Abstract
Engineering cells for adoptive therapy requires overcoming limitations in cell viability and, in the efficiency of transgene delivery, the duration of transgene expression and the stability of genomic integration. Here we report a gene-delivery system consisting of a Sleeping Beauty (SB) transposase encoded into a messenger RNA delivered by an adeno-associated virus (AAV) encoding an SB transposon that includes the desired transgene, for mediating the permanent integration of the transgene. Compared with lentiviral vectors and with the electroporation of plasmids of transposon DNA or minicircle DNA, the gene-delivery system, which we named MAJESTIC (for ‘mRNA AAV–SB joint engineering of stable therapeutic immune cells’), offers prolonged transgene expression, as well as higher transgene expression, therapeutic-cell yield and cell viability. MAJESTIC can deliver chimeric antigen receptors (CARs) into T cells (which we show lead to strong anti-tumour activity in vivo) and also transduce natural killer cells, myeloid cells and induced pluripotent stem cells with bi-specific CARs, kill-switch CARs and synthetic T-cell receptors.
Cellular immunotherapy involves the administration of ‘living drugs’: genetically modified immune cells that can proliferate, adapt to their environment, engage surrounding cells and elicit dynamic responses that directly or indirectly target tumour cells for destruction1. Adoptive cell transfer is one type of cellular immunotherapy, and it involves the transfer of cells that directly target tumour cells in the patient2. A notable adoptive cell transfer approach is chimeric antigen receptor (CAR) T-cell therapy, in which T cells are engineered to express a synthetic membrane receptor specific for a tumour antigen. CAR T-cell therapy has had a remarkable effect in patients with certain haematological malignancies3,4, with five CAR T-cell products currently approved by the US Food and Drug Administration for the treatment of multiple myeloma and B-cell malignancies5. To tackle the myriad challenges that different tumours and tumour environments present, multiple cell-based therapies besides CAR T cells have been generated, such as tumour infiltrating lymphocytes6, T-cell-receptor engineered T cells (TCR-T cells)7,8, CAR natural killer (CAR-NK) cells9–15, CAR macrophages (CAR-MAs)16,17 and human induced pluripotent stem cell (iPSC)-derived therapeutic immune cells15,17,18. Additional components have been added to CAR constructs to enhance therapeutic efficacy or safety, such as kill-switch CARs that can be depleted upon administration of a drug in the case of deleterious CAR toxicity19, or tandem CARs that can target two different antigens20. A vital part of the implementation of such cellular immunotherapies is the therapeutic cell generation process: whether and how stably engineered therapeutic immune cells can be efficiently generated has a critical impact on cell therapy21.
The majority of current CAR T cells used in clinical trials were generated using γ-retroviruses or lentiviruses for gene transfer. However, several limitations of this family of viral vectors exist. For example, transgene expression may be silenced or dampened22. A key limitation of γ-retroviruses specifically is their preference of integration into promoters of active genes23, including proto-oncogenes, as observed in a clinical trial24–26. Genomic studies indeed have found that the insertion profile of γ-retroviruses is associated with a higher likelihood of oncogenic transformation21. Human immunodeficiency virus-derived lentiviral vectors are considered safer than γ-retroviral vectors, on the basis of current studies27, but their pathogenic origin and biosafety level (BSL) 2 or 2+ classification still warrant caution.
An adeno-associated virus (AAV) is a single-stranded DNA virus. An AAV is commonly used in gene therapy and is considered a safer vector28 (consistent with its BSL-1 classification). However, an AAV alone is not an ideal CAR carrier, as it is non-integrating and CAR expression is thus rapidly diluted during T-cell expansion. Although an AAV can serve as a template for the efficient generation of stably integrated CAR T cells29,30, such strategies require genome editing via tools such as clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated proteins (Cas). Recent studies31–33 have shown chromosomal rearrangement or loss following CRISPR/Cas9-mediated genome editing.
All current non-viral cell therapy approaches also have their limitations. Messenger RNA (mRNA) electroporation is another CAR generation strategy, but the duration of CAR expression is extremely short, owing to the instability of mRNA21. Transposon systems such as PiggyBac34,35 and Sleeping Beauty (SB)36 can be used to directly integrate DNA sequences into the genome; this is an advantage over lentiviruses and retroviruses, which rely on the relatively error-prone reverse transcriptase enzyme to convert RNA into DNA37. Transposons are also attractive for their non-pathogenic origin21. However, current transposon/transposase approaches for therapeutic cell generation rely predominantly on DNA electroporation or transfection of plasmids or minicircles (MCs), for which efficiency of delivery varies between different studies, and cellular toxicity is usually high36,38–41. In addition, repeated transposon mobilization by transposase may lead to continuous risks of insertional mutagenesis or local chromosomal rearrangements42.
As it stands, all current existing cell therapy engineering approaches, both viral and non-viral, have important limitations. Our goal is to develop a non-CRISPR gene-delivery system that is capable of generating stably integrated therapeutic immune cells and that (1) achieves efficient genomic integration without reliance on retroviruses or lentiviruses, (2) theoretically limits the extent of transposon mobilization, (3) achieves higher viability compared with current methods and (4) is independent of gene editing. Here we developed a prototype of such a system, MAJESTIC (for ‘mRNA AAV–SB joint engineering of stable therapeutic immune cells’), which combines the merits of mRNA, AAV vector and transposon into one composite system. In MAJESTIC, the mRNA component encodes a transposase that mediates a pulse of genomic integration of the SB transposon, which carries genes of interest and is embedded inside the AAV vector. Plasmid DNA is more stable than mRNA, and each copy may produce multiple copies of mRNA43; thus, supplying the transposase as mRNA may limit remobilization and subsequent chromosomal rearrangement in principle.
We tested this system in standard laboratory settings, alone and side by side with conventional gene-delivery systems, such as lentiviral vector or DNA transposons including plasmid DNA and MC DNA electroporation, measuring transduction efficiency, cell viability, therapeutic cell yield and the stability of transgene expression. We also demonstrated the system’s versatility for engineering different cell-therapy constructs, such as canonical CAR, bi-specific CAR, kill-switch CAR and synthetic TCR, and for therapeutic cell engineering of various immune cell types, including T cells, NK cells, iPSCs and cells in the myeloid lineage.
Results
Establishment of the MAJESTIC system and CAR T-cell generation
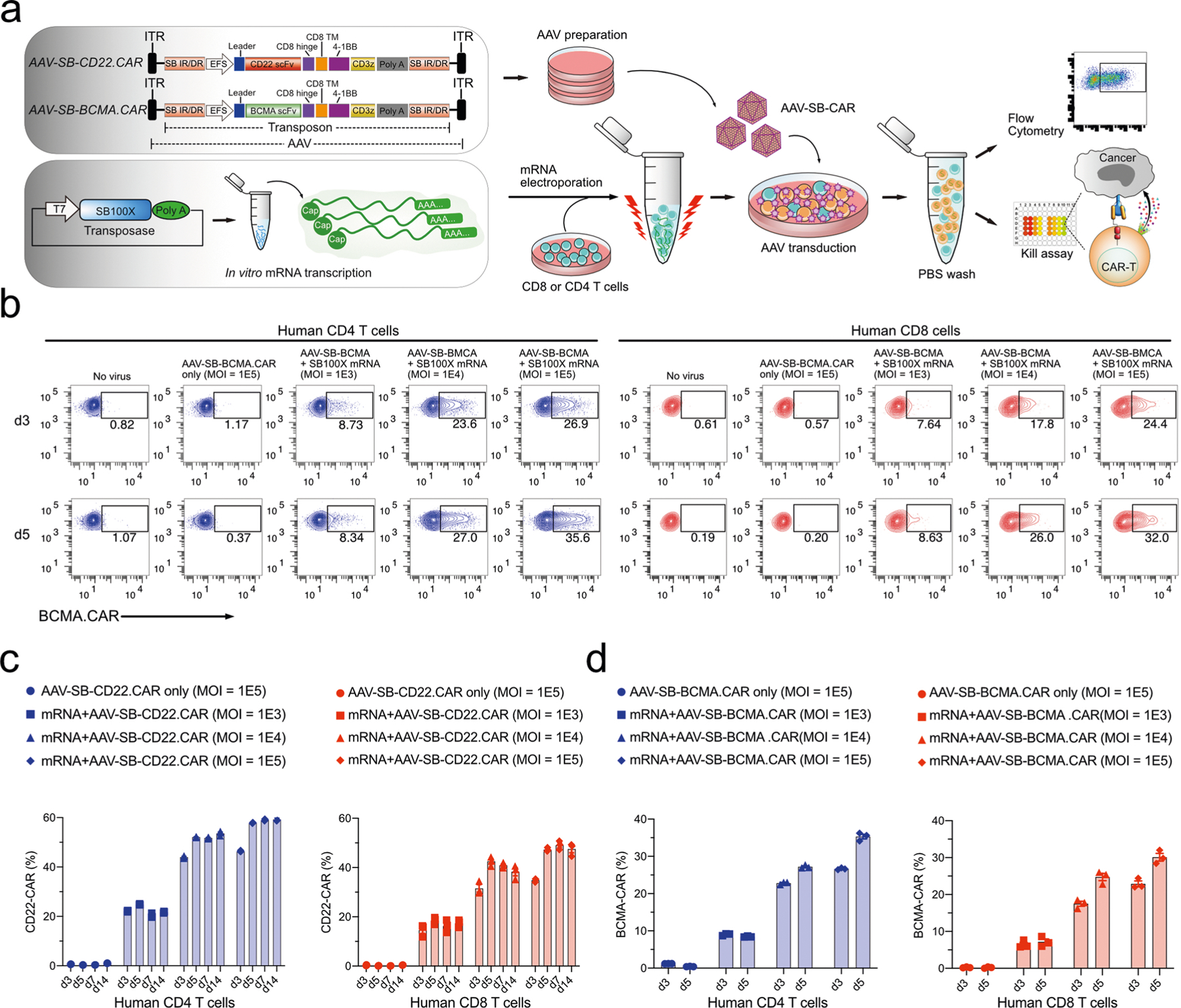
The MAJESTIC system has two core components: (1) the AAV-SB vector carrying desired cell therapy transgenes (AAV-SB-CTx) and (2) mRNA encoding the SB100X transposase (mRNA-transposase) (Fig. 1a). To generate the AAV-SB-CTx plasmid, we first established the AAV-SB plasmid by cloning the SB transposon, which is flanked by inverted repeats and direct repeats (IR/DR), in between the inverted terminal repeats (ITRs) of the AAV plasmid backbone44. Into this chimeric AAV-SB backbone, single single-chain fragment variable (scFv) CAR, tandem scFv CAR, TCR and suicide-gene CAR (CAR.iCasp9) constructs were cloned (Fig. 1a and Extended Data Fig. 1a). The hyperactive SB transposase SB100X45 was delivered to cells via mRNA electroporation to facilitate genomic integration of the SB transposon construct, which was delivered via AAV transduction (Fig. 1a). Importantly, this two-component design achieves integration of the SB transposon while limiting remobilization compared with that for plasmid transfection because the mRNA encoding the transposase is in principle less stable than plasmid DNA transposase.
