Abstract
The 20.9 kDa subunit of NADH:ubiquinone oxidoreductase (complex I) from Neurospora crassa is a nuclear-coded component of the hydrophobic arm of the enzyme. We have determined the primary structure of this subunit by sequencing a full-length cDNA and a cleavage product of the isolated polypeptide. The deduced protein sequence is 189 amino acid residues long and contains a putative membrane-spanning domain. Striking similarity over a 60 amino-acid-residue domain with the M (matrix) protein of para-influenza virus was found. No other relationship with already known sequences could be detected, leaving the function of this subunit in complex I still undefined. The biogenetic pathway of this polypeptide was studied using a mitochondrial import system in vitro. The 20.9 kDa subunit synthesized in vitro is efficiently imported into isolated mitochondria, where it obtains distinct features of the endogenous subunit. Our results suggest that the 20.9 kDa polypeptide is made on cytosolic ribosomes lacking a cleavable targeting sequence, interacts with the mitochondrial outer membrane (in a process that does not require an energized inner membrane), and is imported into mitochondria at contact sites. The 20.9 kDa subunit is then inserted into the inner membrane acquiring a topology similar to that of the already assembled subunit.
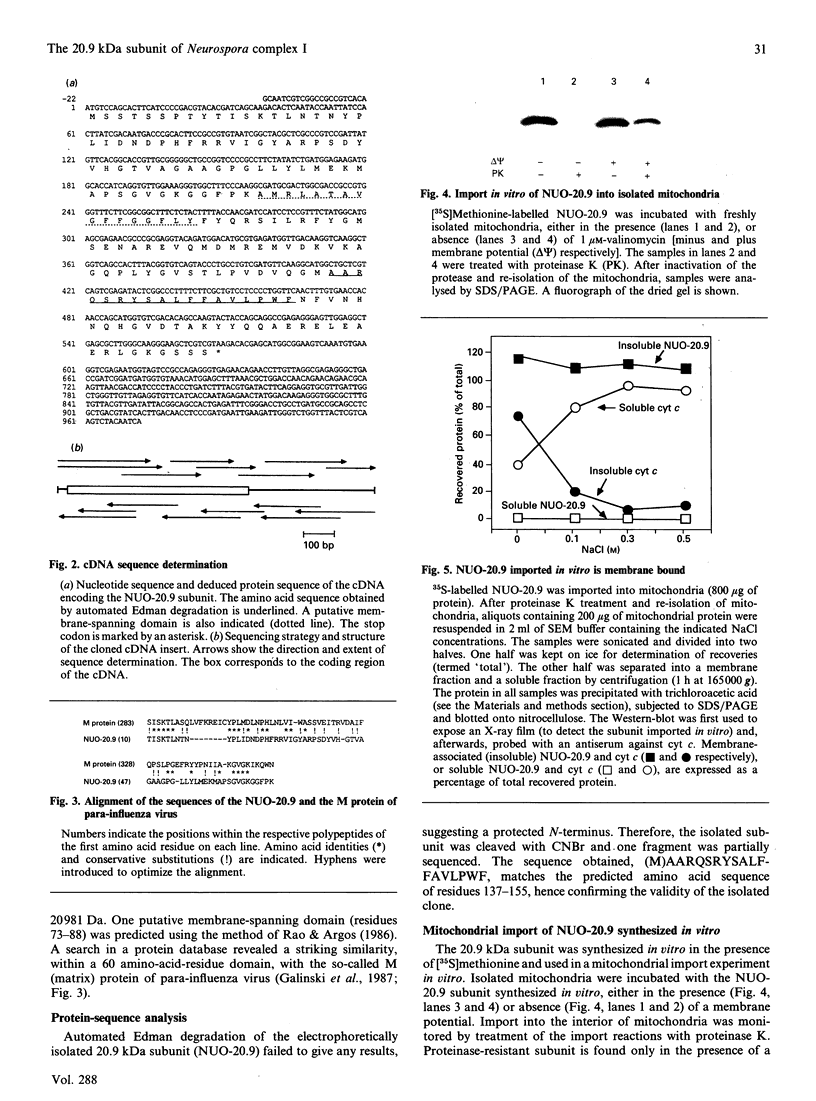
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinert H., Albracht S. P. New insights, ideas and unanswered questions concerning iron-sulfur clusters in mitochondria. Biochim Biophys Acta. 1982 Dec 31;683(3-4):245–277. doi: 10.1016/0304-4173(82)90003-9. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Böhm R., Sauter M., Böck A. Nucleotide sequence and expression of an operon in Escherichia coli coding for formate hydrogenlyase components. Mol Microbiol. 1990 Feb;4(2):231–243. doi: 10.1111/j.1365-2958.1990.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Cleeter M. W., Ragan C. I., Riley M., Doolittle R. F., Attardi G. URF6, last unidentified reading frame of human mtDNA, codes for an NADH dehydrogenase subunit. Science. 1986 Oct 31;234(4776):614–618. doi: 10.1126/science.3764430. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Mariottini P., Cleeter M. W., Ragan C. I., Matsuno-Yagi A., Hatefi Y., Doolittle R. F., Attardi G. Six unidentified reading frames of human mitochondrial DNA encode components of the respiratory-chain NADH dehydrogenase. Nature. 1985 Apr 18;314(6012):592–597. doi: 10.1038/314592a0. [DOI] [PubMed] [Google Scholar]
- Dupuis A., Skehel J. M., Walker J. E. A homologue of a nuclear-coded iron-sulfur protein subunit of bovine mitochondrial complex I is encoded in chloroplast genomes. Biochemistry. 1991 Mar 19;30(11):2954–2960. doi: 10.1021/bi00225a032. [DOI] [PubMed] [Google Scholar]
- Eckerskorn C., Mewes W., Goretzki H., Lottspeich F. A new siliconized-glass fiber as support for protein-chemical analysis of electroblotted proteins. Eur J Biochem. 1988 Oct 1;176(3):509–519. doi: 10.1111/j.1432-1033.1988.tb14308.x. [DOI] [PubMed] [Google Scholar]
- Fearnley I. M., Runswick M. J., Walker J. E. A homologue of the nuclear coded 49 kd subunit of bovine mitochondrial NADH-ubiquinone reductase is coded in chloroplast DNA. EMBO J. 1989 Mar;8(3):665–672. doi: 10.1002/j.1460-2075.1989.tb03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filser M., Werner S. Pethidine analogues, a novel class of potent inhibitors of mitochondrial NADH: ubiquinone reductase. Biochem Pharmacol. 1988 Jul 1;37(13):2551–2558. doi: 10.1016/0006-2952(88)90245-6. [DOI] [PubMed] [Google Scholar]
