was about 62.5% ± 17.1% for the entire cohort (n = 282). As shown in Fig. 1, in the AA group the long-term curve for percentage of excess weight was significantly lower than the CA group (P < .001) at any time interval. Fig. 2 depicts temporal changes in BMI of the 2 groups. Overall and at any time interval, changes in BMI were significantly (P = .001) higher in the CA group. At 3 years follow-up, T2 DM remission rate was similar in AA and CA women (75% and 77%, respectively).
Table 1.
Patient characteristics at baseline
| Characteristic | AA (n = 78) | CA (n = 204) | P value |
|---|---|---|---|
| Age | 40.3 ± 9.1 | 41.1 ± 8.9 | NS |
| Preoperative BMI | 50.6 ± 7.5 | 50.2 ± 7.1 | NS |
| Type 2 diabetes (%) | 20.5 | 21.1 | NS |
| Hypertension (%) | 69.1 | 52 | NS |
| Sleep apnea (%) | 35.9 | 34.8 | NS |
| Insurance status (%) | NS | ||
| Commercial | 89 | 92 | |
| Medicare | 11 | 8 | |
| Medicaid | 0 | 0 | |
| Uninsured | 0 | 0 |
AA = African American; CA = Caucasian; NS = not significant; BMI = body mass index.
Fig. 1.
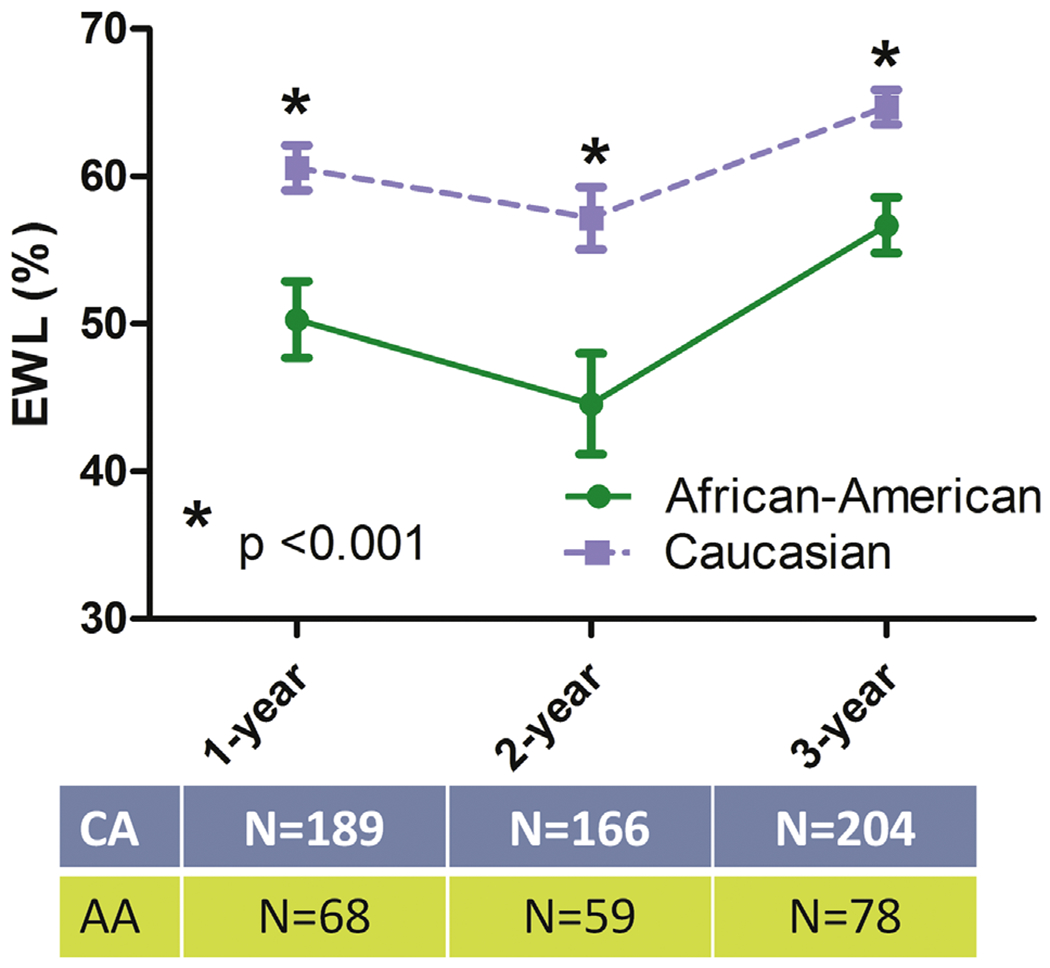
Percentage excess weight loss curves in the 2 groups. EWL = excess weight loss; CA = Caucasian women; AA = African American women.
