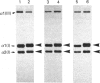
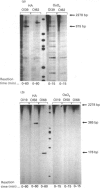
Abstract
Type I collagen alpha 1(I) glycine to serine substitutions, resulting from G-to-A mutations, were defined in three cases of osteogenesis imperfecta (OI). The Gly substitutions displayed a gradient of phenotypic severity according to the location of the mutation in the collagen triple helix. The most C-terminal of these, Gly565 to Ser, led to the lethal perinatal (type II) form of OI, whereas the more N-terminal mutations, Gly415 and Gly352 to Ser, led to severe OI (type III/IV) and moderate OI (type IVB) respectively. These data support the notion that glycine substitutions towards the C-terminus of the alpha 1(I) or alpha 2(I) chains will be more clinically severe than those towards the N-terminus. This results from the more disruptive effect of the mutations at the C-terminus on helix initiation and C- and N-terminal helix directional propagation. This generalization must be modified by considering the nature of the glycine substitution and the surrounding amino acid sequence, since the helix is composed of subdomains of differing stability which will affect the ability of helix re-nucleation and propagation.
Full text
PDF

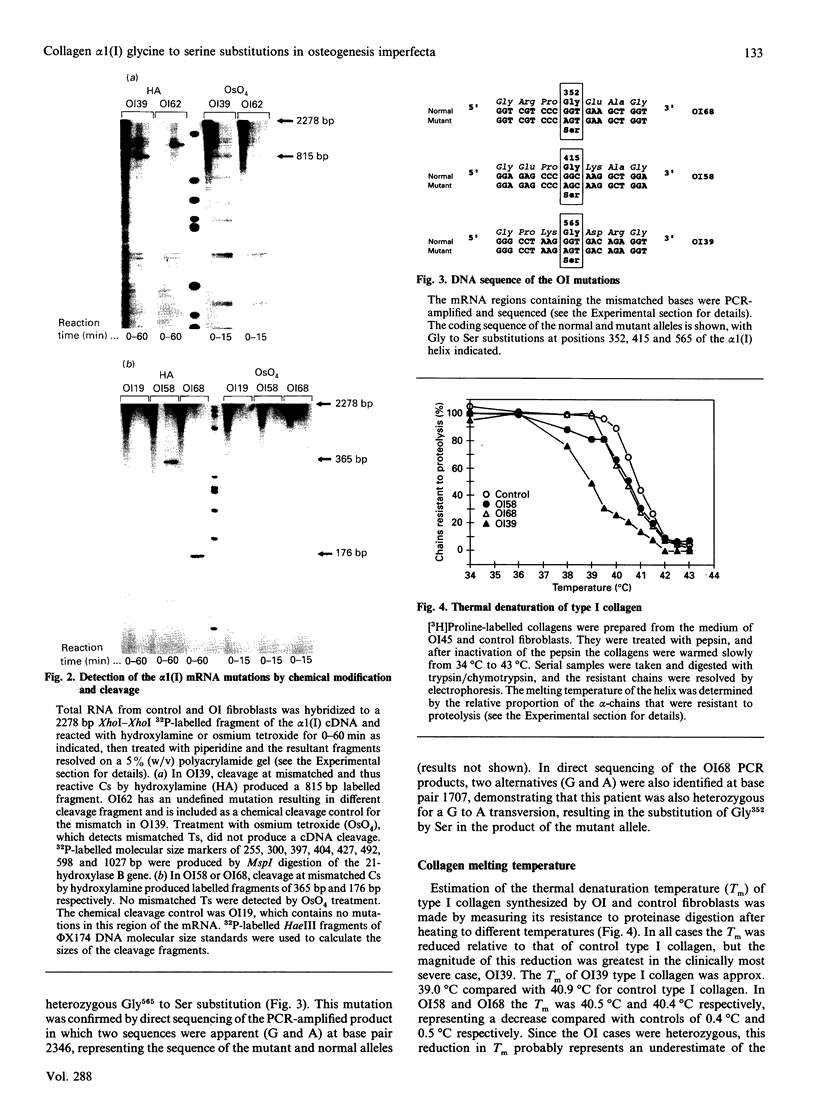


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B., Lüke W., Hunsmann G. Improvement of PCR amplified DNA sequencing with the aid of detergents. Nucleic Acids Res. 1990 Mar 11;18(5):1309–1309. doi: 10.1093/nar/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Lamande S., Mascara T., Cole W. G. Biochemical heterogeneity of type I collagen mutations in osteogenesis imperfecta. Ann N Y Acad Sci. 1988;543:95–105. doi: 10.1111/j.1749-6632.1988.tb55321.x. [DOI] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Mascara T., Rogers J. G., Cole W. G. Collagen defects in lethal perinatal osteogenesis imperfecta. Biochem J. 1986 Dec 15;240(3):699–708. doi: 10.1042/bj2400699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Harley V., Chan D., Cole W. G. Comprehensive analysis of collagen metabolism in vitro using [4(3H)]/[14C]proline dual-labeling and polyacrylamide gel electrophoresis. Anal Biochem. 1988 Jan;168(1):171–175. doi: 10.1016/0003-2697(88)90025-5. [DOI] [PubMed] [Google Scholar]
- Bateman J. F., Lamande S. R., Dahl H. H., Chan D., Mascara T., Cole W. G. A frameshift mutation results in a truncated nonfunctional carboxyl-terminal pro alpha 1(I) propeptide of type I collagen in osteogenesis imperfecta. J Biol Chem. 1989 Jul 5;264(19):10960–10964. [PubMed] [Google Scholar]
- Bateman J. F., Mascara T., Chan D., Cole W. G. Abnormal type I collagen metabolism by cultured fibroblasts in lethal perinatal osteogenesis imperfecta. Biochem J. 1984 Jan 1;217(1):103–115. doi: 10.1042/bj2170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadio J., Byers P. H. Subtle structural alterations in the chains of type I procollagen produce osteogenesis imperfecta type II. Nature. 1985 Jul 25;316(6026):363–366. doi: 10.1038/316363a0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Prockop D. J. Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal Biochem. 1981 Jan 15;110(2):360–368. doi: 10.1016/0003-2697(81)90204-9. [DOI] [PubMed] [Google Scholar]
