Abstract
Stocks of simian immunodeficiency virus (SIV) from the supernatants of infected cell cultures were used to examine the sensitivity of envelope glycoprotein gp120 to enzymatic deglycosylation and the effects of enzyme treatment on infectivity. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and Western blot analysis revealed little or no change in the mobility of virion-associated gp120 after digestion with high concentrations of N-glycosidase F, endoglycosidase F, endoglycosidase H, and endo-β-galactosidase. Soluble gp120, which was not pelletable after the enzymatic reaction, was sensitive to digestion by the same enzymes within the same reaction mix and was only slightly less sensitive than gp120 that had been completely denatured by boiling in the presence of SDS and β-mercaptoethanol. Digestion by three of the seven glycosidases tested significantly changed the infectivity titer compared to that of mock-treated virus. Digestion by endo-β-galactosidase increased infectivity titers by about 2.5-fold, and neuraminidase from Newcastle disease virus typically increased infectivity titers by 8-fold. Most or all of the increase in infectivity titer resulting from treatment with neuraminidase could be accounted for by effects on the virus, not the cells; SIV produced in the presence of the sialic acid analog 2,3-dehydro-2-deoxy-N-acetylneuraminic acid also exhibited increased infectivity, and the effects could not be duplicated by neuraminidase treatment of cells. Digestion with mannosidase reduced infectivity by fivefold. Our results indicate that carbohydrates on native oligomeric gp120 as it exists on the surface of virus particles are largely occluded and are refractory to digestion by glycosidases. Furthermore, the sialic acid residues at the ends of carbohydrate side chains significantly reduce the inherent infectivity of SIV.
The envelope proteins of the primate lentiviruses are heavily glycosylated, with over half of the apparent molecular weight of the external glycoprotein, gp120, contributed by carbohydrates (13, 17, 22, 26, 30). N-linked glycosylation of gp120 is added cotranslationally in the endoplasmic reticulum of the cell and is modified as the protein transits through the golgi (21). Various enzymes trim or add sugar residues to the core carbohydrates to form high-mannose, hybrid or complex oligosaccharide chains (21). In addition, it has been shown that gp120 is further modified by a high degree of sialylation, resulting in an acidic isoelectric point because of the net negative charge of sialic acid (18, 36).
Glycosylation contributes to proper folding of the envelope protein (10, 14, 20). Other studies have demonstrated that it is the overall degree of glycosylation, rather than individual sites, that is important for proper folding (23, 35, 42). Most of the N-linked glycosylation sites can be removed individually without markedly affecting envelope function (3, 23, 35). A number of studies have pointed out that modification of the carbohydrate composition on the viral surface can alter infectivity (11, 18, 32–34, 40, 41, 44, 47). Most prominently, Hu et al. (18) have demonstrated that the degree of virion sialylation affects human immunodeficiency virus type 1 (HIV-1) infectivity.
Previous studies have demonstrated that N-linked carbohydrates on the surface glycoprotein gp120 of the simian immunodeficiency virus (SIV) strain SIVmac can help to shield the virus from antibody recognition (37, 43). Continuing studies that utilize a mutagenic approach will likely provide useful information of a fundamental nature on the contribution of specific carbohydrate attachment sites to the evasion of antibody recognition. More practically, further studies may also lead to improvements in the immunogenicity of envelope-based vaccines for HIV. In our present studies, we have extended previously published observations of the effects of glycosidases by examining a larger panel of glycosidases and their effects on soluble and particle-bound gp120. In addition, enzyme-treated virus stocks were analyzed for changes in infectivity and sensitivity to neutralization.
MATERIALS AND METHODS
Monoclonal antibodies.
The anti-SIVmac gp120 monoclonal antibodies KK43, KK52, and KK54 were the gift of Karen Kent (National Institute for Biological Standards and Control, Potters Bar, Hertfordshire, United Kingdom). Horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) antibody for Western blot detection was purchased from Pierce (Rockford, Ill.).
Cells.
Human CD4+ CEM×174 (National Institutes of Health AIDS Research and Reference Reagent Program, Rockville, Md.) and CEM×174 SIV-SEAP (29) cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). The immortalized rhesus macaque peripheral blood mononuclear cell (PBMC) line, 221 cells, was maintained in RPMI 1640 supplemented with 10% FCS and 5% interleukin-2.
Glycosidase treatment.
Digestions of SIVmac239 and SIVmac316 were carried out in a total volume of 300 μl of RPMI 1640 containing 60 ng of virus p27 and various deglycosylating enzymes. For digestion with N-glycosidase F (NgF) (Calbiochem, La Jolla, Calif.), 4 U of enzyme was used; for N-glycosidase A (Calbiochem), 1 mU was used; for α-mannosidase (Calbiochem), 400 mU was used; for endoglycosidase F (eF) (Calbiochem), 400 mU was used; for endoglycosidase H (Calbiochem), 8 mU was used; for endo-β-galactosidase (Calbiochem), 8 mU was used; and for α2-3,6-neuraminidase, α2-3,8-neuraminidase (from Newcastle disease virus [NDV]), α2-3,6,8-neuraminidase, and α2-3,6,8,9-neuraminidase (Calbiochem), 8 mU was used. Reaction mixtures were incubated for 3 h at 37°C. After the incubation period, 50 μl was removed and used for gel analysis as detailed below. The remainder of the sample was diluted to 800 μl and used for infectivity and neutralization sensitivity measurements as detailed below.
For neuraminidase treatment of cells, CEM×174 SIV-SEAP or parental CEM×174 cells were spun down and resuspended in RPMI 1640 at 1.5 × 105 cells/ml. Neuraminidase (40 mU/ml) was added, and the cells were incubated for 6 h at 37°C. After the incubation period, the cells were washed and resuspended in RPMI 1640 plus 10% FCS and then infected with various amounts of virus. After 15 h, the cells were washed and distributed into 96-well plates. After approximately 60 h, secreted engineered alkaline phosphatase (SEAP) activity was measured using a Phosphalight kit (Tropix, Bedford, Mass.), with slight modifications to the manufacturer's recommendations as described previously (29).
Infectivity assay.
The virus infectivity was measured using the CEM×174 SIV-SEAP cells. A 96-well plate was set up with each row containing two uninfected wells and two sets of five twofold dilutions of virus. To these wells, 3 × 104 CEM×174 SIV-SEAP cells were added, and the plate was transferred to a humidified CO2 incubator at 37°C. After 60 to 72 h, the amount of SEAP activity in the supernatant was measured as described above.
Viral pellets and immunoprecipitation.
To pellet virus, 10 ng of virus was spun for 90 min at high speed in a refrigerated microcentrifuge. Supernatant from the viral pellets was removed and used for immunoprecipitation. To each supernatant, 30 μl of protein A/G (Santa Cruz Biotechnology, Santa Cruz, Calif.) and 5 μl of serum from an SIVmac239-infected rhesus macaque were added. The samples were brought up to 1 ml in volume by addition of RPMI 1640 and incubated with rocking at 4°C for at least 12 h. After the incubation period, the samples were spun for 30 s at high speed. The supernatants were discarded, and the pellets were resuspended in phosphate-buffered saline–0.05% Tween 20. Each sample was then vortexed for 1 min and spun, and the supernatant was removed. This washing procedure was repeated two additional times.
Digestion of SDS-treated virus by various glycosidases.
Equal aliquots of SIVmac239, containing 60 ng of p27, were subjected to high-speed centrifugation for 90 min at 4°C. The supernatants were removed, and the pellets were resuspended in 7 μl of a solution containing 10% sodium dodecyl sulfate (SDS) and 1% β-mercaptoethanol. Samples were boiled for 5 min, and then 63 μl of a solution containing 1% NP-40 and 1% β-mercaptoethanol was added. The appropriate glycosidase was added, and samples were incubated at 37°C for 3 h. At the end of the incubation period, the samples were electrophoresed in a 5% polyacrylamide–SDS gel and subjected to Western blotting as described below.
Western blotting.