Fig. 2.
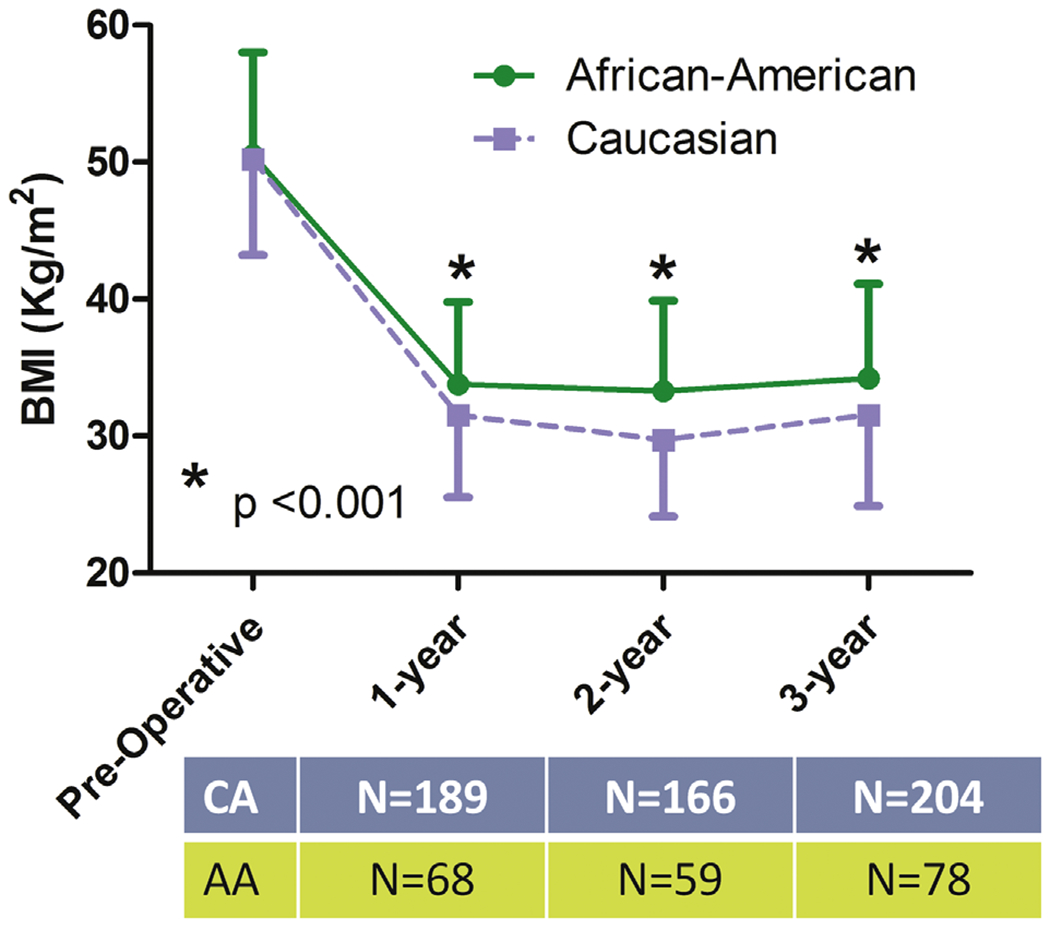
BMI changes in the 2 groups. BMI = body mass index; CA = Caucasian women; AA = African American women.
The AA cohort was divided into 2 subgroups: adequate EWL (n = 60) and inadequate EWL (n = 18), according to a cutoff value of 45.4% of EWL at 3 years derived from the entire cohort data (defined above). As shown in Table 2, demographic (age), anthropometric (preoperative BMI), and clinical (presence of co-morbidities) data were compared in the 2 subgroups. On univariate analysis, preoperative diagnosis of T2D had a significant (P = .01) counterintuitive effect on EWL. Preoperative diagnosis of T2D remained an independent and significant (P = .002) predictor in the multivariate logistic regression (adjusted odds ratio = 6.1 E8) of adequate EWL at 3 years, after controlling for relevant confounder (preoperative BMI). Other covariates did not significantly impact the model.
Table 2.