- Byers P. H. Brittle bones--fragile molecules: disorders of collagen gene structure and expression. Trends Genet. 1990 Sep;6(9):293–300. doi: 10.1016/0168-9525(90)90235-x. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Wallis G. A., Willing M. C. Osteogenesis imperfecta: translation of mutation to phenotype. J Med Genet. 1991 Jul;28(7):433–442. doi: 10.1136/jmg.28.7.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächinger H. P., Davis J. M. Sequence specific thermal stability of the collagen triple helix. Int J Biol Macromol. 1991 Jun;13(3):152–156. doi: 10.1016/0141-8130(91)90040-2. [DOI] [PubMed] [Google Scholar]
- Cotton R. G., Rodrigues N. R., Campbell R. D. Reactivity of cytosine and thymine in single-base-pair mismatches with hydroxylamine and osmium tetroxide and its application to the study of mutations. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4397–4401. doi: 10.1073/pnas.85.12.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl H. H., Lamande S. R., Cotton R. G., Bateman J. F. Detection and localization of base changes in RNA using a chemical cleavage method. Anal Biochem. 1989 Dec;183(2):263–268. doi: 10.1016/0003-2697(89)90477-6. [DOI] [PubMed] [Google Scholar]
- Kadler K. E., Torre-Blanco A., Adachi E., Vogel B. E., Hojima Y., Prockop D. J. A type I collagen with substitution of a cysteine for glycine-748 in the alpha 1(I) chain copolymerizes with normal type I collagen and can generate fractallike structures. Biochemistry. 1991 May 21;30(20):5081–5088. doi: 10.1021/bi00234a035. [DOI] [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in collagen genes: causes of rare and some common diseases in humans. FASEB J. 1991 Apr;5(7):2052–2060. doi: 10.1096/fasebj.5.7.2010058. [DOI] [PubMed] [Google Scholar]
- Lamande S. R., Dahl H. H., Cole W. G., Bateman J. F. Characterization of point mutations in the collagen COL1A1 and COL1A2 genes causing lethal perinatal osteogenesis imperfecta. J Biol Chem. 1989 Sep 25;264(27):15809–15812. [PubMed] [Google Scholar]
- Liu C. P., Slate D. L., Gravel R., Ruddle F. H. Biological detection of specific mRNA molecules by microinjection. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4503–4506. doi: 10.1073/pnas.76.9.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini J. C., Grange D. K., Gottesman G. S., Lewis M. B., Koeplin D. A. Osteogenesis imperfecta type IV. Detection of a point mutation in one alpha 1(I) collagen allele (COL1A1) by RNA/RNA hybrid analysis. J Biol Chem. 1989 Jul 15;264(20):11893–11900. [PubMed] [Google Scholar]