Viral pellets and immunoprecipitated samples were resuspended in Laemmli sample buffer and boiled for 3 min. The samples were then electrophoresed through a 5% polyacrylamide–SDS gel and transferred onto Immobilon-P membranes (Millipore, Bedford, Mass.). The membranes were blocked with 5% skim milk in phosphate-buffered saline–0.05% Tween 20 for 1 h. The blots were then incubated sequentially with a mixture of anti-SIVmac gp120 antibodies KK43, KK52, and KK54 and then with horseradish peroxidase-labeled anti-mouse IgG (Pierce). The antibodies were visualized using a PicoWest chemiluminescence kit (Pierce) and then were either placed against film or visualized using an LAS-1000 charge-coupled device camera (Fuji, Inc., Tokyo, Japan).
Growth of virus in the presence of glycosidase inhibitors.
Four flasks of CEM×174 cells (106 per flask) were infected with 10 ng of SIVmac239 p27. At 6 days postinfection the cells were spun down and resuspended in RPMI 1640 plus 10% FCS containing swainsonine (58 μM) (Calbiochem), deoxygalactonojirimycin (dGJ) (1 mM) (Calbiochem), 2,3-dehydro-2-deoxy-N-acetylneuraminic acid (DANA) (1.72 mM) (Calbiochem), or no inhibitor. The medium was completely replaced with fresh, inhibitor-containing medium every day for the next 2 days. On the third day the cells were spun down, washed, and resuspended in RPMI 1640 plus 10% FCS but lacking inhibitor. Cell-free viral stocks were collected 20 h later, and the amount of p27 antigen was measured.
RESULTS
Glycosidases have little effect on the electrophoretic mobility of virion-associated gp120 of SIVmac.
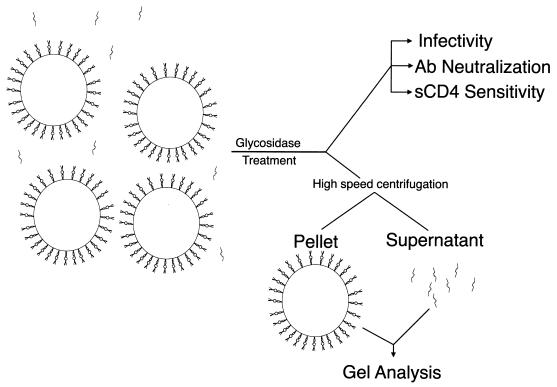
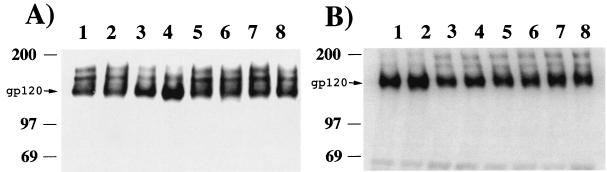
Stocks of SIVmac239 and SIVmac316 produced in CEM×174 cells were treated with a number of different glycosidases. A schematic of the experiments performed with each glycosidase is shown in Fig. 1. After digestion, a portion of the treated material was microcentrifuged at 4°C to pellet the intact virus. The supernatant was removed from each sample, the pellet was subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and immunoreactive gp120 was detected by Western blotting. Shifts in the mobility of gp120 in the pelletable material compared with that for mock-treated virus were either marginal or nondetectable (Fig. 2). Endoglycosidase H and α-mannosidase occasionally caused a small shift in SIVmac316 gp120 (Fig. 2B, lanes 4 and 6) but not in SIVmac239 gp120 (Fig. 2A, lanes 4 and 6). However, this slight shift was not consistently observed.
FIG. 1.
Schematic of experimental design. Stocks of virus containing both free and virion-bound gp120 were incubated with various glycosidases. After digestion, a portion of the virus was used to test for infectivity and neutralization sensitivity using CEM×174 SIV-SEAP cells. Another portion of the treated virus was subjected to high-speed centrifugation. Supernatant was removed from the resulting pellet, and the gp120 within it was immunoprecipitated with sera from SIVmac239-infected rhesus monkeys. The pellet and immunoprecipitated materials were then analyzed by SDS-PAGE and Western blotting. Ab, antibody.
FIG. 2.
Mobilities of virion-associated, glycosidase-treated gp120 of SIVmac239 (A) and SIVmac316 (B). Virus stock containing 60 ng of p27 was incubated at 37°C with one of the various glycosidases or buffer alone for 3 h. Each of the samples was then spun for 90 min at high speed in a refrigerated microcentrifuge. Supernatant was drawn off from each sample and set aside for immunoprecipitation experiments. Each of the pellets was boiled for 5 min in loading buffer containing 10% SDS and 1% β-mercaptoethanol. Equal amounts of each sample were then subjected to SDS-PAGE and blotted onto Immobilon-P membranes. The blots were sequentially incubated with a mixture of anti-gp120 monoclonal antibodies (KK43, KK52, and KK54) and a horseradish peroxidase-conjugated anti-mouse IgG antibody. Localization of the antibodies was visualized with a SuperSignal Pico West kit (Pierce) according to the manufacturer's recommendations. Lanes: 1, mock treatment; 2, NgF-treatment; 3, N-glycosidase A treatment; 4, α-mannosidase treatment; 5, eF treatment; 6, endoglycosidase H treatment; 7, endo-β-galactosidase-treatment; 8, neuraminidase-treatment. Numbers on the left indicate the relative positions of molecular mass markers and are shown in kilodaltons. An arrow shows the location of gp120.
Glycosidases can change the mobility of free gp120.
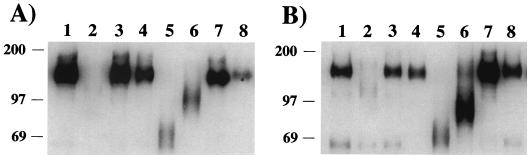
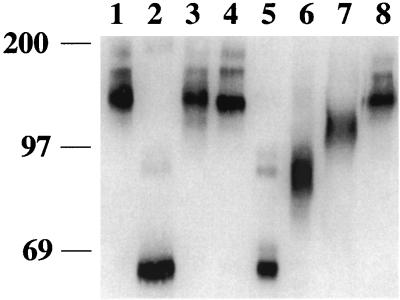
Nonpelletable gp120 was immunoprecipitated from the supernatants of the glycosidase-treated, microcentrifuged samples described above and analyzed by SDS-PAGE and Western blotting. In this case, mobility shifts were readily apparent with four of the seven enzymes that were tested (Fig. 3). Digestion with eF resulted in the largest shift in mobility, producing a diffuse band at around 69 kDa (Fig. 3, lanes 5). The diffuse band is likely a result of either different numbers of N-linked carbohydrates remaining on the individual gp120 molecules due to incomplete digestion or microheterogeneity in the remaining carbohydrates. Endoglycosidase H digestion produced a diffuse band at around 80 to 97 kDa (Fig. 3, lanes 6). A greater band width was consistently seen with SIVmac316 gp120 (Fig. 3B, lane 6, and data not shown) than with SIVmac239 gp120 (Fig. 3A, lane 6, and data not shown), reflecting differences in either carbohydrate addition or envelope folding. Endo-β-galactosidase treatment produced only a small shift in both SIVmac239 and SIVmac316 gp120 (Fig. 3, lanes 7). Treatment with NgF resulted in about a 20-kDa shift that was seen in Fig. 3, lanes 2, after longer exposure. Incubation with NgF appeared to decrease gp120 protein stability or increase proteolytic activity, as the amount of nonpelletable material was always decreased after digestion compared to mock digestion. This was not the result of a loss of recognition by the antibodies used for immunoprecipitation, because SDS-boiled, NgF-digested gp120 was efficiently precipitated (data not shown). In addition, an increase in HIV-1 envelope sensitivity to proteolysis after digestion with NgF has been previously reported (38). The results in Fig. 2 and 3 indicate that the state or structure of gp120 influenced sensitivity to digestion by glycosidases. Both the pelletable and nonpelletable materials were in the same enzymatic reaction, and only the nonpelletable material was sensitive, while the pelletable material was refractory, to glycosidase digestion. Based on this, we next examined the sensitivity of completely denatured gp120 to enzymatic deglycosylation.
FIG. 3.