Fig. 1 |. Development of MAJESTIC, a composite mRNA:AAV-SB system for highly efficient generation of therapeutic immune cells.
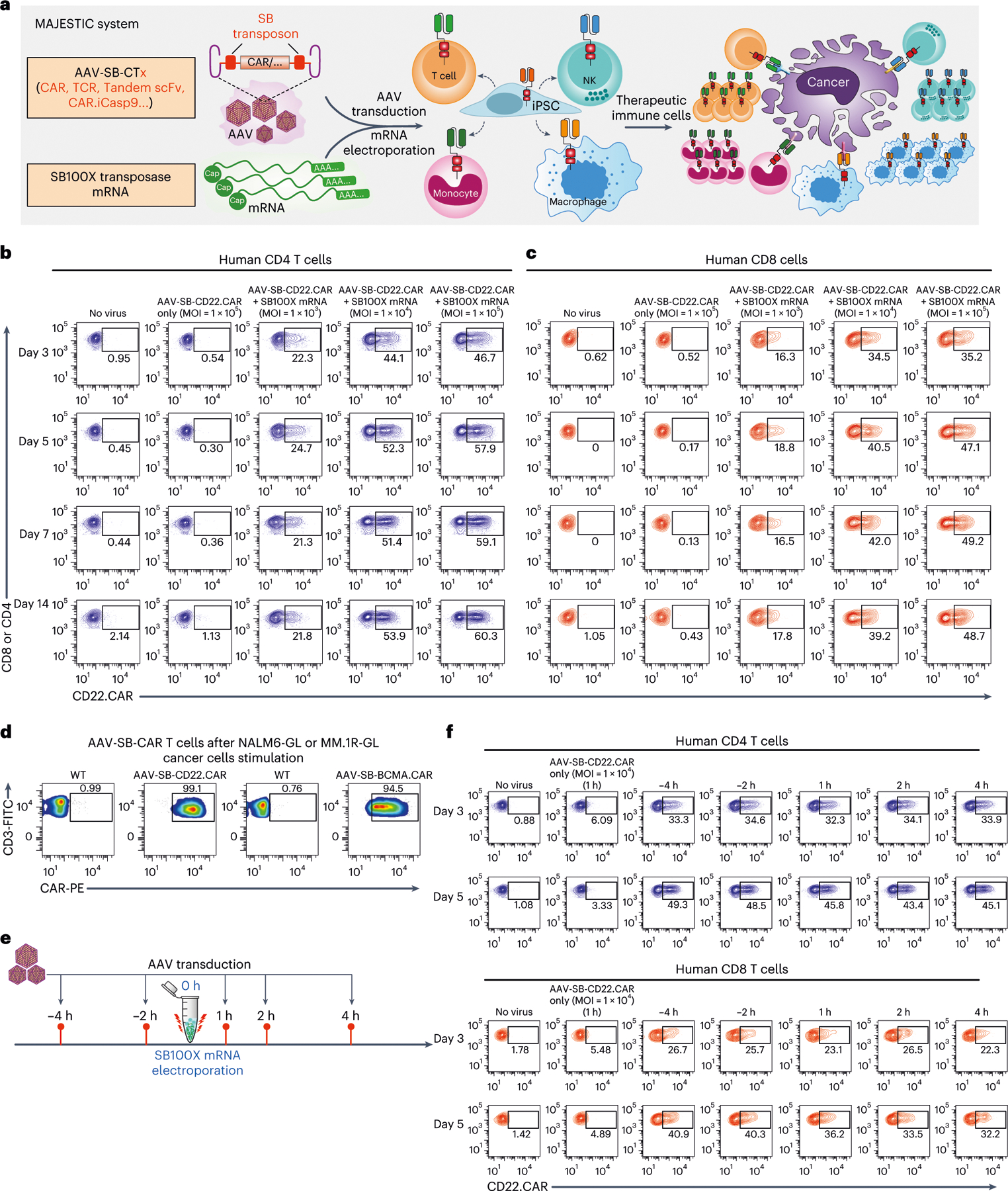
a, Schematics of the hybrid AAV-SB construct, SB100X mRNA electroporation and CAR T/NK/macrophage/iPSC generation. b, Representative flow cytometry plots of human CD4 (gated for CD3+CD8−cells) AAV-SB-CD22.CAR T cells. Human CD3 T cells were first electroporated with SB100X mRNA, then transduced with a titration series of AAV-SB-CD22.CAR virus. CAR expression levels were evaluated at various time points from day 3 to day 14. c, Representative flow cytometry plots of human CD8 (gated for CD3+CD8+ cells) AAV-SB-CD22.CAR T cells. Human CD3 T cells were first electroporated with SB100X mRNA, then transduced with a titration series of AAV-SB-CD22.CAR virus. Data were collected at several time points from day 3 to day 14. d, Representative flow cytometry plots of AAV-SB-CD22.CAR and AAV-SB-BCMA.CAR T cells after cancer stimulation. e, Schematic representation of various AAV-SB-CD22.CAR transduction time points relative to SB100X mRNA electroporation. f, Representative flow cytometry plots of CD22.CAR T cells quantifying CAR-percentages of CD4 (top) and CD8 (bottom) T cells which were transduced with AAV-SB-CD22.CAR virus at various time points relative to when SB100X mRNA electroporation occurred (at 0 h). In this figure, for optimization of conditions, each assay was done with one donor with three technical replicates. Donor 2 T cells were used in this figure. Cells were not purified for CD8/CD4 populations before electroporation.
We first examined the feasibility of this system for CAR T-cell generation. We electroporated human CD4 and CD8 T cells with SB100X mRNA, then transduced them with a titration series of AAV-SB-CD22.CAR virus (Extended Data Fig. 1a), using multiplicities of infection (MOIs) of 1 × 103, 1 × 104 and 1 × 105 (Fig. 1b,c). We then monitored CD22.CAR expression via flow cytometry from day 3 to day 14. CD22.CAR-positive ratio correlated positively with virus titre, and CAR constructs were stably expressed in all time points tested. On day 14, for MOIs of 1 × 103, 1 × 104 and 1 × 105, respectively, 21.8%, 53.9% and 60.3% of the total CD4 T-cell population were CAR positive (Fig. 1b and Extended Data Fig. 1c), and 17.8%, 39.2% and 48.7% of CD8 T cells were CAR positive (Fig. 1c and Extended Data Fig. 1c). We next tested a different CAR transgene, a B-cell maturation antigen (BCMA)-targeting CAR on day 5, and at an MOI of 1 × 105, 35.6% and 32% of CD4 and CD8 T cells were BCMA.CAR positive, respectively (Extended Data Fig. 1b,d). Furthermore, T cells in the AAV-SB-CAR condition can be quickly enriched for CAR-positive cells to nearly a pure CAR population following one-time antigen-specific cancer cell stimulation (day 12 post-stimulation), where CD22.CAR+ and BCMA. CAR+ T cells were 99.1% and 94.5% of cell populations, respectively (Fig. 1d). These titration and condition-optimization experiments suggested that the MAJESTIC system can efficiently generate stable CAR T cells from a single human donor.