Characteristics of the African American groups with or without adequate EWL
| Characteristic | Adequate EWL (n = 60) | Inadequate EWL (n = 18) | P value |
|---|---|---|---|
| Age | 40.1 ± 9.3 | 40.6 ± 8.6 | NS |
| Preoperative BMI | 49.7 ± 7.1 | 53.2 ± 8.3 | NS |
| Type 2 diabetes (%) | 26.7 | 0 | .01 |
| Hypertension (%) | 61.7 | 55.6 | NS |
| Sleep apnea (%) | 38.3 | 27.8 | NS |
EWL = excess weight loss; NS = not significant; BMI = body mass index.
Table 3 shows data relative to the 2 subgroups of CA women with adequate EWL (n = 177) and inadequate EWL (n = 24). CA women with inadequate weight loss were characterized by significantly higher preoperative BMI and prevalence of T2D. Interestingly, diagnosis of T2D is a negative prognostic factor for adequate EWL in CA women, a result that is completely contrasting with what was observed in AA women.
Table 3.
Characteristics of the Caucasian groups with or without adequate EWL
| Characteristic | Adequate EWL (n = 177) | Inadequate EWL (n = 27) | P value |
|---|---|---|---|
| Age | 41.1 ± 8.9 | 40.7 ± 9.0 | NS |
| Preoperative BMI | 49.4 ± 6.8 | 55.2 ± 6.3 | .001 |
| Type 2 diabetes (%) | 18.1 | 40.7 | .007 |
| Hypertension (%) | 49.7 | 66.7 | NS |
| Sleep apnea (%) | 36.2 | 25.9 | NS |
EWL = excess weight loss; NS = not significant; BMI = body mass index.
Discussion
Morbid obesity is more prevalent in the AA population than in the CA population [13]. The mechanism for this higher prevalence is poorly understood. There is some evidence to support hypotheses based on dietary, lifestyle, and metabolic influences. [14]. Moreover, AAs appear to be more resistant to weight loss than CAs [10]. This resistance to weight loss seems to also be present after bariatric surgery, as several short-term studies suggest [5,6,15]. These interesting findings deserve further investigation considering that overall rates of bariatric surgery are highest for black females (29.4/10,000) compared with white females (21.3/10,000) and other racial minority (8.6/10,000) females [16].
Harvin et al., using a multivariate logistic regression mode, determined that the CA race significantly predicted adequate weight loss at 2 years after controlling for relevant confounders [4]. The authors speculated that racial differences seen in their study and the other previous studies [5] may be related to environmental factors such as access to a healthy diet. Our study was not designed to look after socioeconomic factors that play a role in the weight loss outcome. The only socioeconomic factor that we analyzed was insurance status, and this was found not found to be significant. However, the importance of these factors is well known in the bariatric surgery community. The Buffington and Marema study [6] offers a different explanation using anthropometric data. In this study, AA women have greater adiposity than CA women and lose significantly less body fat after LRYGB, suggesting a possible ethnic difference in fat metabolism. Madan et al.’s work challenges the aforementioned studies: In one study, no ethnic differences were found in the percentage of patients with successful weight loss and resolution/remission of T2D and hypertension [15]. In another study, they found little ethnic differences between cytokine release by omental adipose tissue explants in vitro or the messenger ribonucleic acid (mRNA) content in omental adipose tissue of interleukin (IL)-6, IL-8, or cyclooxygenase (COX)-2 [11].
The present study, at longer-term follow-up, confirms previous short-term data that AA women undergoing LRYGB have lower EWL than CAs. Compared with other studies [4–6], the present conclusions are better supported by a nested case-control design, higher follow-up rate, and longer follow-up. The major novelty of the present study resides in the fact that preoperative diagnosis of T2D was identified as an independent and significant predictor of adequate 3-year EWL in AA women undergoing LRYGB. To reinforce the significance of these findings, T2D was instead a significant predictor of inadequate EWL in CA women. In fact, a study from Carbonell et al. determined that obese patients with T2D had a lower percentage of EWL and greater BMI than the patients without T2D at 1 year after LRYGB [17]. In another study, Campos et al. determined that T2D and larger pouch size are independently associated with poor weight loss after LRYGB [18]. The authors speculate that one possible explanation is that most of T2D patients take insulin, promoting lipogenesis, stimulation of triglyceride synthesis, and adipocyte differentiation. These are all factors that may reduce the degree of weight loss after LRYGB. The population studied by Carbonell et al. and Campos et al. was predominantly CA. In the present study, the preoperative diagnosis of T2D significantly affects EWL in the AA population and in a counterintuitive way. The present study was not designed to determine the mechanisms responsible for this interesting outcome. Future research should be directed at determining potential genetic reasons for these differences, including genes associated with T2D.