Mobilities of nonpelletable, glycosidase-treated gp120 of SIVmac239 (A) and SIVmac316 (B). Supernatants from viral pellets (described in the legend to Fig. 2) were subjected to immunoprecipitation with serum from an SIVmac239-infected rhesus macaque. The samples were then electrophoresed in a 5% polyacrylamide–SDS gel, transferred to a membrane, and reacted with a mixture of anti-gp120 monoclonal antibodies KK43, KK52, and KK54. Lanes: 1, mock treatment; 2, NgF treatment; 3, N-glycosidase A treatment; 4, α-mannosidase treatment; 5, eF treatment; 6, endoglycosidase H treatment; 7, endo-β-galactosidase treatment; 8, neuraminidase treatment. Numbers on the left indicate the relative positions of molecular mass markers (in kilodaltons).
Denaturation increases gp120 deglycosylation.
Equal amounts of SIVmac239 virus were pelleted in a microcentrifuge, and the supernatant was removed. The viral pellets were resuspended in 10% SDS plus β-mercaptoethanol and boiled for 5 min. The ionic SDS detergent was complexed by addition of an excess of NP-40, a nonionic detergent. Glycosidase digestions were then performed as described above, using the same amount of enzyme and the same incubation period. At the end of the digestion, sample buffer was added and the sample was analyzed directly by SDS-PAGE without further manipulation. Envelope protein was visualized following Western blotting as described above.
If all 24 potential N-linked glycosylation sites of SIVmac239 and SIVmac316 are used and each N-linked carbohydrate contributes an average of 2.5 kDa (21), then complete deglycosylation of gp120 will give an expected molecular mass of around 60 kDa. Based on these assumptions, NgF and eF removed all or almost all of the N-linked carbohydrates present on gp120, as evidenced by an increase in mobility to around 60 kDa (Fig. 4, lanes 2 and 5). This is a much greater shift in mobility than was seen after treatment of the nonboiled samples with NgF, which resulted in a weak band of about 97 kDa (Fig. 3A, lane 2). The shift caused by eF treatment of SDS-denatured gp120 was slightly greater than that caused by treatment of the nonboiled sample (Fig. 4, lane 5, versus Fig. 3A, lane 5), and the band was less diffuse, indicating removal of additional N-linked carbohydrates. Endo-β-galactosidase digestion also caused a greater mobility shift in the boiled (Fig. 4, lane 7) than in the nonboiled (Fig. 3A, lane 7) samples. The mobility of the endoglycosidase H-treated sample was also increased by boiling, but the sample still migrated as a diffuse band, indicating incomplete digestion or microheterogeneity. The α-mannosidase-treated sample showed a slight increase in mobility after boiling, while both N-glycosidase A- and neuraminidase-treated samples migrated identically with or without boiling. The lack of detectable changes in mobility after digestion with these enzymes can be explained by the sensitivity of these enzymes to blocking by certain terminal carbohydrate residues. Table 1 shows a summary of the expected versus observed shifts in molecular mass of the virion-associated gp120, free gp120, and SDS-denatured gp120 for each of the glycosidases tested.
FIG. 4.
Mobility of SIVmac239 gp120 after SDS denaturation and then glycosidase treatment. Equal amounts of SIVmac239 were subjected to high-speed centrifugation for 90 min at 4°C. The supernatant was removed, and the pellets were resuspended in 10% SDS and 1% β-mercaptoethanol. Samples were boiled for 5 min, and then excess NP-40 was added to complex the SDS. Samples were digested with various glycosidases and then electrophoresed in a 5% polyacrylamide–SDS gel, transferred to a membrane, and reacted with a mixture of anti-gp120 monoclonal antibodies KK43, KK52, and KK54. Antibody localization was visualized by Western blotting as described in Materials and Methods. Lanes: 1, mock treatment; 2, NgF treatment; 3, N-glycosidase A treatment; 4, α-mannosidase treatment; 5, eF treatment; 6, endoglycosidase H treatment; 7, endo-β-galactosidase treatment; 8, neuraminidase treatment. Numbers on the left indicate the relative positions of molecular mass markers and are shown in kilodaltons.
TABLE 1.
Summary of expected and observed shifts in molecular masses of virion-associated, free, and SDS-denatured gp120 for each of the glycosidases tested
| Glycosidase | Specificitya | Mobility (kDa) of:
|
Predicted mobility (kDa) of gp120 after complete digestion | ||
|---|---|---|---|---|---|
| Virion-bound gp120d | Free gp120e | SDS-denatured gp120f | |||
| NgF | R1-GlcNAc-GlcNAc⇓Asnb | 110–115 | 80 | 60 | 60h |
| N-glycosidase A | R1-GlcNAc-GlcNAc⇓Asnc | 120 | 120 | 120 | 60i |
| α-Mannosidase | α1-2,3,6-linked mannose | 115–120 | 115–120 | 115 | NPj |
| eF | R2-GlcNAc⇓GlcNAc-Asn | 120 | 67–72 | 60g | 67k |
| Endoglycosidase H | R3-GlcNAc⇓GlcNAc-Asn | 110 | 75–110 | 97–85 | NP |
| Endo-β-galactosidase | R4-(GlcNAc-Galβ1)n⇓4R5 | 120 | 110 | 100 | NP |
| Neuraminidase | Terminal α2-3,6,8,9-Neu5Ac | 120 | 120 | 120 | 105–91l |
The arrow indicates the site of cleavage. Asn, asparagine; Gal, galactose; Neu5Ac, N-acetylneuraminic acid; R1, high-mannose, hybrid-, or complex-form oligosaccharides; R2, high-mannose, hybrid-, or biantennary-complex-form oligosaccharides; R3, high-mannose or hybrid-form oligosaccharides; R4, Gal or Neu5Ac-Gal; R5, GlcNAc or Gal.
Blocked by core fucosylation.
Blocked by Neu5Ac.
Mobility of pelletable, glycosidase-treated gp120 as described in the text.
Mobility of immunoprecipitated, nonpelletable, glycosidase-treated gp120 as described in the text.
Mobility of SDS-denatured and then glycosidase-treated gp120 as described in the text.
The mobility of denatured, eF-treated SIVmac239 is greater than predicted because of NgF contamination, as described in the text.
Based on the assumption that all 24 potential N-linked carbohydrates, each contributing about 2.5 kDa, are completely removed.
Based on the assumption that all 24 potential N-linked carbohydrates, each contributing about 2.5 kDa, are completely removed.
NP, not possible to predict.
Based on the assumption that all 24 potential N-linked carbohydrates are removed by cleavage in the chitobiose core, removing about 2.2 kDa each.
Based on the assumption that all 24 potential N-linked carbohydrates have two to four sialic residues, each contributing 309 Da, that are completely removed.
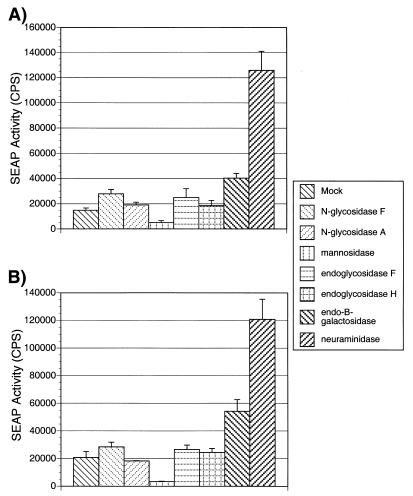
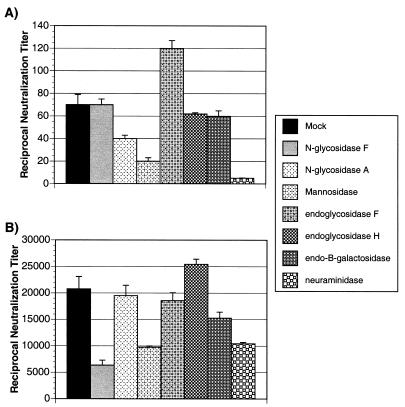
Glycosidase digestion can increase the infectivity of SIVmac.