Next, we surveyed the optimal time point(s) for AAV-SB-CTx viral transduction relative to SB100X mRNA electroporation. Setting SB100X mRNA electroporation as the 0 h time point, we transduced cells with AAV-SB-CTx along a series of time points between −4h and 4 h (Fig. 1e). We observed that the AAV-only group showed low CAR expression, although notable over the no-virus control background, potentially because of the transient expression of AAV (Fig. 1f and Extended Data Fig. 2a). In contrast, the MAJESTIC (AAV-SB-CTx) group has substantially higher CAR expression (Fig. 1f and Extended Data Fig. 2a). While the time points have similarly high CAR+ rates, the −4 h time point (that is, AAV-SB-CTx transduction 4 h before mRNA-SB100X electroporation) appeared to be the most efficient numerically, which was true for both CD4 and CD8 T cells (Fig. 1f and Extended Data Fig. 2a,b). For practical convenience, we used a time point of 0–1 h for all experiments moving forward, because the efficiency differences between AAV transduction time points are moderate.
We then examined the optimal concentration of SB100X transposase. We titrated SB100X mRNA concentration by using a fixed amount of virus (MOI = 1 × 105) and varying amounts of mRNA (Fig. 2a and Extended Data Fig. 2c,d). Both CD4 and CD8 T cells electroporated with 1 μg mRNA per 2 × 106 T cells yielded around 40% CAR-positive T cells. The CAR ratio is higher with 2 μg compared with 1 μg mRNA in both CD4 and CD8 T cells; beyond 2 μg of mRNA the CAR ratio appeared to be saturated (Fig. 2a and Extended Data Fig. 2c,d). We used a ratio of 2 μg of mRNA per 2 × 106 cells hereafter.
Fig. 2 |. Comparison between MAJESTIC and conventional CAR T-cell generation approaches.
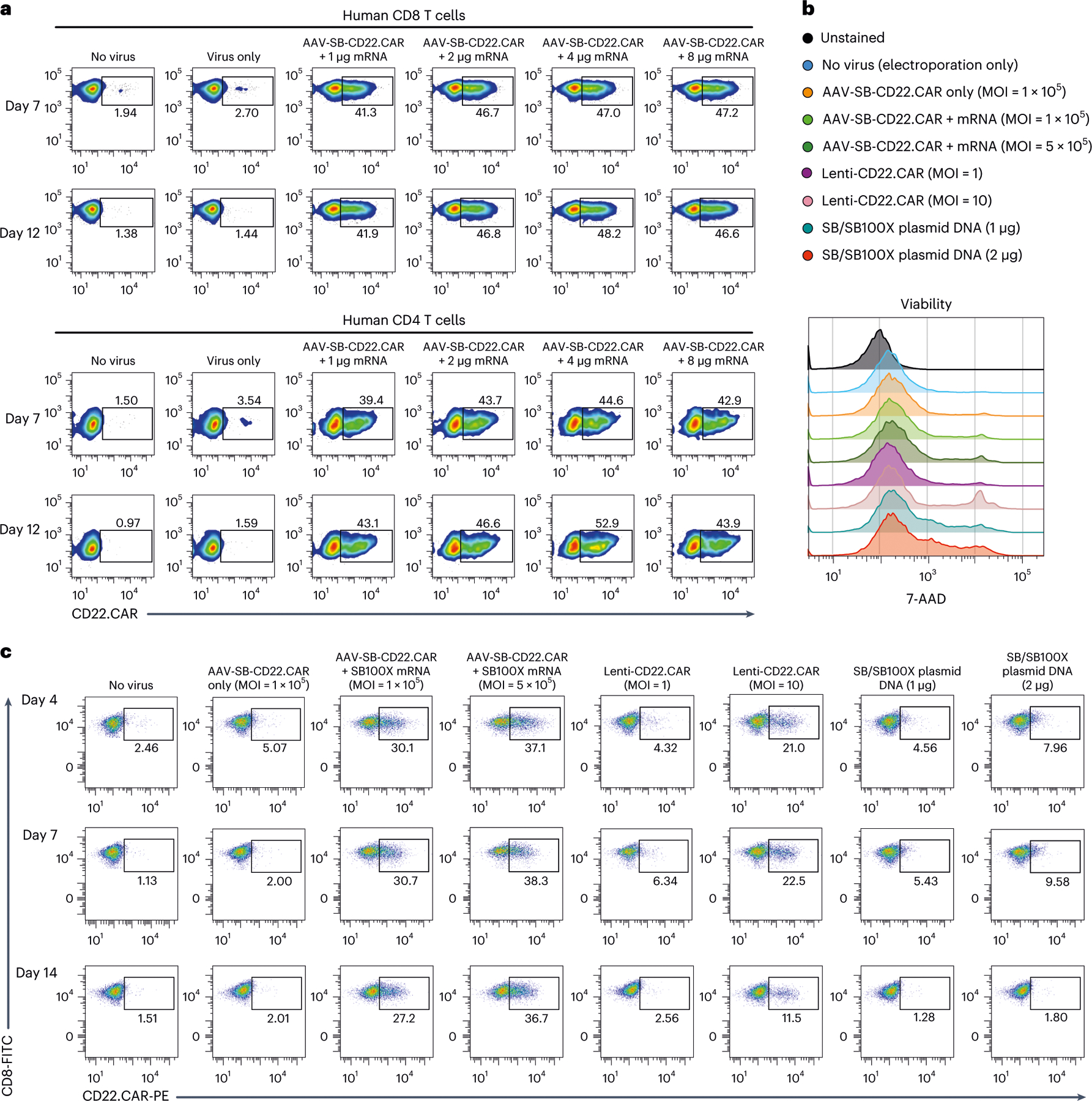
a, Representative flow cytometry plots of CD8 and CD4 CD22.CAR T cells were produced via AAV-SB and a titrated series of SB100X mRNA. b, Representative flow cytometry plots measuring the viability of CD8 T cells. 7-AAD staining was performed to measure cell viability after mRNA electroporation, plasmid DNA electroporation and lentivirus transduction. Media were changed 1 day after electroporation to remove the virus. c, Representative flow cytometry plots of human CD8 T cells transduced with AAV-SB-CD22.CAR virus (MOI = 1 × 105 and 5 × 105), transduced with CD22.CAR lentivirus (MOI = 1 and 10) or electroporated with plasmid DNA (1 μg = 0.5 μg transposon plasmid + 0.5 μg transposase plasmid) at three time points. In this figure, each assay was done with one donor with at least three technical replicates, donor 0286 T cells were used in this figure.
MAJESTIC system produces stable and functional CAR T cells
We then compared CAR T-cell generation with the MAJESTIC system to lentiviral vector and SB transposon and transposase plasmid DNA electroporation approaches. It is difficult to uniformly compare these methods, as the underlying principles for each method differ. However, we still sought to systematically evaluate the performance of each method in parallel under standard laboratory settings (Methods). It is important to note that for AAV, the functional MOI (in transduction units) is usually 3–4 orders of magnitude lower than that of genomic MOI (in genome copies)46; therefore, in our experiments we used AAV genomic MOIs between 1 × 104 and 5 × 105. We used lentiviral MOIs between 1 and 10, according to current standards for lentiviral CAR T cells in the literature47. For DNA-plasmid electroporation, we used 1–4 μg total DNA per 1 × 106 cells, which is similar to the range of concentrations tested previously38,48.
After CAR T-cell generation with all three systems (MAJESTIC, lentivirus and SB transposon plasmid electroporation), we first determined cell viability via 7-Aminoactinomycin D (7-AAD) staining 48 h after electroporation. Flow cytometry results showed that the MAJESTIC (AAV-SB-CD22.CAR + SB100X-mRNA) groups had viability above 85%. Lenti-CD22.CAR cells were over 90% viable at an MOI of 1 but only about 75% viable at a higher MOI of 10 (Fig. 2b and Extended Data Fig. 2e). Cells electroporated with plasmid DNA showed lower viability: 75% and 70% for 1 μg and 2 μg of plasmid DNA, respectively (Fig. 2b and Extended Data Fig. 2e). Under these conditions, flow cytometry on day 4 revealed that Lenti-CD22.CAR at a high MOI (MOI = 10) yielded 21% CD22.CAR-positive CD8 T cells and only 4.32% at a low MOI (MOI = 1) (Fig. 2c). Even lower CAR-positive population percentages were observed for the plasmid DNA electroporation groups (4.56% at 1 μg and 7.96% at 2 μg) compared with lentivirus (Fig. 2c). Substantially higher CAR expression was observed in the MAJESTIC groups, with 30.1% (MOI = 1 × 105) and 37.1% (MOI = 5 × 105) CD22.CAR-positive T cells (Fig. 2c). We proceeded to monitor the stability of CAR-positive T cell populations by examining CD22.CAR T populations on day 7 and day 14 by flow cytometry. CAR-positive populations were largely stable in the MAJESTIC groups but substantially declined in the lentivirus and plasmid groups (Fig. 2c and Extended Data Fig. 2f).