- Pack M., Constantinou C. D., Kalia K., Nielsen K. B., Prockop D. J. Substitution of serine for alpha 1(I)-glycine 844 in a severe variant of osteogenesis imperfecta minimally destabilizes the triple helix of type I procollagen. The effects of glycine substitutions on thermal stability are either position of amino acid specific. J Biol Chem. 1989 Nov 25;264(33):19694–19699. [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins. Proteins which do not present a single cooperative system. Adv Protein Chem. 1982;35:1–104. [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey A., Bateman J., Choi T., Mascara T., Cole W., Jaenisch R. Perinatal lethal osteogenesis imperfecta in transgenic mice bearing an engineered mutant pro-alpha 1(I) collagen gene. Nature. 1988 Mar 10;332(6160):131–136. doi: 10.1038/332131a0. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Avigad G., Brodsky B., Eikenberry E. F. Glycation induces expansion of the molecular packing of collagen. J Mol Biol. 1988 Sep 20;203(2):495–505. doi: 10.1016/0022-2836(88)90015-0. [DOI] [PubMed] [Google Scholar]
- Tromp G., Kuivaniemi H., Stacey A., Shikata H., Baldwin C. T., Jaenisch R., Prockop D. J. Structure of a full-length cDNA clone for the prepro alpha 1(I) chain of human type I procollagen. Biochem J. 1988 Aug 1;253(3):919–922. doi: 10.1042/bj2530919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel B. E., Doelz R., Kadler K. E., Hojima Y., Engel J., Prockop D. J. A substitution of cysteine for glycine 748 of the alpha 1 chain produces a kink at this site in the procollagen I molecule and an altered N-proteinase cleavage site over 225 nm away. J Biol Chem. 1988 Dec 15;263(35):19249–19255. [PubMed] [Google Scholar]
- Wake S. A., Mercer J. F. Induction of metallothionein mRNA in rat liver and kidney after copper chloride injection. Biochem J. 1985 Jun 1;228(2):425–432. doi: 10.1042/bj2280425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis G. A., Starman B. J., Zinn A. B., Byers P. H. Variable expression of osteogenesis imperfecta in a nuclear family is explained by somatic mosaicism for a lethal point mutation in the alpha 1(I) gene (COL1A1) of type I collagen in a parent. Am J Hum Genet. 1990 Jun;46(6):1034–1040. [PMC free article] [PubMed] [Google Scholar]
- Wallis G. A., Starman B. J., Zinn A. B., Byers P. H. Variable expression of osteogenesis imperfecta in a nuclear family is explained by somatic mosaicism for a lethal point mutation in the alpha 1(I) gene (COL1A1) of type I collagen in a parent. Am J Hum Genet. 1990 Jun;46(6):1034–1040. [PMC free article] [PubMed] [Google Scholar]
- Westerhausen A., Kishi J., Prockop D. J. Mutations that substitute serine for glycine alpha 1-598 and glycine alpha 1-631 in type I procollagen. The effects on thermal unfolding of the triple helix are position-specific and demonstrate that the protein unfolds through a series of cooperative blocks. J Biol Chem. 1990 Aug 15;265(23):13995–14000. [PubMed] [Google Scholar]