After digestion of viral stock by glycosidase, a portion was examined for changes in infectivity. Enzymatically treated and mock-treated viruses were used to infect CEM×174 SIV-SEAP cells. These cells secrete an engineered, placental alkaline phosphatase (SEAP) into the medium in response to infection by SIV (29). The amount of SEAP secreted is in direct relation to the amount of infecting virus and can be sensitively and rapidly measured using a chemiluminescence assay. The results of a representative experiment are shown in Fig. 5. For both SIVmac239 and SIVmac316, treatment with NDV neuraminidase and endo-β-galactosidase resulted in an increase of infectivity as evidenced by increased SEAP activity in the medium of infected CEM×174 SIV-SEAP cells. NDV neuraminidase treatment increased SEAP activity by around 8-fold in typical experiments but by up to 12-fold in some experiments. This is similar to the increases in infectivity seen in the experiments of Hu et al. (18). NgF, endoglycosidase H, and eF treatments consistently increased the infectivity of both SIVmac239 and SIVmac316, but by a smaller amount, less than twofold (Fig. 5 and data not shown). Treatment with α-mannosidase consistently reduced viral infectivity by fourfold or more (Fig. 5 and data not shown). Finally, N-glycosidase A treatment had little or no effect on viral infectivity (Fig. 5 and data not shown). In all cases except for neuraminidase treatment, cell viability, examined microscopically by trypan blue exclusion, and cell morphology were comparable to those for cells infected with mock-treated virus (data not shown). After infection with neuraminidase-treated virus, the cell viability remained unchanged, but the cells aggregated to a slightly greater degree than the cells infected with mock-treated virus. Since there was a large effect of neuraminidase treatment on infectivity, we examined the phenomenon further.
FIG. 5.
Infectivity of glycosidase-treated virus. After digestion with the indicated glycosidase, SIVmac239 (A) or SIVmac316 (B) was used to infect CEM×174 SIV-SEAP cells. Twofold dilutions of virus were added to equal amounts of CEM×174 SIV-SEAP cells and then transferred to a 37°C CO2 incubator. At approximately 60 h postinfection, the cell-free supernatant was harvested and the amount of SEAP expression was measured as described in Material and Methods. The amount of SEAP expression for each dilution was used to generate a curve from which the amount of SEAP activity per nanogram of p27 was calculated. Panel A shows the average SEAP activity per nanogram of p27 SIVmac239 after treatment with the indicated glycosidase, and panel B shows infectivity of SIVmac316 after treatment with the same glycosidases. The standard error for each experiment is indicated.
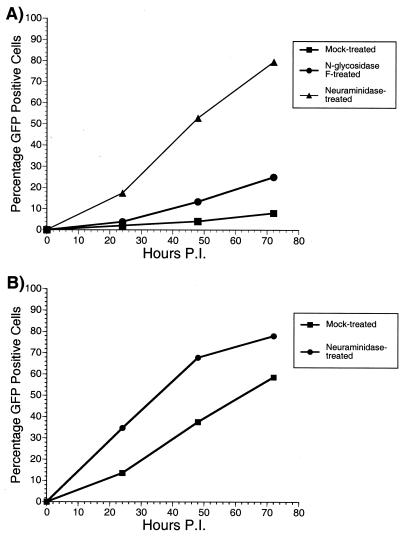
NDV neuraminidase treatment of SIVmac239-EGFP increases infectivity as measured by flow cytometry.
SIVmac239-EGFP is a clone of SIVmac239 that has been engineered to express the enhanced green fluorescent protein (EGFP) in place of the nef gene (2). This allows the number of infected cells in a culture to be counted easily by flow cytometry. Figure 6 shows the results of two experiments performed with this virus. For Fig. 6A, equal amounts of virus were either mock digested or digested with NgF or NDV neuraminidase. The treated viruses were then used to infect CEM×174 cells. At various times postinfection, cells were removed and the number of EGFP-expressing cells was counted by flow cytometry. Over time the quantity of EGFP-positive cells increased in each culture, irrespective of treatment. At each time point, however, the numbers of EGFP-positive cells in the cultures infected with neuraminidase-treated virus were greater than those in the other cultures, around 8- to 10-fold higher than for mock-treated virus. Similarly, the numbers of positive cells in the cultures infected with NgF-treated virus were about twofold greater than the amounts in the mock-treated cultures. These increased numbers of infected cells agree with the results of the CEM×174 SIV-SEAP infections (Fig. 5). We performed a similar experiment with 221 cells, a herpesvirus saimiri-transformed rhesus macaque T-lymphoid cell line (1). Cells were infected with mock-treated or neuraminidase-treated SIVmac239-EGFP. As with CEM×174 cells, the numbers of EGFP-positive cells were counted at various times postinfection. Throughout the course of infection, the cultures infected with neuraminidase-treated virus had a greater number of EGFP-positive cells than the cultures infected with mock-treated virus. This difference, however, was only two- to threefold (Fig. 6B), not as great as was seen after infection of CEM×174 cells (Fig. 6A).
FIG. 6.
Neuraminidase treatment increases the infectivity of SIVmac239-EGFP. Equal amounts of SIVmac239-EGFP were either mock treated, treated with neuraminidase, or treated with NgF. The treated virus was used to infect CEM×174 cells (A) or 221 cells (B). At various time points postinfection, the amounts of EGFP-positive cells were quantitated by flow cytometry analysis.
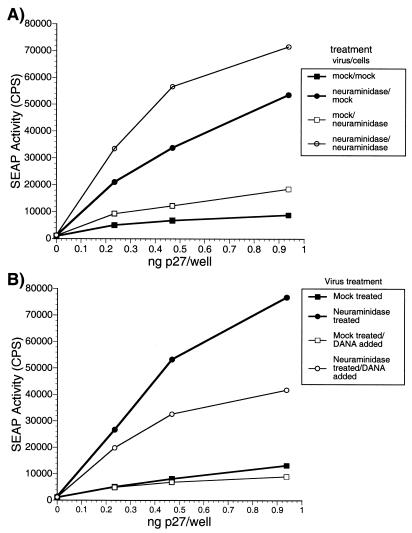
Small effect of neuraminidase on susceptibility of cells to infection.
As described above, infection of cells with neuraminidase-treated virus resulted in a slight aggregation of the cells. To determine whether residual neuraminidase in the inoculum acting on cell surface proteins was responsible for the apparent increase in viral infectivity, we performed the following experiment. Cells were treated with a concentration of neuraminidase equal to 10 times the largest amount they would receive during the normal infectivity assay. After a 6-h incubation, the cells were washed in neuraminidase-free medium and immediately inoculated with mock- or neuraminidase-treated virus. After 15 h, the cells were washed to remove free virus and the medium was replaced with neuraminidase-containing medium. Comparison of SEAP activities from untreated cells infected with the mock- and neuraminidase-treated viruses gave an expected increase (sevenfold) in infectivity (Fig. 7A). Infection of neuraminidase-treated cells with neuraminidase-treated virus gave about a fourfold increase in SEAP activity in the medium compared with infection by mock-treated virus (Fig. 7A). Overall, neuraminidase treatment of cells increased SEAP activity in the medium only slightly, about twofold, compared with infection of untreated cells. When neuramindase-treated virus was used to infect cells in the presence and absence of DANA, a sialic acid analog inhibitor, the majority of the infectivity enhancement could again be accounted for by an effect of the enzyme on the virus (Fig. 7B).
FIG. 7.
Effects of neuraminidase on cells. CEM×174 SIV-SEAP cells were treated for 6 h with 40 mU of neuraminidase, which is 10 times the largest amount of neuraminidase present during the measurement of neuraminidase-treated virus in the infectivity assay. These cells were then washed with neuraminidase-free medium and infected with either mock- or neuraminidase-treated SIVmac239. SEAP activity in the medium was assayed at approximately 60 h postinfection as described in Materials and Methods.
Sialic acid linked α2-3, α2-6, α2-8, and α2-9 to the viral envelope affects infectivity.
As a further exploration of the effect of sialic acid on viral infectivity, we next treated SIVmac239 with neuraminidases which differ in their ability to cleave specific sialic acid linkages. Aliquots of SIVmac239 containing 60 ng of p27 were mock treated or treated with 8 mU of α2-3,6-, α2-3,8-, α2-3,6,8-, or α2-3,6,8,9-neuraminidase for 3 h at 37°C. The treated virus was then tested for infectivity using the CEM×174 SIV-SEAP cells (Fig. 8). Treatment of the virus with α2-3,6,8,9-neuraminidase resulted in the greatest increase in infectivity in this assay, approximately sixfold. Treatment with α2-3,6,8-neuraminidase resulted in a diminished increase in infectivity, only about fivefold. Digestion with α2-3,8-neuraminidase increased infectivity only about threefold, and treatment with α2-3,6-neuraminidase did not significantly affect viral infectivity. Overall, this experiment indicates that multiple sialic acid residues, with different linkage specificities, influence viral infectivity enhancement; neuraminidases with increasing breadth of specificities showed increasing enhancement of infectivity.