We performed another experiment to specifically analyse the phenomenon that Lenti-CAR% declined across time in culture. After viral transduction and/or electroporation, we sorted Lenti-CAR and MAJESTIC-CAR T cells on day 2. Both normal Lenti-CD22.CAR and spin-infected Lenti-CD22.CAR groups showed reduction of CAR+ percentages by day 5 (68.3% and 61.3%), falling even further by day 13 (52% and 43%). MAJESTIC-CAR T cells maintained a stable CAR+ ratio of around 85% (89.3% right after sorting) (Extended Data Fig. 2g). Although it is not entirely certain how lentiviral CAR ratios decline, it has been known that lentivirus transgenes can often get silenced22,49, potentially leading to reduced CAR percentages. CAR T-cell yield is an important joint outcome of viability, efficiency and proliferation (yield = CAR positive percentage × total live cell count), all of which can be affected by the cell states post CAR transgene delivery. We estimated the yield on days 5, 9 and 14. As a result, starting from approximately equal quantities of human CD8 T cells, the yields of CAR+ T cells from the MAJESTIC groups were much higher than those of lentivirus and of plasmid electroporation (that is, 4.5 times and 73.7 times higher, comparing high dose conditions, respectively) (Extended Data Fig. 2h). These data suggested that, in the laboratory settings specified (Methods), the MAJESTIC system is much more efficient in generating viable and stable CAR T cells than lentiviral or DNA transposon electroporation approaches. Moreover, we also tested MAJESTIC using the Maxcyte electroporation approach: the procedure of MAJESTIC using both Neon and Maxcyte electroporators for introduction of the SB transposase (SB100X) mRNA component into cells yielded CAR percentages of over 36% (Extended Data Fig. 2i), suggesting that MAJESTIC is not limited to one electroporation approach.
Characterization of CAR T cells generated by MAJESTIC
We then sought to estimate the vector copy number (VCN) of CAR transgenes per cell in CAR T cells generated by MAJESTIC, choosing to use a later time point (day 21) with the aim to measure stable transgenes. Using a standard approach similar to others in the field50 (Methods), we estimated the VCN of MAJESTIC- and MC-SB + SB100X mRNA-generated CD22 CAR T cells in four different human donors. The data showed that, under this experimental condition, the AAV-only group shows an average VCN of approximately 1. This detected background level could be caused by prolonged episomal persistence of the AAV vector and/or random genomic integration of the AAV vector DNA. The MC-SB + SB100X mRNA group had a VCN of approximately 1–9 copies per cell. The MAJESTIC group was observed with VCNs of approximately 1–4 copies per cell (Extended Data Fig. 3a–c). VCN measurement using both left arm and right arm probes showed consistent results (Extended Data Fig. 3a–c). We also quantified excision circles as a proxy for the excision efficiency of the SB100X transposase. AAV-SB-CAR constructs were substantially processed after 1 day of SB100X mRNA electroporation. We used quantitative PCR (qPCR) primers amplifying the junction between the AAV arms and SB arms, for both the left and right sides separately to verify the assay. In amplifying the left arm, excision efficiency was around 55% on day 2 and 36% on day 3 (Extended Data Fig. 3d). With the right arm, excision efficiency was consistent, at around 53% on day 2 and 34% on day 3 (Extended Data Fig. 3d). It is noteworthy that the excision efficiency was slightly higher on day 2 compared with day 3, which may be because of the degradation of SB100X mRNA. This aligns the goal of using mRNA in MAJESTIC system to minimize unnecessary transposon excision after transgene delivery.
To address whether the CAR T cells generated by MAJESTIC were functional, we first performed cancer-killing assays by co-culturing cancer cells and CAR T cells, generated via either MAJESTIC or lentivirus. We excluded the DNA plasmid electroporation group, as plasmid-electroporated cells did not expand well and the yields were too low to be practically useful. Two forms of CAR were evaluated in co-culture: CD22.CAR versus NALM6-GL cancer cells and BCMA.CAR versus MM.1R-GL cancer cells. In counting T cells for kill assays, we normalized the number of T cells applied by CAR T ratio to ensure each group received the same number of CAR T cells. While both MAJESTIC-generated CAR T cells (AAV-SB-CAR) and lentiviral-transduced CAR T cells (Lenti-CAR) showed tumour cell killing across the board, both AAV-SB-CD22.CAR and AAV-SB-BCMA. CAR T cells manifested stronger killing over their lentivirus-mediated counterparts, for matched effector:target (E:T) ratios (Extended Data Fig. 3e,f). These data show that the CAR T cells generated by MAJESTIC were indeed functional, with a potential advantage over the lentiviral CAR system in the settings tested.
We then further characterized the surface markers of MAJESTIC-generated CD8 CAR T cells and explored whether virus and mRNA introduction would affect the immune phenotypes of T cells. We evaluated T-cell exhaustion and memory markers before and after electroporation and viral transduction by staining for CD22.CAR and HER2.CAR T cells. The flow cytometry data showed that PD-1 was slightly decreased post MAJESTIC, whereas CTLA-4, TIM-3 and LAG-3 were increased; nevertheless, PD-1, CLTA-4 and TIM-3 all remained at baseline level as measured by mean fluorescence intensity (Extended Data Fig. 3g). For the memory markers, only CCR7 showed consistent increase in both HER2.CAR and CD22.CAR T cells. IL-7Ra remained at baseline for CD22.CAR and decreased for HER2.CAR (Extended Data Fig. 3g). CXCR3 showed differing expression patterns, potentially because of differences in the CAR constructs (for example, regarding co-stimulatory domains, CD22.CAR has 4–1BB, whereas HER2.CAR has 4–1BB and CD28). To further assess whether the CAR T cells generated by MAJESTIC were effective against cancer, we performed a CAR T-cell efficacy testing experiment in vivo using an animal model of B-cell leukaemia with adoptive cell transfer treatment. It is important to note that this in vivo experiment was intended only to validate that MAJESTIC-generated CAR T cells were indeed functional—not to compare with cells generated by other systems. The results showed strong anti-tumour efficacy, where MAJESTIC produced CAR T cells but not unmodified CD8 T cells, substantially suppressed cancer progression as measured by in vivo imaging system (IVIS)-bioluminescence imaging (P < 0.0001) (Extended Data Fig. 3h,i) and notably extended the overall survival of treated mice (P = 0.0002) (Extended Data Fig. 3j). The animals in this cohort were solely used for efficacy study (IVIS imaging and survival) and thus were not euthanized concurrently. In the future, it will be feasible and informative to use MAJESTIC to generate CAR T cells and evaluate their phenotypes such as persistence potential in vivo. Together, these data showed that the MAJESTIC system can efficiently produce stable and functional CAR T cells with high viability and yield.
MAJESTIC system applications in human T cells
We next applied this technology for engineering other types of therapeutic transgene in human primary T cells, such as solid tumour CAR T cells, tandem scFv (bi-specific) CAR T cells, TCR T cells and suicide-gene CAR T cells (Fig. 3a). For solid-tumour-specific CARs, we generated HER2-specific CAR T cells, again comparing MAJESTIC, lentivirus and plasmid DNA electroporation gene-transfer methods (Fig. 3a,b and Extended Data Fig. 4a). We observed that MAJESTIC produced the highest percentage of HER2.CAR-positive cells (28.1%) (Fig. 3c and Extended Data Fig. 4b). The Lenti-HER2.CAR and plasmid DNA groups were less efficient, at 8.99% and 13.7%, respectively (Fig. 3c and Extended Data Fig. 4b). We also generated EGFRvIII-specific CAR T cells in a similar manner. Although efficiency was generally lower, MAJESTIC’s CAR T-cell production efficiency was again significantly higher than those of lentivirus (Extended Data Fig. 4c,d).
Fig. 3 |. Application of MAJESTIC for delivery of different therapeutic transgenes into T human cells.