- Friedrich T., Hofhaus G., Ise W., Nehls U., Schmitz B., Weiss H. A small isoform of NADH:ubiquinone oxidoreductase (complex I) without mitochondrially encoded subunits is made in chloramphenicol-treated Neurospora crassa. Eur J Biochem. 1989 Mar 1;180(1):173–180. doi: 10.1111/j.1432-1033.1989.tb14629.x. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinski M. S., Mink M. A., Lambert D. M., Wechsler S. L., Pons M. W. Molecular cloning and sequence analysis of the human parainfluenza 3 virus gene encoding the matrix protein. Virology. 1987 Mar;157(1):24–30. doi: 10.1016/0042-6822(87)90309-6. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Pfanner N., Nicholson D. W., Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989 Jan 18;988(1):1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Schmidt B., Wachter E., Weiss H., Neupert W. Transport into mitochondria and intramitochondrial sorting of the Fe/S protein of ubiquinol-cytochrome c reductase. Cell. 1986 Dec 26;47(6):939–951. doi: 10.1016/0092-8674(86)90809-3. [DOI] [PubMed] [Google Scholar]
- Hofhaus G., Weiss H., Leonard K. Electron microscopic analysis of the peripheral and membrane parts of mitochondrial NADH dehydrogenase (complex I). J Mol Biol. 1991 Oct 5;221(3):1027–1043. doi: 10.1016/0022-2836(91)80190-6. [DOI] [PubMed] [Google Scholar]
- Ise W., Haiker H., Weiss H. Mitochondrial translation of subunits of the rotenone-sensitive NADH:ubiquinone reductase in Neurospora crassa. EMBO J. 1985 Aug;4(8):2075–2080. doi: 10.1002/j.1460-2075.1985.tb03894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Masui R., Wakabayashi S., Matsubara H., Hatefi Y. The amino acid sequence of the 9 kDa polypeptide and partial amino acid sequence of the 20 kDa polypeptide of mitochondrial NADH:ubiquinone oxidoreductase. J Biochem. 1991 Oct;110(4):575–582. doi: 10.1093/oxfordjournals.jbchem.a123622. [DOI] [PubMed] [Google Scholar]
- Mohana Rao J. K., Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986 Jan 30;869(2):197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Ragan C. I., Hatefi Y. EPR studies of iron-sulfur clusters in isolated subunits and subfractions of NADH-ubiquinone oxidoreductase. J Biol Chem. 1985 Mar 10;260(5):2782–2788. [PubMed] [Google Scholar]
- Paluh J. L., Orbach M. J., Legerton T. L., Yanofsky C. The cross-pathway control gene of Neurospora crassa, cpc-1, encodes a protein similar to GCN4 of yeast and the DNA-binding domain of the oncogene v-jun-encoded protein. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3728–3732. doi: 10.1073/pnas.85.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. The mitochondrial protein import apparatus. Annu Rev Biochem. 1990;59:331–353. doi: 10.1146/annurev.bi.59.070190.001555. [DOI] [PubMed] [Google Scholar]
- Pilkington S. J., Skehel J. M., Gennis R. B., Walker J. E. Relationship between mitochondrial NADH-ubiquinone reductase and a bacterial NAD-reducing hydrogenase. Biochemistry. 1991 Feb 26;30(8):2166–2175. doi: 10.1021/bi00222a021. [DOI] [PubMed] [Google Scholar]
- Preis D., Weidner U., Conzen C., Azevedo J. E., Nehls U., Röhlen D., van der Pas J., Sackmann U., Schneider R., Werner S. Primary structures of two subunits of NADH: ubiquinone reductase from Neurospora crassa concerned with NADH-oxidation. Relationship to a soluble NAD-reducing hydrogenase of Alcaligenes eutrophus. Biochim Biophys Acta. 1991 Aug 27;1090(1):133–138. doi: 10.1016/0167-4781(91)90049-r. [DOI] [PubMed] [Google Scholar]