FIG. 8.
Differential effects of sialic acid residues linked α2-3, α2-6, or α2-8 on viral infectivity. SIVmac239 was treated with the indicated neuraminidases as described in Materials and Methods. The treated virus was then used to infect CEM×174 SIV-SEAP cells, and the amount of SEAP activity was quantitated at approximately 60 h postinfection. The standard deviation for each experiment is indicated.
Treatment with deglycosylation enzymes has little effect on neutralization sensitivity.
After treatment with each of the glycosidases, a portion of virus was tested for a change in neutralization sensitivity versus that of mock-treated virus in the SEAP neutralization assay. Briefly, a fixed amount of each virus, 1 ng of p27/well, was added to assay plates containing twofold serial dilutions of sera pooled from naive or SIVmac239-infected rhesus macaques. After a 1-h incubation, 3 × 104 CEM×174 SIV-SEAP cells were added to each well. The plates were then transferred to a humidified, 37°C CO2 incubator. The amount of SEAP activity in the medium was measured approximately 60 h later. Figure 9 shows the reciprocal dilution of the titer of serum required to neutralize 50% of the virally induced SEAP activity compared with a nonneutralized control. The neutralization sensitivity of SIVmac239 did not change appreciably after digestion with any of the glycosidases (Fig. 9A). In all cases the 50% neutralization titer against the treated SIVmac239 was 1:50 ± 2.5-fold, identical to that against untreated SIVmac239. There was a slight (less than twofold) increase in sensitivity after digestion with endoglycosidase F (Fig. 9A). Treatment with α-mannosidase or N-glycosidase A resulted in a decrease in neutralization sensitivity, as evidenced by a larger amount of serum being necessary to neutralize 50% of viral infectivity (Fig. 9A). However, this change in sensitivity was only slight compared with untreated SIVmac239. Digestion with neuraminidase caused the greatest decrease in neutralization sensitivity (Fig. 9A). The neutralization sensitivity of SIVmac316 also did not appreciably change after digestion with the various glycosidases (Fig. 9B), although the variation in neutralization titer was greater than that seen with treated versus untreated SIVmac239 (Fig. 9A). Digestion with endoglycosidase H caused a less-than-twofold increase in neutralization sensitivity (Fig. 9B). Of the other enzymes, only NgF decreased sensitivity by more than threefold (Fig. 9B). Like the case for SIVmac239, treatment of SIVmac316 with α-mannosidase and neuraminidase decreased neutralization sensitivity (Fig. 9B), but not to as great an extent as for SIVmac239 (Fig. 9A). Overall, under the conditions used in these experiments, digestion of SIVmac by glycosidases had little or no effect on neutralization sensitivity to sera from SIVmac239-infected rhesus macaques.
FIG. 9.
Neutralization of viruses by sera from SIVmac239-infected rhesus macaques. SIVmac239 (A) and SIVmac316 (B), treated with the indicated glycosidases as described in Materials and Methods, were used as virus inocula in SEAP neutralization assays as described in Materials and Methods. From the neutralization curves the amount of sera, pooled from SIVmac239-infected rhesus macaques, required to neutralize 50% of the virus-induced SEAP activity as compared with nonneutralized virus was determined. These results are representative of those from three separate experiments performed with each set of viruses, and the standard deviations are indicated.
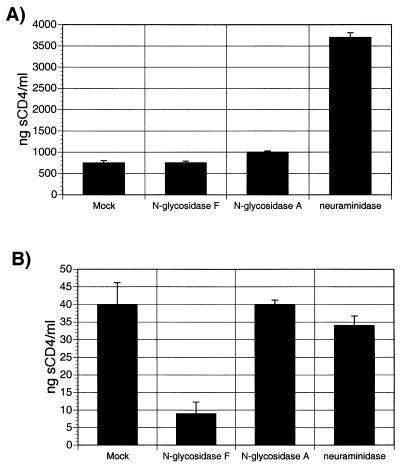
Treatment with glycosidases alters viral sensitivity to soluble CD4.
Next, several of the treated viruses were tested for changes in sensitivity to neutralization by soluble CD4 (sCD4). Neutralization assays were set up as described above, but instead of rhesus macaque sera, twofold dilutions of sCD4 were added. The amount of sCD4 required to neutralize 50% of viral infectivity compared with that for a mock-neutralized control was calculated, and Fig. 10 shows the results of a representative experiment. The amount of sCD4 necessary to reduce SIVmac239 infectivity by 50% was approximately the same for the mock-treated, NgF-treated and N-glycosidase A-treated viruses, about 750 ng/ml (Fig. 10A). The amount required to neutralize 50% of the infectivity of neuraminidase-treated SIVmac239 was almost fivefold higher, at about 3,700 ng/ml (Fig. 10A). Mock-treated SIVmac316 was much more sensitive to neutralization by sCD4 than mock-treated SIVmac239. Only 40 ng/ml was required to reduce mock-treated SIVmac316 viral infectivity by 50% (Fig. 10B). About fourfold less sCD4 was required for 50% neutralization of NgF-treated SIVmac316 (Fig. 10B). Digestion of SIVmac316 by either N-glycosidase A or neuraminidase had no effect on neutralization by sCD4.
FIG. 10.
Neutralization of viruses by sCD4. Neutralization of SIVmac239 (A) or SIVmac316 (B) was tested as described in the legend to Fig. 9, using sCD4 instead of macaque sera to neutralize viral infectivity. The concentration of sCD4 required to reduce SIVmac239- or SIVmac316-induced SEAP activity by 50% is shown. These results are representative of those from three separate experiments performed with each set of viruses, and the standard deviations are indicated.
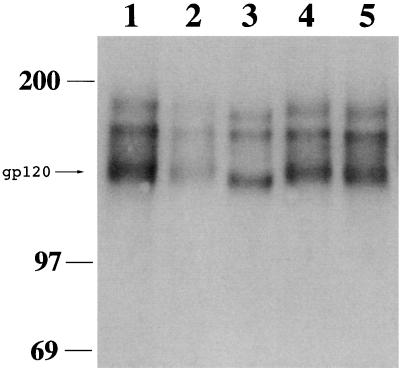
Production of SIVmac239 in the presence of glycosidation inhibitors alters gp120 mobility.
To further examine the effects of glycosylation on SIVmac239 infectivity and neutralization, virus was produced in CEM×174 cells grown in the presence of various glycosidation inhibitors. Swainsonine, a Golgi mannosidase inhibitor, blocks the processing of high-mannose-form N-glycans to complex-form carbohydrates. A second inhibitor, dGJ, which blocks α-galactosidase activity, was also used. Finally, virus was produced in the presence of DANA, a sialic acid analog inhibitor. Equal amounts of virus were centrifuged, and the pelleted gp120 was visualized by SDS-PAGE and Western blotting as described above. Growth in the presence of swainsonine caused a shift in gp120 mobility compared with virus grown in the absence of inhibitors (Fig. 11, lane 3 versus lanes 1 and 5). The inhibitors dGJ and DANA had no effect on gp120 mobility (Fig. 11, lanes 2 and 4 versus lanes 1 and 5). However, growth in the presence of dGJ resulted in a decrease in the amount of pelletable envelope, reflecting either decreased protein stability or, as previously reported for N-butyldeoxynojirimycin (11) and castanospermine (45), a decrease in the amount of envelope protein per virion.
FIG. 11.
Mobility of gp120 from SIVmac239 grown in the presence of glycosylation inhibitors. CEM×174 cells were infected with equal amounts of SIVmac239. At 6 days postinfection, medium alone or medium containing dGJ (1 mM), swainsonine (50 μM), or DANA (1.72 mM) was added to the cultures. The medium was completely replaced with fresh inhibitor-containing medium every 24 h for 3 days. At 10 days postinfection, the cells were washed and resuspended in medium without inhibitor. Cell-free virus stocks were collected 24 h later, and the amount of p27 antigen was quantitated by enzyme-linked immunosorbent assay. Equal amounts of each virus were spun for 90 min at high speed in a microcentrifuge. The supernatant was removed, and gp120 was electrophoresed and visualized as described in the legend to Fig. 2. Lanes: 1 and 5, mock treatment; 2, dGJ treatment; 3, swainsonine treatment; 4, DANA treatment.