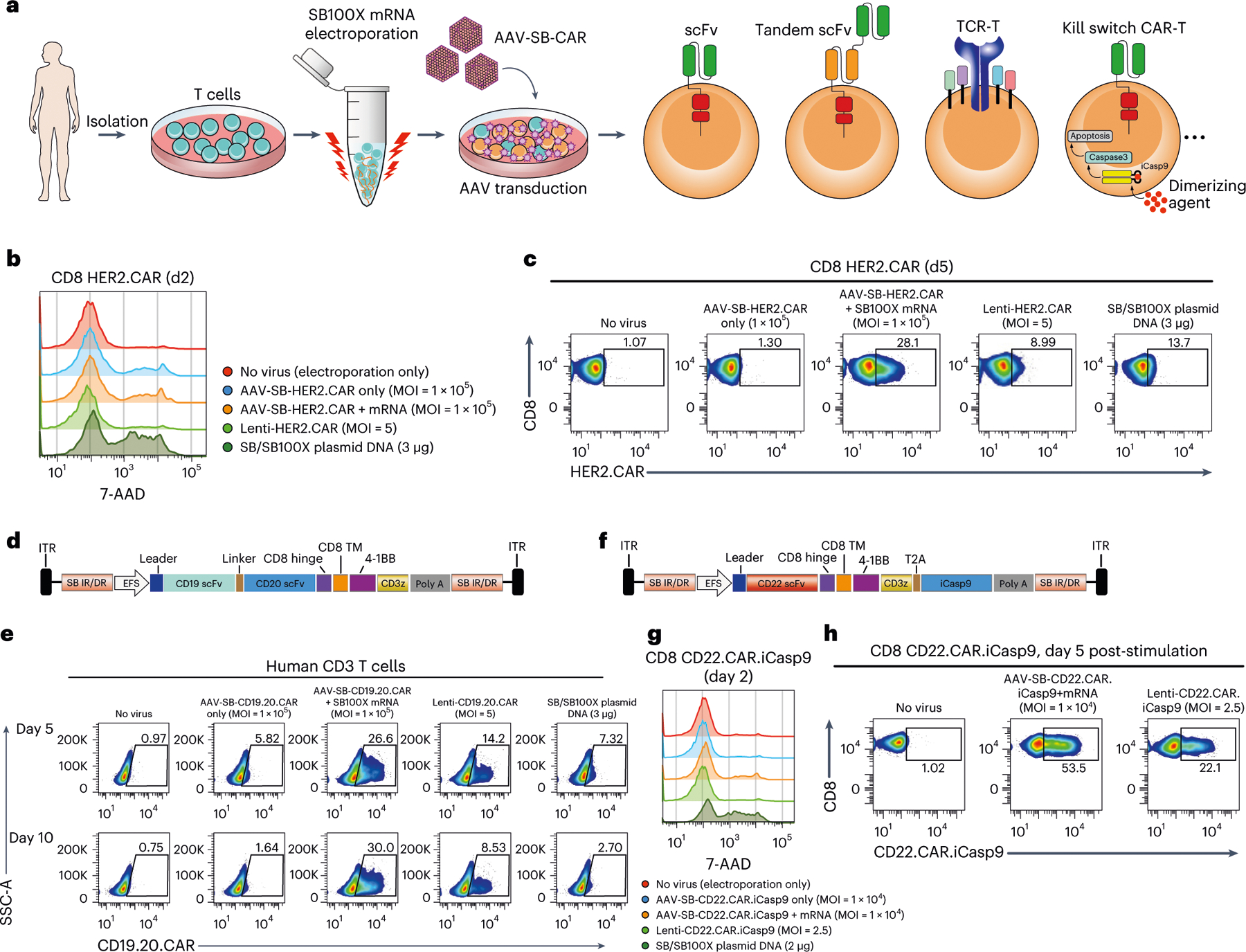
a, Schematic of various therapeutic cells (that is, single scFv CAR, tandem scFv CAR, TCR-T and kill-switch CAR) that can be generated via the AAV-SB system. b, Flow cytometry and histogram overlays of cell viability post-electroporation as measured with 7-AAD staining. c, Representative flow cytometry plots of HER2.CAR T cells. d, Schematic representation of the AAV-SB-CD19.20.CAR construct. CD19 scFv and CD20 scFv CAR sequences are joined by a linker and are expressed together as a tandem scFv CAR. e, Representative flow cytometry plots to evaluate CAR expression of CD19.20.CAR T cells. f, Schematic representation of the AAV-SB-HER2.CAR. iCasp9 construct. g, Flow cytometry and histogram overlays of cell viability post-electroporation as measured with 7-AAD staining. h, Representative flow cytometry plots of CD22.CAR.iCasp9 T cells post antigen-specific cancer cells stimulation. In this figure, each assay was done with one donor with three to five technical replicates; donor 2 T cells were used for b and c; donor VP2 T cells were used for d and e; donor 2 T cells were used for g and h.
Bi-specific CAR T cells can recognize two antigens and may thereby reduce the chance of immune escape; such systems have shown potent efficacy against relapsed B-cell malignancies that down-regulated single-target antigen expression20. To test whether MAJESTIC can be used for bi-specific CAR T-cell generation, we designed an anti-CD19/anti-CD20 tandem scFv construct and used it to transduce primary human T cells, again comparing MAJESTIC with lentivirus and DNA transposon systems in parallel (Fig. 3d). We performed flow cytometry on day 5 and day 10 after electroporation and viral transduction. As with single CARs, we observed that the efficiency of MAJESTIC was higher than that of lentivirus, under the conditions tested (Fig. 3e and Extended Data Fig. 4e). Moreover, the CAR+% of the bi-specific CAR T cells was stable in the MAJESTIC group but was substantially reduced for both the lentivirus and DNA transposon systems (Fig. 3e and Extended Data Fig. 4e). In evaluating antigen expression of the cognate leukaemia cancer cells, we noted high CD19 expression but weak CD20 expression (Extended Data Fig. 4f), consistent with previous reports51. The CD19.CD20 bi-specific CAR T cells showed strong killing ability, with nearly 100% and around 80% cytolysis after 17 h at E:T ratios of 1:4 and 1:10, respectively, for both MAJESTIC and lentiviral CAR T cells (Extended Data Fig. 4g). These data suggested that MAJESTIC can efficiently deliver a bi-specific CAR construct into human T cells.
To test the utility of MAJESTIC for TCR-T cell production, we next cloned a New York esophageal squamous cell carcinoma 1 (NY-ESO-1) TCR construct along with a green fluorescent protein (GFP) marker into the AAV-SB backbone. AAV-SB-NY-ESO-1.GFP transduction plus SB100X mRNA electroporation was able to generate a fraction of NY-ESO-1 TCR-T cells, which was still substantially higher than that using SB/SB100X plasmid DNA electroporation (17% versus 11%) (Extended Data Fig. 5a,b). Conditional inactivation of CAR T cells, for example through kill-switch elements such as induced caspase 9 (iCasp9)52, may be clinically important for controlling potential toxicity. We thus generated conditional control CAR T cells (CD22. CAR.iCasp9 T cells) with two transgenes, CD22 CAR and a suicide gene (Fig. 3f). Flow cytometry data showed that these cells were more efficiently generated by MAJESTIC than by lentiviral and plasmid systems under the experimental conditions as above (Fig. 3g and Extended Data Fig. 5c–e). CAR-positive T cells were further enriched in the AAV-SB-CD22.CAR.iCasp9 + mRNA group (53.5%) on day 5 after antigen-specific cancer cells stimulation (Fig. 3h and Extended Data Fig. 5f). These data together showed the versatility of the MAJESTIC system for delivering a variety of payloads to generate various therapeutic T cells.
Comparison of MAJESTIC system with MC DNA system
The MC vector is a recently developed non-viral strategy that has shown notable improvement compared with conventional plasmid vector—specifically, the SB MC delivery35 of a CAR transgene has been proven to be more effective and less toxic compared with SB plasmid gene transfer. We performed a head-to-head comparison of the MAJESTIC system with both MC and plasmid DNA systems, using CD3 T cells as a source. The 7-AAD staining data of the SB/SB100X plasmid DNA and MC-SB/MC-SB100X groups showed similar cell viability (~70%), with the MC-SB + SB100X mRNA group showing slightly higher cell viability (Extended Data Fig. 6a). In comparison, the AAV-SB-CD22. CAR + SB100X mRNA group (MAJESTIC) attained 86% viability, which was higher than the SB/SB100X plasmid DNA, MC-SB/MC-SB100X and MC-SB + SB100X mRNA groups (Extended Data Fig. 6a). CAR T-cell ratio on day 4 after electroporation confirmed higher CAR T-cell production efficiency compared with MC versus plasmid DNA electroporation (19.7% versus 8.01%). Efficiency could be further improved if MC-SB was electroporated with transposase supplied as SB100X mRNA (24.4%) (Extended Data Fig. 6b,c). In comparison, the MAJESTIC (AAV-SB-CD22. CAR + SB100X mRNA) group yielded the highest CAR T-cell ratio (51.8%) on day 4, substantially higher than those of MC/MC-transposase, MC/mRNA-transposase and transposon plasmids (Extended Data Fig. 6b,c).
To further verify these results, we performed another independent set of MC versus MAJESTIC comparisons in an independent human donor, using CD4 and CD8 T cells separately in this case. Flow cytometry revealed CD22.CAR T ratios of 16.1%, 20.0% and 24.2% for the MC-SB + SB100X mRNA group in donor 6760 CD4 T cells on days 3, 8 and 14, respectively (Fig. 4a,c). The MAJESTIC group showed CD22. CAR T ratios of 43.4%, 60.9% and 81.9% in donor 6760 CD4 T cells on days 3, 8 and 14, respectively (Fig. 4a,c). Similar results were also observed in donor 6760 CD8 T cells. The MC-SB + SB100X mRNA group showed CD22.CAR T ratios of 29.1%, 29.4% and 37.0% on days 3, 8 and 14, respectively (Fig. 4b,d), and the AAV-SB-CD22.CAR + SB100X mRNA group showed CD22.CAR T ratios of 60.4%, 76.3% and 82.4% on days 3, 8 and 14, respectively (Fig. 4b,d). The yield of MAJESTIC and MC/mRNA-transposase is shown from aggregated replicates (Extended Data Fig. 6d).
Fig. 4 |. Comparison of MC transposon and MAJESTIC methods.