- Rassow J., Guiard B., Wienhues U., Herzog V., Hartl F. U., Neupert W. Translocation arrest by reversible folding of a precursor protein imported into mitochondria. A means to quantitate translocation contact sites. J Cell Biol. 1989 Oct;109(4 Pt 1):1421–1428. doi: 10.1083/jcb.109.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runswick M. J., Fearnley I. M., Skehel J. M., Walker J. E. Presence of an acyl carrier protein in NADH:ubiquinone oxidoreductase from bovine heart mitochondria. FEBS Lett. 1991 Jul 29;286(1-2):121–124. doi: 10.1016/0014-5793(91)80955-3. [DOI] [PubMed] [Google Scholar]
- Sackmann U., Zensen R., Röhlen D., Jahnke U., Weiss H. The acyl-carrier protein in Neurospora crassa mitochondria is a subunit of NADH:ubiquinone reductase (complex I). Eur J Biochem. 1991 Sep 1;200(2):463–469. doi: 10.1111/j.1432-1033.1991.tb16205.x. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stueber D., Ibrahimi I., Cutler D., Dobberstein B., Bujard H. A novel in vitro transcription-translation system: accurate and efficient synthesis of single proteins from cloned DNA sequences. EMBO J. 1984 Dec 20;3(13):3143–3148. doi: 10.1002/j.1460-2075.1984.tb02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., King T. E. Evidence of an ubisemiquinone radical(s) from the NADH-ubiquinone reductase of the mitochondrial respiratory chain. J Biol Chem. 1983 Jan 10;258(1):352–358. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuschen G., Sackmann U., Nehls U., Haiker H., Buse G., Weiss H. Assembly of NADH: ubiquinone reductase (complex I) in Neurospora mitochondria. Independent pathways of nuclear-encoded and mitochondrially encoded subunits. J Mol Biol. 1990 Jun 20;213(4):845–857. doi: 10.1016/S0022-2836(05)80268-2. [DOI] [PubMed] [Google Scholar]
- Videira A., Tropschug M., Werner S. Primary structure and expression of a nuclear-coded subunit of complex I homologous to proteins specified by the chloroplast genome. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1168–1174. doi: 10.1016/0006-291x(90)90807-y. [DOI] [PubMed] [Google Scholar]
- Videira A., Tropschug M., Werner S. Primary structure, in vitro expression and import into mitochondria of a 29/21-kDa subunit of complex I from Neurospora crassa. Biochem Biophys Res Commun. 1990 Jan 15;166(1):280–285. doi: 10.1016/0006-291x(90)91942-l. [DOI] [PubMed] [Google Scholar]
- Videira A., Tropschüg M., Wachter E., Schneider H., Werner S. Molecular cloning of subunits of complex I from Neurospora crassa. Primary structure and in vitro expression of a 22-kDa polypeptide. J Biol Chem. 1990 Aug 5;265(22):13060–13065. [PubMed] [Google Scholar]
- Videira A., Werner S. Assembly kinetics and identification of precursor proteins of complex I from Neurospora crassa. Eur J Biochem. 1989 May 1;181(2):493–502. doi: 10.1111/j.1432-1033.1989.tb14751.x. [DOI] [PubMed] [Google Scholar]
- Wachter E., Werhahn R. Attachment of tryptophanyl peptides to 3-aminopropyl-glass suited for subsequent solid-phase Edman degradation. Anal Biochem. 1979 Aug;97(1):56–64. doi: 10.1016/0003-2697(79)90327-0. [DOI] [PubMed] [Google Scholar]
- Wang D. C., Meinhardt S. W., Sackmann U., Weiss H., Ohnishi T. The iron-sulfur clusters in the two related forms of mitochondrial NADH: ubiquinone oxidoreductase made by Neurospora crassa. Eur J Biochem. 1991 Apr 10;197(1):257–264. doi: 10.1111/j.1432-1033.1991.tb15906.x. [DOI] [PubMed] [Google Scholar]
- Weiss H., Friedrich T., Hofhaus G., Preis D. The respiratory-chain NADH dehydrogenase (complex I) of mitochondria. Eur J Biochem. 1991 May 8;197(3):563–576. doi: 10.1111/j.1432-1033.1991.tb15945.x. [DOI] [PubMed] [Google Scholar]
- Werner S. Preparation of polypeptide subunits of cytochrome oxidase from Neurospora crassa. Eur J Biochem. 1977 Sep 15;79(1):103–110. doi: 10.1111/j.1432-1033.1977.tb11788.x. [DOI] [PubMed] [Google Scholar]