Glycosidation inhibitors have slight effects on infectivity and neutralization sensitivity of SIVmac239.
Viruses grown in the presence of the various inhibitors were next examined for differences in infectivity. Equal amounts of virus (2 ng of p27) were used to infect CEM×174 SIV-SEAP cells. The level of SEAP activity was measured at 60 h postinfection. Virus produced in the presence of DANA was about twofold more infectious per nanogram of p27 Gag antigen than the untreated virus (Fig. 12A). Virus subjected to any of the other treatments had essentially unchanged infectivity compared with untreated virus (Fig. 12A). These viruses were next tested for sensitivity to sera pooled from SIVmac239-infected rhesus macaques. Swainsonine-treated and DANA-treated viruses both showed slight increases in neutralization sensitivity that are likely not significant (Fig. 12B). Thus, while changes in carbohydrate addition caused by DANA had effects on infectivity, the changes in glycosylation caused by growth in the presence of the other two glycosylation inhibitors had little effect on viral properties.
FIG. 12.
Infectivity and neutralization sensitivity of SIVmac239 grown in the presence of glycosylation inhibitors. (A) Stocks of SIVmac239 grown in the presence of the indicated inhibitor were used to generate infectivity curves as described in the legend to Fig. 5. The average amounts of SEAP activity induced per nanogram of p27 of each stock from CEM×174 SIV-SEAP cells are shown, along with the standard error from three separate experiments. (B) The same stocks of virus were used as inocula for SEAP neutralization assays, and the 50% reciprocal neutralization titer of pooled SIVmac239-infected rhesus macaque sera against each stock is shown. Similar results were obtained in three separate experiments, and the standard deviations are indicated.
DISCUSSION
A variety of activities have been attributed to the carbohydrate side chains of SIV envelope. In this study, we have examined the accessibility of N-linked glycans, their role in viral infectivity, and their effects on neutralization. Virus was digested with a variety of glycosidases and then tested for phenotypic changes by SDS-PAGE analysis and in SEAP reporter gene assays. Our studies show that in the context of the viral particle, gp120 that is presumably in oligomeric form is largely occluded from digestion by a number of endo- and exoglycosidases (Fig. 2). Both SIVmac239 and SIVmac316 demonstrated this resistance to digestion. This was not simply due to an inability of any of the enzymes to recognize N-glycans on the envelope proteins of these two viruses. This was shown by examining nonpelletable, presumably monomeric, gp120 present in the same reactions for changes in mobility (Fig. 3). Several of the enzymes, i.e., NgF, α-mannosidase, eF, endoglycosidase H, and endo-β-galactosidase, caused changes in the mobility of the monomeric gp120. This demonstrates that the enzymes used were capable of removing glycans from both envelope proteins. This also suggests that there is greater exposure of the N-linked carbohydrates of free gp120 than of oligomeric gp120. However, we cannot completely rule out the possibility that removal of carbohydrate from oligomeric envelope on the viral surface caused gp120 to be released into the supernatant. In parallel studies (R. E. Means, M. Li, and J. Jung, unpublished data), a highly glycosylated envelope protein of herpesvirus saimiri, ORF51, was completely deglycosylated under equivalent nondenaturing conditions (data not shown). This demonstrates that these conditions were sufficient to remove N-linked carbohydrates from a membrane-associated protein and that membrane association itself did not prevent deglycosylation. In the presented experiments the mobility of completely denatured gp120 shifted even more than the mobility of nondenatured, monomeric gp120 after digestion with the same enzymes (Fig. 4). Since the digestions were set up to contain equivalent amounts of envelope proteins and enzymes, the results in Fig. 4 also demonstrate that a sufficient amount of enzyme was added to the digestion of the nondenatured viral particles to remove all susceptible carbohydrate side chains. The shifts in mobility seen in Fig. 4 are in agreement with other studies of the carbohydrates of SIV envelope (9, 24, 46).
NgF digestion of N-linked carbohydrates is blocked by the presence of fucose on the asparagine-linked N-acetylglucosamine (GlcNAc) of the chitobiose core. Digestion of the SDS-denatured gp120 of both SIVmac239 and SIVmac316 with NgF resulted in a shift of approximately 60 kDa. This is consistent with the removal of the majority, if not all, of the N-linked glycosylation. Other studies have reported HIV-1, HIV-2, and SIVsm gp120 chitobiose fucosylation (26, 27, 31), but the results presented here suggest that fucose is either not present or present on only a minority of SIVmac239 gp120 and SIVmac316 gp120 N-linked carbohydrates produced in CEM×174 cells. Holschbach et al. have reported that SIVsm gp130 produced in human H9 cells contains some core fucosylation (17), so it is possible that envelope from SIVmac grown in cells other than CEM×174 may contain chitobiose fucosylation.
The eF used in these experiments also contained NgF activity. The mobility of denatured gp120 treated with eF and NgF was equal to that of denatured gp120 treated with NgF alone, while the mobility of free gp120 treated with both enzymes was much greater than that of free gp120 treated only with NgF. This additional shift in mobility is explained by the difference in the eF and NgF cleavage sites. NgF cleaves the bond between the chitobiose GlcNAc and the protein asparagine, while eF cleaves between the two chitobiose GlcNAc residues. This slight shift in position likely allows eF easier access to its cleavage site, even in the context of a partially native protein.
Surprisingly, even in the absence of a detectable shift in mobility of the pelletable, virion-associated gp120 after treatment with either endo-β-galactosidase or neuraminidase (Fig. 2), there was an increase in viral infectivity (Fig. 5). Neuraminidase removes terminal sialic acid residues from the carbohydrate side chains. Since sialic acid has a low molecular mass of about 300 Da, a large number of these residues would have to be removed to result in a detectable shift in the mobility of the relatively large, 120-kDa gp120. However, mobility differences were seen between mock-treated and SDS-denatured, endo-β-galactosidase-treated gp120. This suggests that modification of only a small number of envelope proteins present on the surface of the virus is sufficient to change viral infectivity. It is also possible that deglycosylation of virion-associated producer cell proteins such as adhesins, which are known to play a role in viral infectivity (7, 8, 12, 15, 16, 25, 39), contributed to the differences in infectivity seen in these experiments.
The increased infectivity of neuraminidase-treated virus as measured on CEM×174 SIV-SEAP cells in these experiments is in close agreement with the fourfold enhancement of HIV-1NL4-3 infectivity measured on MT-4 cells in the experiments of Hu et al. (18). We confirmed that the results we observed were not cell type or measurement system specific by measuring viral infectivity for 221 cells, a rhesus T-cell line, using SIVmac239-EGFP (Fig. 6). Increases in viral infectivity measured this way were similar to those seen in the single-cycle SEAP reporter gene assay. The experiments presented in our report extend previous studies by examining the effects of a number of additional deglycosylating enzymes on both infectivity and neutralization sensitivity. Our studies show that neuraminidase treatment of virus also affects its sensitivity to neutralization by sCD4 and, to a lesser extent, neutralizing antibodies from infected animals.
Since neuraminidase-treated virus was added directly to cells without removal of enzyme to test viral infectivity in the SEAP assay, it was possible that the neuraminidase was acting on the cells and not the virus to increase infectivity. Two previous studies have shown that incubation of patient PBMCs with neuraminidase increased the efficiency of viral isolation (44, 47). However, in those studies it was unclear whether the effects were on PBMC or virus. We examined the effects of pretreating cells with neuraminidase and then infecting them with virus (Fig. 7). This treatment of cells resulted in only a small increase in the infectivity of mock-treated virus (Fig. 5), despite the fact that considerably larger amounts of neuraminidase were incubated with the cells than was used for treatment of virus (Fig. 7).