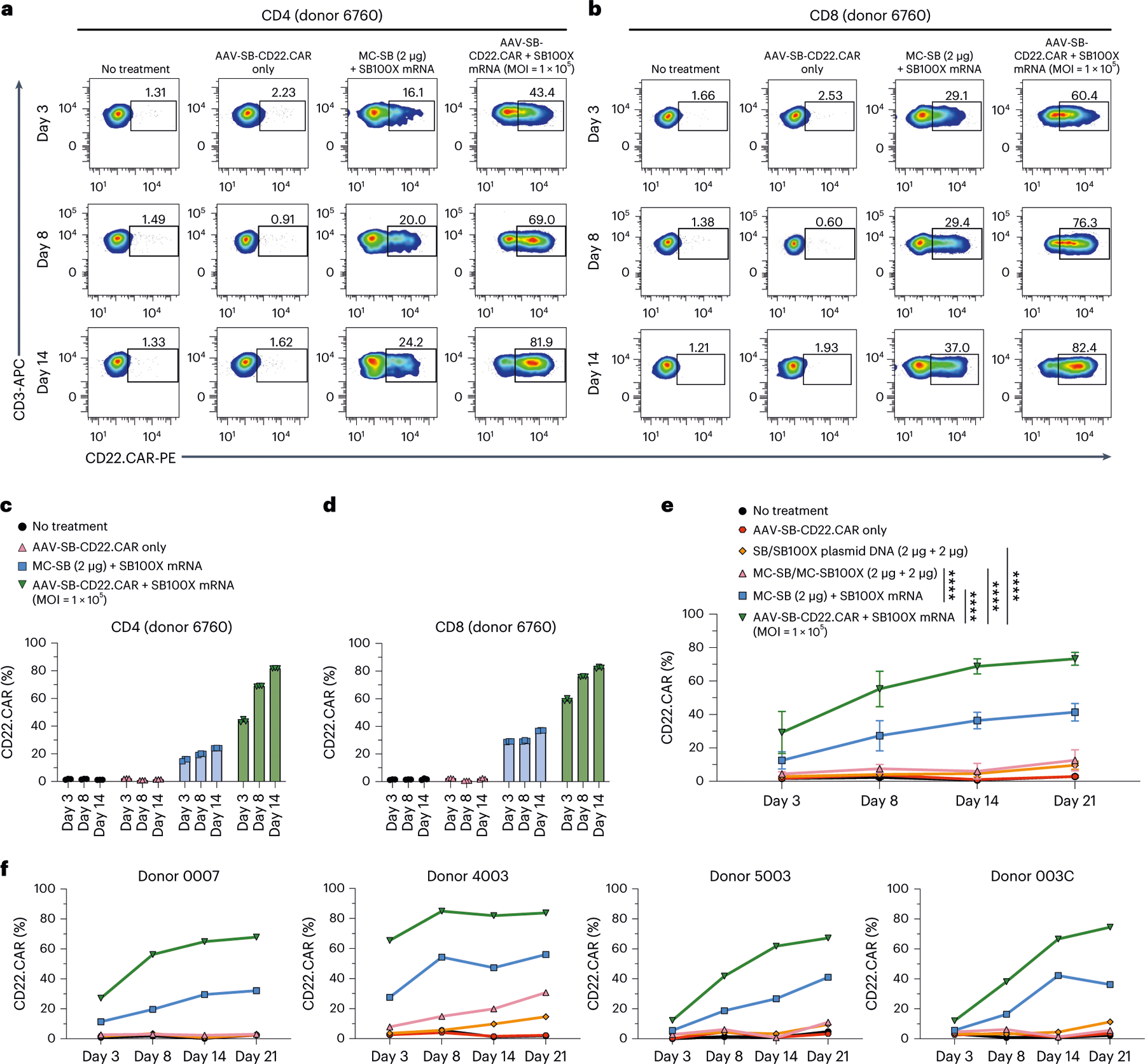
a, Flow cytometry of CD22.CAR ratio in donor 6760 CD4 T cells. b, Flow cytometry of CD22.CAR ratio in donor 6760 CD8 T cells. c,d, Quantification of flow cytometry data of CD22.CAR T cells from human primary CD4 (c) and CD8 (d) T cells, from an independent donor, with CAR T cells produced by plasmid transposon plasmid, transposon MC, transposon MC with mRNA-transposase and MAJESTIC (AAV-SB-CD22.CAR + SB100X mRNA). e,f, Quantification of time-course flow cytometry data of CD22.CAR T cells produced by MAJESTIC and other systems, generated from PBMCs of four independent healthy human donors (n = 4) (e, showing mean ± s.e.m., n = 4 for all time points; f, showing individual donors in separate panels). In this figure, data in a–d were sourced from one donor with three technical replicates; data in e and f were sourced from four independent donors (donor 0007, donor 4003, donor 5003 and donor 003C). Two-way ANOVA with multiple comparisons tests was used to evaluate statistical significance, ****P < 0.0001.
To further test the variability of the MAJESTIC system, we applied this technology along with other systems to four new healthy donors (donor 0007, donor 4003, donor 5003 and donor 003C) in head-to-head comparisons. Consistent with the results above, across all four donors, the CAR T-cell ratio was the highest in the MAJESTIC group (AAV-SB-CD22.CAR + SB100X mRNA) compared to the MC/MC and MC/mRNA-transposase groups (Fig. 4e,f and Extended Data Fig. 6e). Specifically, the CD22.CAR% was on average 29.20% ± 12.57% on day 3 (n = 4, mean ± s.e.m.) and as high as 73.3% ± 3.83% on day 21 for MAJESTIC (n = 4, mean ± s.e.m.) (Fig. 4e and Extended Data Fig. 6e). Importantly, although there is donor-to-donor variability as expected, in each respective donor, MAJESTIC is consistently the group with the highest efficiency in matched comparisons, across each donor and in all time points (Fig. 4f). Electroporation involving either the plasmid or the MC form of DNA appears to result in lower viability and CAR% versus MAJESTIC even in different cell types and donors (Fig. 4a–f and Extended Data Fig. 6). Together, the data showed the efficiency and reduced cellular toxicity of the MAJESTIC gene transfer system compared with plasmid and MC gene transfer methods.
Genomic integration profiling of transposon in CAR T cells
To examine the genomic integration profile of the MAJESTIC system in T cells, we performed splinkerette library preparation followed by next-generation sequencing (Methods). We isolated genomic DNA from one donor (three technical replicates) of MAJESTIC-generated and MC/mRNA-generated CAR T-cells collected on day 14 after electroporation. We fragmented the gDNA for this donor and then conducted a two-step splinkerette PCR to generate insertion site libraries. Analysis of next-generation sequencing data allowed us to map insertion locations in karyograms (Fig. 5a). These data showed that MAJESTIC indeed mediates cargo integration into the genome of human T cells, across all major chromosomes.
Fig. 5 |. Genomic insertion and safe harbour preference of MC transposon and MAJESTIC methods.
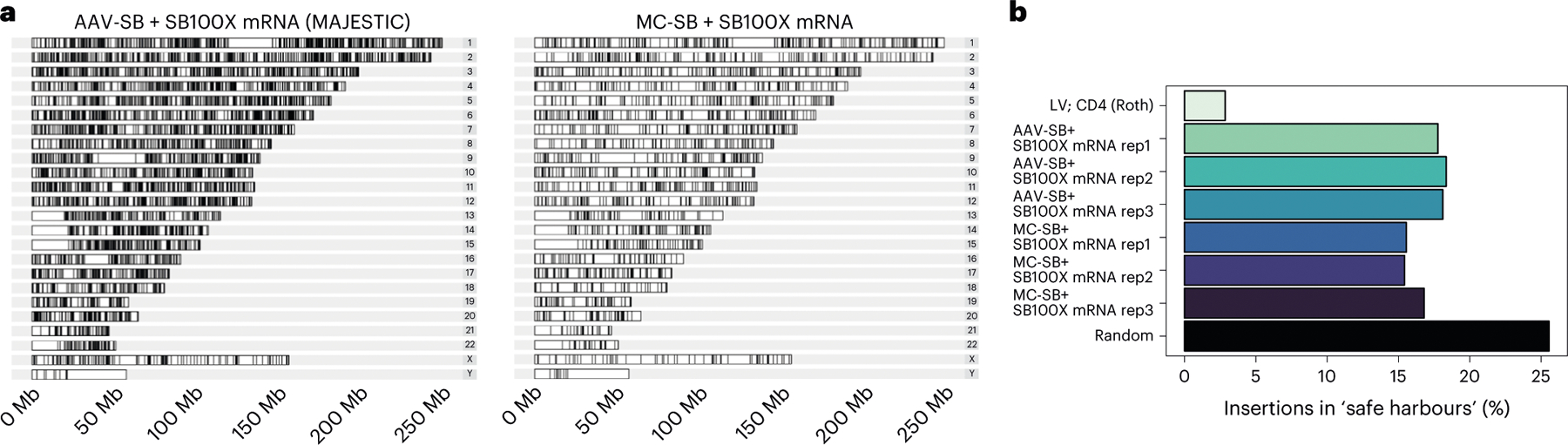
a, Karyogram of genomic insertions for one representative technical replicate from the MAJESTIC and MC-SB + SB100X mRNA groups. b, A bar plot of frequencies of insertions in safe harbours. Three technical replicates were mapped for the MAJESTIC and MC-SB + SB100X mRNA groups. Random refers to a set of one million randomly generated genomic coordinates. Insertion coordinates for LV and CD4 (Roth) were obtained from literature (see main text for citations). LV, lentivirus.