Conclusion
Overall, the present study has several potential limitations. The AA cohort was relatively small, thereby limiting the study’s power. The data was analyzed retrospectively from a single center, thereby limiting its generalizability. Nevertheless, race significantly affected long-term EWL at 3 years for patients undergoing LRYGB at the authors’ institution; and in the AA group, a preoperative diagnosis of T2D was an independent and significant predictor of adequate 3-year EWL.
Acknowledgments
This study was supported by a National Institutes of Health award (NIH K23 DK07597) to Dr. Alfonso Torquati.
Footnotes
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
References
- [1].Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292(14):1724–37. [DOI] [PubMed] [Google Scholar]
- [2].Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg 2009;19(12):1605–11. [DOI] [PubMed] [Google Scholar]
- [3].Attiah MA, Halpern CH, Balmuri U, et al. Durability of Roux-en-Y gastric bypass surgery: a meta-regression study. Ann Surg 2012;256(2):251–4. [DOI] [PubMed] [Google Scholar]
- [4].Harvin G, DeLegge M, Garrow DA. The impact of race on weight loss after Roux-en-Y gastric bypass surgery. Obes Surg 2008;18(1):39–42. [DOI] [PubMed] [Google Scholar]
- [5].Anderson WA, Greene GW, Forse RA, Apovian CM, Istfan NW. Weight loss and health outcomes in African Americans and whites after gastric bypass surgery. Obesity (Silver Spring) 2007;15(6):1455–63. [DOI] [PubMed] [Google Scholar]
- [6].Buffington CK, Marema RT. Ethnic differences in obesity and surgical weight loss between African-American and Caucasian females. Obes Surg 2006;16(2):159–65. [DOI] [PubMed] [Google Scholar]
- [7].Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 2003;237(6):751–6. discussion 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Parikh M, Lo H, Chang C, Collings D, Fielding G, Ren C. Comparison of outcomes after laparoscopic adjustable gastric banding in African-Americans and whites. Surg Obes Relat Dis 2006;2(6):607–10. discussion 10–2. [DOI] [PubMed] [Google Scholar]
- [9].Lynch CS, Chang JC, Ford AF, Ibrahim SA. Obese African-American women’s perspectives on weight loss and bariatric surgery. J Gen Intern Med 2007;22(7):908–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rickel KA, Milsom VA, Ross KM, Hoover VJ, Peterson ND, Perri MG. Differential response of African American and Caucasian women to extended-care programs for obesity management. Ethn Dis 2011;21(2):170–5. [PMC free article] [PubMed] [Google Scholar]
- [11].Madan AK, Tichansky DS, Coday M, Fain JN. Comparison of IL-8, IL-6 and PGE(2) formation by visceral (omental) adipose tissue of obese Caucasian compared to African-American women. Obes Surg 2006;16(10):1342–50. [DOI] [PubMed] [Google Scholar]
- [12].Lutfi R, Torquati A, Sekhar N, Richards WO. Predictors of success after laparoscopic gastric bypass: a multivariate analysis of socioeconomic factors. Surg Endosc 2006;20(6):864–7. [DOI] [PubMed] [Google Scholar]
- [13].Freedman DS, Khan LK, Serdula MK, Galuska DA, Dietz WH. Trends and correlates of class 3 obesity in the United States from 1990 through 2000. JAMA 2002;288(14):1758–61. [DOI] [PubMed] [Google Scholar]
- [14].Kumanyika S. Obesity in black women. Epidemiol Rev 1987;9:31–50. [DOI] [PubMed] [Google Scholar]
- [15].Madan AK, Whitfield JD, Fain JN, et al. Are African-Americans as successful as Caucasians after laparoscopic gastric bypass? Obes Surg 2007;17(4):460–4 [DOI] [PubMed] [Google Scholar]
- [16].Birkmeyer NJ, Race Gu N. socioeconomic status, and the use of bariatric surgery in Michigan. Obes Surg 2012;22(2):259–65. [DOI] [PubMed] [Google Scholar]
- [17].Carbonell AM, Wolfe LG, Meador JG, Sugerman HJ, Kellum JM, Maher JW. Does diabetes affect weight loss after gastric bypass? Surg Obes Relat Dis 2008;4(3):441–4. [DOI] [PubMed] [Google Scholar]
- [18].Campos GM, Rabl C, Mulligan K, et al. Factors associated with weight loss after gastric bypass. Arch Surg 2008;143(9):877–83. discussion 84. [DOI] [PMC free article] [PubMed] [Google Scholar]