Sialic acid can be linked to the terminal galactose of the N-linked carbohydrate side chain either α2-3 or α2-6. In addition, sialic acid can be linked α2-8 or α2-9 to other sialic acid residues. Because the neuraminidases used in these experiments are all exosialidases, sialic acid attached α2-3 or α2-6 can be removed only if it is the terminal residue and not blocked by additional α2-8- or α2-9-linked sialic acid residues. The NDV neuraminidase is an α2-3,8-neuraminidase and so could not remove sialic acid either linked α2-6 or blocked by residues attached α2-9. In the experiment presented in Fig. 8, neuraminidases capable of removing sialic residues added α2-3,6 or α2-3,8 had only a slight effect on infectivity, while one that could remove residues added α2-3,6,8 increased infectivity to a much greater extent. This indicates that side chains linked α2-6 or blocked from removal by α2-8-linked terminal residues contribute to the effects of sialic acid on viral infectivity.
There are a number of possible explanations for the large increase in infectivity resulting from neuraminidase treatment. One previous study demonstrated that removal of sialic acid residues from gp120 did not enhance CD4 affinity (9). However, desialylation might allow for an increased affinity or ability to bind the viral coreceptor, which could translate into greater infectivity. Several other reports have suggested that removal of sialic acid from HIV-1 gp120 allows the virus to utilize galactose receptors on the cell surface for cell entry, potentially increasing infectivity (28, 32, 34). Another possibility is that a decrease in the electrostatic charge on the surface of the virus could allow for increased interaction between the virus and the negatively charged cell surface. Yet another possible mechanism for the increase in infectivity could be greater cell-to-cell spread. Greater cell aggregation was seen in cultures infected with neuraminidase-treated virus than in cultures infected with mock-treated virus. However, since infectivity measurements were done at a time point when viral spread would not be expected, this mechanism is unlikely. The cell aggregation seen after neuraminidase treatment also suggests the possibility that the increase in virion infectivity might be due to deglycosylation of virion-associated producer cell molecules that are involved in viral entry, a possibility that is not ruled out by the present experiments.
Although these experiments demonstrated a slight decrease in SIVmac neutralization sensitivity after neuraminidase treatment, experiments with myxomavirus have demonstrated the opposite. Increased, not decreased, sialylation decreases neutralization sensitivity (19). It is possible that the conditions used in these experiments also changed the envelope conformation, leading to the decrease in neutralization sensitivity. The fact that infectivity was increased by such a degree could also explain this difference in the experimental results. Since the infectivity per nanogram of p27 is higher, the molar ratio between infectious particles and antibody is, in effect, higher. There is an increased number of infectious viral particles per antibody molecule; therefore, a larger amount of antibody is needed to neutralize viral infectivity. This would also be true for neutralization by sCD4, as is seen in Fig. 9.
What advantage might there be for a virus to decrease its inherent infectivity by maintaining the complement of N-linked sugars that it carries? One possible explanation comes from our earlier work demonstrating that N-linked carbohydrates can affect both antibody elicitation and sensitivity to antibody-mediated neutralization (43). Several groups have reported that desialylation increases the antigenicity of HIV-1 gp120-derived peptides (4–6). Viral fitness may require a balance between inherent infectivity and avoidance of antibody recognition. This of course does not exclude positive effects of carbohydrates on folding, processing, and function.
ACKNOWLEDGMENTS
We thank Karen Kent for the gift of monoclonal antibodies used for Western blot detection of SIVmac gp120 and Louis Alexander for the gift of the SIVmac239-EGFP construct. We also thank Jae Jung for valuable advice, Welkin Johnson and Susan Czajak for critical reading, and the members of the Desrosiers lab for helpful discussions.
This work was supported by Public Health Service grants AI25328, AI35365, and RR00168 from the National Institutes of Health.
REFERENCES
- 1.Alexander L, Du Z, Rosenzweig M, Jung J U, Desrosiers R C. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J Virol. 1997;71:6094–6099. doi: 10.1128/jvi.71.8.6094-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander L, Lee H, Rosenzweig M, Jung J U, Desrosiers R C. An EGFP-containing vector system that facilitates stable and transient expression assays. BioTechniques. 1997;23:64–66. doi: 10.2144/97231bm12. [DOI] [PubMed] [Google Scholar]
- 3.Benjouad A, Babas T, Montagnier L, Bahraoui E. N-linked oligosaccharides of simian immunodeficiency virus envelope glycoproteins are dispensable for the interaction with the CD4 receptor. Biochem Biophys Res Commun. 1993;190:311–319. doi: 10.1006/bbrc.1993.1049. [DOI] [PubMed] [Google Scholar]
- 4.Benjouad A, Gluckman J C, Montagnier L, Bahraoui E. Specificity of antibodies produced against native or desialylated human immunodeficiency virus type 1 recombinant gp160. J Virol. 1993;67:1693–1697. doi: 10.1128/jvi.67.3.1693-1697.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjouad A, Gluckman J C, Rochat H, Montagnier L, Bahraoui E. Influence of carbohydrate moieties on the immunogenicity of human immunodeficiency virus type 1 recombinant gp160. J Virol. 1992;66:2473–2483. doi: 10.1128/jvi.66.4.2473-2483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjouad A, Mabrouk K, Gluckman J C, Fenouillet E. Effect of sialic acid removal on the antibody response to the third variable domain of human immunodeficiency virus type-1 envelope glycoprotein. FEBS Lett. 1994;341:244–250. doi: 10.1016/0014-5793(94)80465-6. [DOI] [PubMed] [Google Scholar]
- 7.Cantin R, Fortin J F, Lamontagne G, Tremblay M. The acquisition of host-derived major histocompatibility complex class II glycoproteins by human immunodeficiency virus type 1 accelerates the process of virus entry and infection in human T-lymphoid cells. Blood. 1997;90:1091–1100. [PubMed] [Google Scholar]
- 8.Cosma A, Blanc D, Braun J, Quillent C, Barassi C, Moog C, Klasen S, Spire B, Scarlatti G, Pesenti E, Siccardi A G, Beretta A. Enhanced HIV infectivity and changes in GP120 conformation associated with viral incorporation of human leucocyte antigen class I molecules. AIDS. 1999;13:2033–2042. doi: 10.1097/00002030-199910220-00005. [DOI] [PubMed] [Google Scholar]
- 9.Fenouillet E, Clerget-Raslain B, Gluckman J C, Guetard D, Montagnier L, Bahraoui E. Role of N-linked glycans in the interaction between the envelope glycoprotein of human immunodeficiency virus and its CD4 cellular receptor. Structural enzymatic analysis. J Exp Med. 1989;169:807–822. doi: 10.1084/jem.169.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer P B, Collin M, Karlsson G B, James W, Butters T D, Davis S J, Gordon S, Dwek R A, Platt F M. The alpha-glucosidase inhibitor N-butyldeoxynojirimycin inhibits human immunodeficiency virus entry at the level of post-CD4 binding. J Virol. 1995;69:5791–5797. doi: 10.1128/jvi.69.9.5791-5797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer P B, Karlsson G B, Dwek R A, Platt F M. N-Butyldeoxynojirimycin-mediated inhibition of human immunodeficiency virus entry correlates with impaired gp120 shedding and gp41 exposure. J Virol. 1996;70:7153–7160. doi: 10.1128/jvi.70.10.7153-7160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fortin J F, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geyer H, Holschbach C, Hunsmann G, Schneider J. Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J Biol Chem. 1988;263:11760–11767. [PubMed] [Google Scholar]
- 14.Gruters R A, Neefjes J J, Tersmette M, de Goede R E, Tulp A, Huisman H G, Miedema F, Ploegh H L. Interference with HIV-induced syncytium formation and viral infectivity by inhibitors of trimming glucosidase. Nature. 1987;330:74–77. doi: 10.1038/330074a0. [DOI] [PubMed] [Google Scholar]