We then examined the frequency of insertions into safe harbours, which are generally defined as regions of the genome where transgene insertions lead to predictable expression and do not interrupt existing gene activity53. We used a list of safe harbour coordinates from ref. 54. Using a random set of 1 million genomic sites, we estimated that approximately 25% of these random sites intersect with safe harbours. Using this value as a reference point, we compared MAJESTIC with other gene-transfer methods, using integration profile data from the literature for lentivirus transduction55. We sought to understand trends in the safety profile of MAJESTIC compared with other methods. While MC/mRNA-mediated insertions were similar to MAJESTIC to be within safe harbours (around 15% versus 17% on average), MAJESTIC-mediated insertions were much more likely to be within safe harbours compared with that of lentivirus (around 2–3%) (Fig. 5b). Furthermore, we determined the proportion of insertions into functional gene regions, including exons, introns and cancer genes and calculated the frequencies as fold change relative to the randomly generated sites (Supplementary Fig. 1). Using this functional-gene region profile as a proxy for insertion safety, MAJESTIC insertions occupy a reasonably favourable performance, comparable with MC/mRNA and better than lentiviral vector (Fig. 5a,b and Supplementary Fig. 1a). Together, these data reveal the integration site profile of the MAJESTIC system and show a trend towards safer insertions compared with lentiviral transduction.
Unexpectedly, we noticed certain differences in the frequencies of integration into exons, coding exons and 5′ untranslated regions in the MC-SB + SB100X mRNA group, compared with a previous study38. This may be because of technical reasons (such as different methods of sample preparation or different time points). For example, it is possible that the selection of the CAR+ cell population in this analysis might introduce a difference (because CAR+ selection enriches T cells with the CAR transgene inserted in genomic loci that avoid transgene silencing), compared with the unselected bulk samples in the previous study38. Regarding the integration profile differences observed between MAJESTIC and MC systems, because both have the same SB transposon, the differences could be because of technical reasons (such as differences in sample preparation) and/or biological reasons associated with the differences in transposon delivery approaches (for example, the single-stranded DNA SB template provided by AAV in the episome and the double-stranded DNA SB template provided by MC in transient extrachromosomal DNA).
Application of MAJESTIC across multiple immune cell types
Although CAR T cells have strong clinical potential, they also have inherent limitations4. It is well known that the suppressive tumour microenvironment of solid tumours creates hurdles for T cells56. However, unlike T cells, myeloid cells such as macrophages and monocytes naturally infiltrate tumours57. In addition, NK cells have been explored as an alternative to T cells for immunotherapy, because they utilize a different set of signalling pathways, have rapid activation, can show TCR-independent cytotoxicity and are relatively easier to develop into an off-the-shelf product58. Therefore, it is of interest to expand cell therapy to other immune cell types, such as NK cells and myeloid cells59, to overcome the inherent limitations of T-cell-based therapy.
To test the utility of MAJESTIC in other immune cell types, we used MAJESTIC for delivery of CAR transgenes to generate CAR-NKs, CAR monocytes and CAR-MAs. We again performed lentivirus transduction and/or plasmid electroporation in parallel. NK92 is an immortalized NK cell line that has been used to produce CAR-NKs, which have achieved use in clinical trials60. We transduced NK92 cells to engineer HER2-specific CAR-NK cells. Flow cytometry revealed that the MAJESTIC system efficiently generated HER2 CAR-NK cells (nearly 50% at a high dose on day 14) at significantly higher rates than that by lentiviral or DNA transposon electroporation in the conditions tested (Fig. 6a,b). For example, on day 4, Lenti-HER2.CAR at a high MOI (MOI = 2.5) yielded 13.9% HER2.CAR-positive NK92 cells but only 2.71% with low MOI (MOI = 1) (Fig. 6a,b). The plasmid DNA transposon electroporation groups achieved 16.1% and 8.06% HER2.CAR-positive NK92 cells (Fig. 6a). The AAV-SB-HER2.CAR + mRNA groups yielded 41.6% and 13.2% HER2.CAR-positive NK92 cells in high (1 × 105) and low (1 × 104) MOI conditions, respectively (Fig. 6a). Notably, the proportion of the HER2.CAR-positive population did not substantially decline in long-term cultures of MAJESTIC-generated CAR-NK cells: the percentage of HER2.CAR+% NK cells was 44.9–49.0% on day 14 (Fig. 6a,b) and was sustained at 43.8% on day 33 (Fig. 6c,d). It is noteworthy that the dose-dependence effect is strong for CAR-NK generation via MAJESTIC, as high (1 × 105) MOI resulted in higher CAR+% NK cells than low (1 × 104) MOI (Fig. 6a–d). Because NK cells can kill cancer cells independent of cancer-specific antigens, we tested the function of the resultant HER2. CAR-NK cells in a kill assay with co-culture of MCF-7 breast cancer cells. Results showed that the HER2.CAR-NK cells are efficient killer cells, showing stronger killing compared with untreated NK92 cells, with around 70% target cell death 24 h post co-culture (Fig. 6e). These data show that the MAJESTIC system can be used to efficiently generate stable and functional CAR-NK cells.
Fig. 6 |. CAR-NK generation via MAJESTIC.
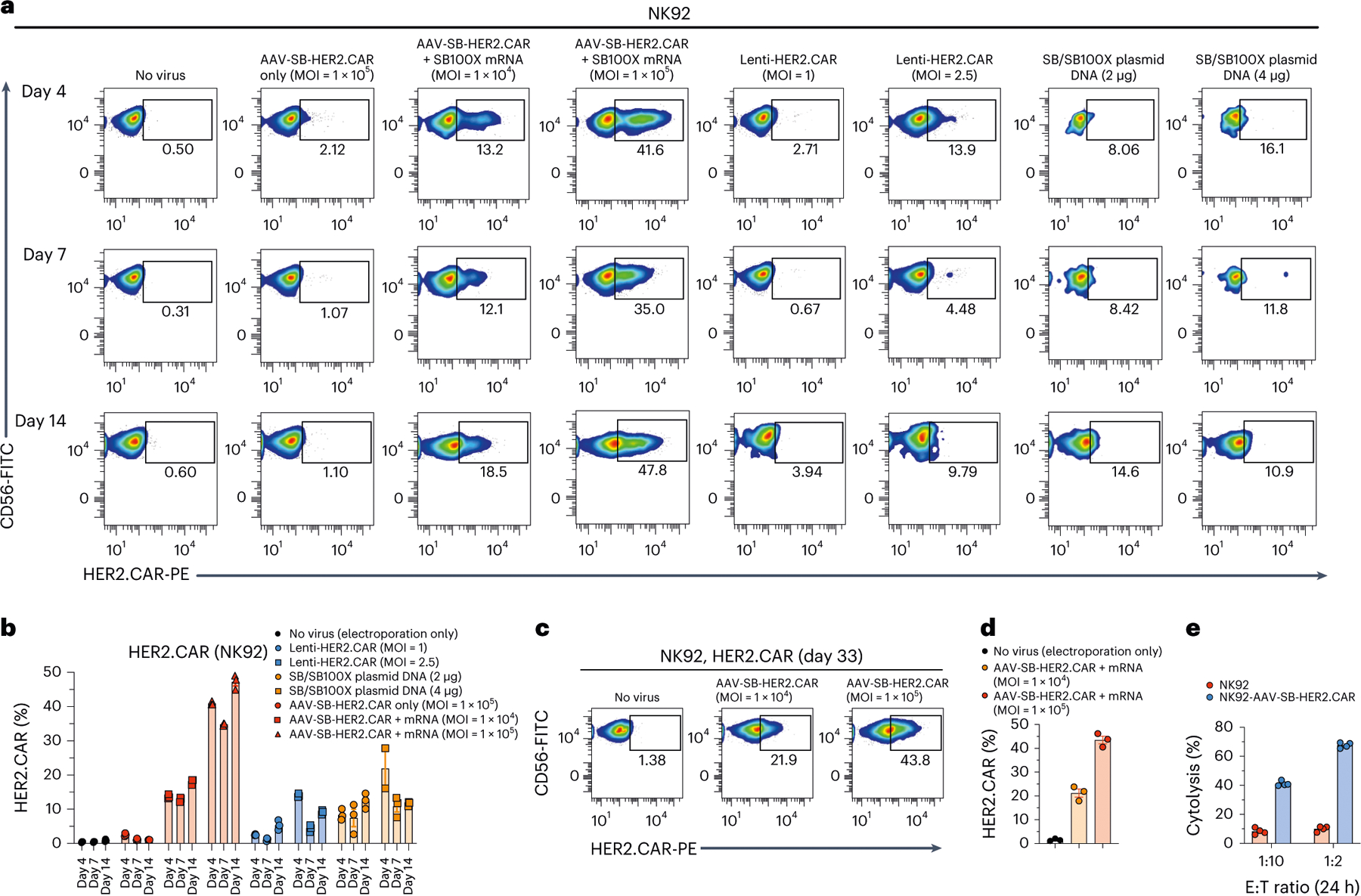
a, Representative flow cytometry plots of NK92 cells transduced with AAV-SB-HER2.CAR virus (MOI = 1 × 104 and 1 × 105), transduced with HER2.CAR lentivirus (MOI = 1 and 2.5), or electroporated with plasmid DNA (2 μg = 1 μg transposon plasmid + 1 μg transposase plasmid) at three different time points. b, Quantification of a. c, Flow-cytometry measurement of HER2.CAR expression in NK92 cells on day 33 after mRNA electroporation and AAV-SB-HER2.CAR viral transduction. d, Quantification of c. e, Cytolysis analysis of MCF7-PL (MCF7 with puromycin resistance and luciferase expression) cancer cells that were co-cultured with NK92-AAV-SBHER2.CAR cells. CAR-NKs were seeded at two E:T ratios, and a luciferase assay was performed at two time points (24 h and 48 h). Error bars, mean ± s.e.m. of three biological replicates.