- 15.Guo M M, Hildreth J E. HIV acquires functional adhesion receptors from host cells. AIDS Res Hum Retroviruses. 1995;11:1007–1013. doi: 10.1089/aid.1995.11.1007. [DOI] [PubMed] [Google Scholar]
- 16.Hioe C E, Bastiani L, Hildreth J E, Zolla-Pazner S. Role of cellular adhesion molecules in HIV type 1 infection and their impact on virus neutralization. AIDS Res Hum Retroviruses. 1998;14(Suppl. 3):S247–S254. [PubMed] [Google Scholar]
- 17.Holschbach C, Schneider J, Geyer H. Glycosylation of the envelope glycoprotein gp130 of simian immunodeficiency virus from sooty mangabey (Cercocebus atys) Biochem J. 1990;267:759–766. doi: 10.1042/bj2670759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu H, Shioda T, Moriya C, Xin X, Hasan M K, Miyake K, Shimada T, Nagai Y. Infectivities of human and other primate lentiviruses are activated by desialylation of the virion surface. J Virol. 1996;70:7462–7470. doi: 10.1128/jvi.70.11.7462-7470.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson R J, Hall D F, Kerr P J. Myxoma virus encodes an α2,3-sialyltransferase that enhances virulence. J Virol. 1999;73:2376–2384. doi: 10.1128/jvi.73.3.2376-2384.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlsson G B, Butters T D, Dwek R A, Platt F M. Effects of the imino sugar N-butyldeoxynojirimycin on the N-glycosylation of recombinant gp120. J Biol Chem. 1993;268:570–576. [PubMed] [Google Scholar]
- 21.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 22.Kozarsky K, Penman M, Basiripour L, Haseltine W, Sodroski J, Krieger M. Glycosylation and processing of the human immunodeficiency virus type 1 envelope protein. J Acquir Immune Defic Syndr. 1989;2:163–169. [PubMed] [Google Scholar]
- 23.Lee W R, Syu W J, Du B, Matsuda M, Tan S, Wolf A, Essex M, Lee T H. Nonrandom distribution of gp120 N-linked glycosylation sites important for infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1992;89:2213–2217. doi: 10.1073/pnas.89.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Luo L, Rasool N, Kang C Y. Glycosylation is necessary for the correct folding of human immunodeficiency virus gp120 in CD4 binding. J Virol. 1993;67:584–588. doi: 10.1128/jvi.67.1.584-588.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao Z, Roos J W, Hildreth J E. Increased infectivity of HIV type 1 particles bound to cell surface and solid-phase ICAM-1 and VCAM-1 through acquired adhesion molecules LFA-1 and VLA-4. AIDS Res Hum Retroviruses. 2000;16:355–366. doi: 10.1089/088922200309232. [DOI] [PubMed] [Google Scholar]
- 26.Liedtke S, Adamski M, Geyer R, Pfutzner A, Rubsamen-Waigmann H, Geyer H. Oligosaccharide profiles of HIV-2 external envelope glycoprotein: dependence on host cells and virus isolates. Glycobiology. 1994;4:477–484. doi: 10.1093/glycob/4.4.477. [DOI] [PubMed] [Google Scholar]
- 27.Liedtke S, Geyer R, Geyer H. Host-cell-specific glycosylation of HIV-2 envelope glycoprotein. Glycoconj J. 1997;14:785–793. doi: 10.1023/a:1018577619036. [DOI] [PubMed] [Google Scholar]
- 28.Manca F. Galactose receptors and presentation of HIV envelope glycoprotein to specific human T cells. J Immunol. 1992;148:2278–2282. [PubMed] [Google Scholar]
- 29.Means R E, Greenough T, Desrosiers R C. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Means R E, Reitter J, Desrosiers R C. N-glycosylation and the avoidance of humoral immunity. In: Girard M, Dodet B, editors. Colloque Des Cent Gardes. Vol. 11. ParisParis, France: Elsevier; 1997. pp. 137–143. [Google Scholar]
- 31.Mizuochi T, Matthews T J, Kato M, Hamako J, Titani K, Solomon J, Feizi T. Diversity of oligosaccharide structures on the envelope glycoprotein gp 120 of human immunodeficiency virus 1 from the lymphoblastoid cell line H9. Presence of complex-type oligosaccharides with bisecting N-acetylglucosamine residues. J Biol Chem. 1990;265:8519–8524. [PubMed] [Google Scholar]
- 32.Montefiori D C, Robinson W E, Jr, Mitchell W M. Antibody-independent, complement-mediated enhancement of HIV-1 infection by mannosidase I and II inhibitors. Antiviral Res. 1989;11:137–146. doi: 10.1016/0166-3542(89)90025-9. [DOI] [PubMed] [Google Scholar]
- 33.Montefiori D C, Robinson W E, Jr, Mitchell W M. Role of protein N-glycosylation in pathogenesis of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1988;85:9248–9252. doi: 10.1073/pnas.85.23.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montefiori D C, Stewart K, Ahearn J M, Zhou J. Complement-mediated binding of naturally glycosylated and glycosylation-modified human immunodeficiency virus type 1 to human CR2 (CD21) J Virol. 1993;67:2699–2706. doi: 10.1128/jvi.67.5.2699-2706.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohgimoto S, Shioda T, Mori K, Nakayama E E, Hu H, Nagai Y. Location-specific, unequal contribution of the N glycans in simian immunodeficiency virus gp120 to viral infectivity and removal of multiple glycans without disturbing infectivity. J Virol. 1998;72:8365–8370. doi: 10.1128/jvi.72.10.8365-8370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olofsson S, Eriksson S, Karlsson A, Oberg B. The HIV replication inhibitor 3′-fluoro-3′-deoxythymidine blocks sialylation of N-linked oligosaccharides. Antiviral Res. 1992;19:71–80. doi: 10.1016/0166-3542(92)90057-c. [DOI] [PubMed] [Google Scholar]
- 37.Overbaugh J, Rudensey L M. Alterations in potential sites for glycosylation predominate during evolution of the simian immunodeficiency virus envelope gene in macaques. J Virol. 1992;66:5937–5948. doi: 10.1128/jvi.66.10.5937-5948.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papandreou M J, Fenouillet E. Effect of various glycosidase treatments on the resistance of the HIV-1 envelope to degradation. FEBS Lett. 1997;406:191–195. doi: 10.1016/s0014-5793(97)00273-1. [DOI] [PubMed] [Google Scholar]
- 39.Paquette J S, Fortin J F, Blanchard L, Tremblay M J. Level of ICAM-1 surface expression on virus producer cells influences both the amount of virion-bound host ICAM-1 and human immunodeficiency virus type 1 infectivity. J Virol. 1998;72:9329–9336. doi: 10.1128/jvi.72.11.9329-9336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ratner L. Glucosidase inhibitors for treatment of HIV-1 infection. AIDS Res Hum Retroviruses. 1992;8:165–173. doi: 10.1089/aid.1992.8.165. [DOI] [PubMed] [Google Scholar]
- 41.Ratner L, vander Heyden N, Dedera D. Inhibition of HIV and SIV infectivity by blockade of alpha-glucosidase activity. Virology. 1991;181:180–192. doi: 10.1016/0042-6822(91)90483-r. [DOI] [PubMed] [Google Scholar]
- 42.Reitter J N, Desrosiers R C. Identification of replication-competent strains of simian immunodeficiency virus lacking multiple attachment sites for N-linked carbohydrates in variable regions 1 and 2 of the surface envelope protein. J Virol. 1998;72:5399–5407. doi: 10.1128/jvi.72.7.5399-5407.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reitter J N, Means R E, Desrosiers R C. A role for carbohydrates in immune evasion in AIDS. Nat Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 44.Stamatos N M, Gomatos P J, Cox J, Fowler A, Dow N, Wohlhieter J A, Cross A S. Desialylation of peripheral blood mononuclear cells promotes growth of HIV-1. Virology. 1997;228:123–131. doi: 10.1006/viro.1996.8373. [DOI] [PubMed] [Google Scholar]
- 45.Walker B D, Kowalski M, Goh W C, Kozarsky K, Krieger M, Rosen C, Rohrschneider L, Haseltine W A, Sodroski J. Inhibition of human immunodeficiency virus syncytium formation and virus replication by castanospermine. Proc Natl Acad Sci USA. 1987;84:8120–8124. doi: 10.1073/pnas.84.22.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willey R L, Shibata R, Freed E O, Cho M W, Martin M A. Differential glycosylation, virion incorporation, and sensitivity to neutralizing antibodies of human immunodeficiency virus type 1 envelope produced from infected primary T-lymphocyte and macrophage cultures. J Virol. 1996;70:6431–6436. doi: 10.1128/jvi.70.9.6431-6436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xin X, Shioda T, Fukushima M, Hu H, Oka S, Iwamoto A, Nagai Y. Facilitation of HIV-1 isolation from patients by neuraminidase. Arch Virol. 1998;143:85–95. doi: 10.1007/s007050050270. [DOI] [PubMed] [Google Scholar]