Next, we tested this system for CAR delivery to THP-1 cells, a human monocytic and myeloid cell line61. We achieved over 93% HER2. CAR-positive THP-1 with AAV-SB-HER2 (MOI 3 × 105) on days 5 and 10, compared with ~30% with lentiviral transduction at MOI 5 (Fig. 7a,b). For the DNA transposon plasmid electroporation group, cell viability was extremely low, with the vast majority of cells (>99%) dead as a result of the high cellular toxicity of plasmid electroporation (Fig. 7a,b), making it impossible to generate sufficient cells for subsequent analysis. We then set out to engineer CAR-MAs using primary human CD14+ macrophages, again comparing MAJESTIC with lentivirus and DNA transposon systems. Flow cytometry revealed a 25.5% CD22-specific CAR-MA population for the AAV-SB-CD22.CAR + mRNA group, which increased to 69.1% by day 11 (Fig. 7c,d). Lentiviral transduction worked reasonably well in primary macrophages, with CAR-positive percentages of 52.9% on day 5 and 48.1% on day 11 (Fig. 7c,d). Interestingly, AAV-SB-CAR alone without the mRNA-transposase yielded nearly 30% CAR+ MAs by day 5, which fell by more than half by day 11 (Fig. 7c,d). On day 11, the CAR+% of CAR-MAs in the MAJESTIC group was the highest among all groups tested (versus AAV-SB-CAR alone, lentivirus and DNA transposon). These data suggest that the MAJESTIC system can be used to efficiently generate stable CAR-MA cells. Finally, because human iPSCs can be used as a source for derivation of various types of cellular lineage15,17,18, we set out to test whether MAJESTIC can be applied to iPSCs. Results showed MAJESTIC can transduce iPSCs carrying a HER2. CAR transgene at high efficiency (>75% HER2.CAR+) (Supplementary Fig. 1b,c). Along with MAJESTIC, lentiviral vector can also transduce iPSCs at high efficiency, but not transposon DNA electroporation (Supplementary Fig. 1b,c). Although differentiation of iPSCs into other cell types takes additional time15,18,62, the delivery of cell therapy transgenes into iPSCs by MAJESTIC provides another avenue to generate various therapeutic immune cells.
Fig. 7 |. CAR-monocyte and CAR-MA generation via MAJESTIC.
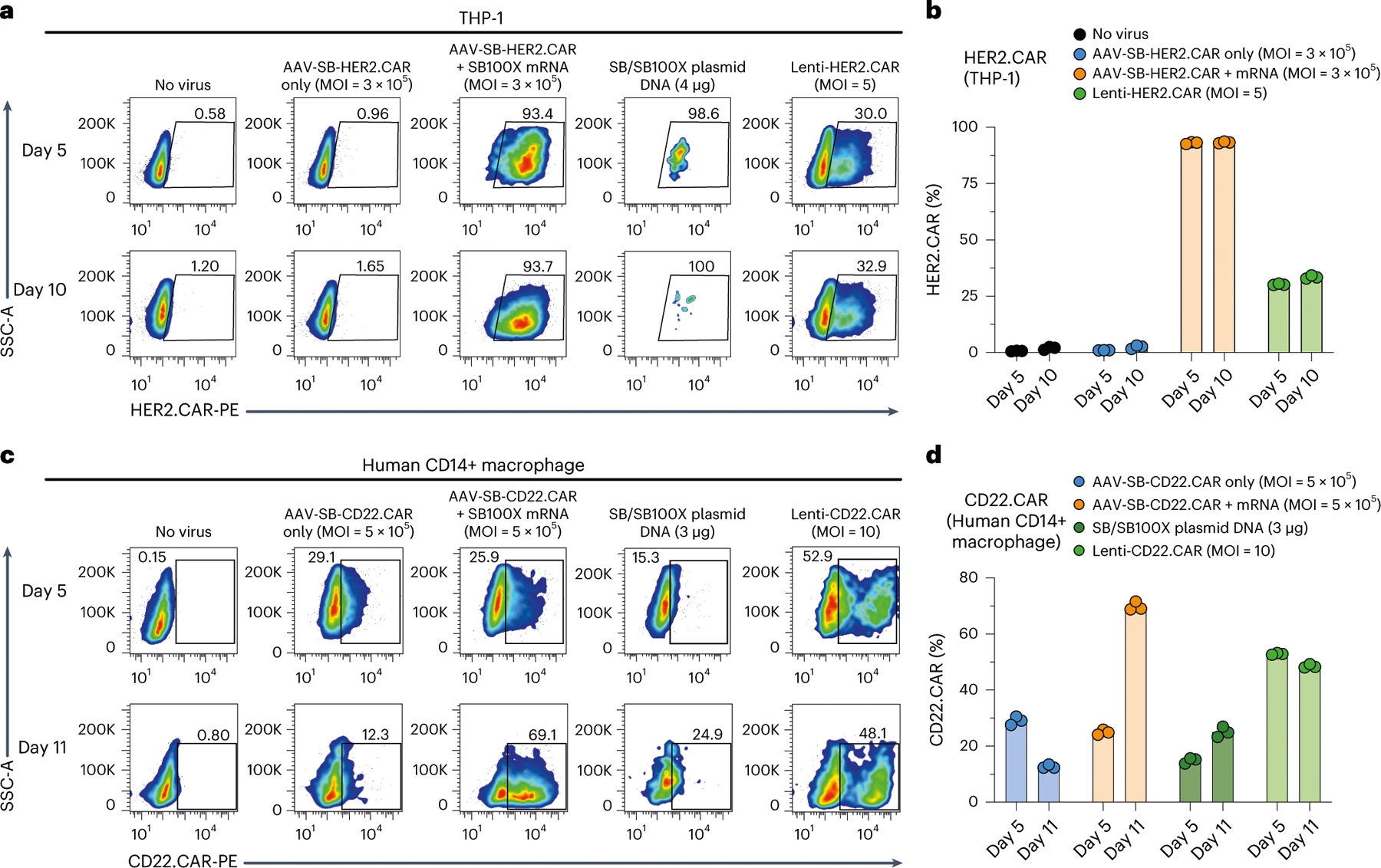
a, Representative flow-cytometry plots of THP-1 cells (human monocytic cell line) transduced with AAV-SB-HER2.CAR virus (MOI = 3 × 105), transduced with HER2.CAR lentivirus (MOI = 5) or electroporated with plasmid DNA (4 μg total). b, Quantification of a. c, Representative flow-cytometry plots of human CD14+ macrophages transduced with AAV-SB-CD22.CAR virus (MOI = 5 × 105), transduced with CD22.CAR lentivirus (MOI = 10) or electroporated with plasmid DNA (3 μg). d, Quantification of c. Error bars, mean ± s.e.m. of three biological replicates.
Altogether, these data suggested that the MAJESTIC system is capable of efficiently engineering stable functional therapeutic immune cells and is applicable to various types of transgene and across multiple lineages of immune cells.
Discussion
Adoptive cell therapy, most notably CAR T-cell therapy, has shown clinical success in patients with several indications of haematological malignancies. A vital and potentially limiting step of this therapy is the manufacturing of engineered immune cells: to produce sufficient therapeutic cells for therapy would ideally require a sizeable pool of patient immune cells to begin with and a highly efficient genetic engineering technology. Although low transfer efficiency can be alleviated in part by increased culturing time, high initial efficiency would expedite the manufacturing process. In addition, increasing culturing time may necessitate extended exposure of the cells to their target antigens during production, which may shift the cells in favour of a differentiated phenotype, reducing long-term memory function21 and thus the overall quality of the engineered cells.
γ-Retroviral vectors are indeed capable of efficient genome integration, but their tendency to insert into promoters of actively transcribed genes raises concerns about potential genotoxicity. Lentiviruses are commonly used in clinical trials today, and recent advances have improved their safety. However, there are still certain safety risks associated with the pathogenic origin of such viruses, and a recent study has suggested a higher preference of lentiviral insertion into active genes compared with SB-based gene transfer methods63,54.
The field has developed various alternative gene transfer methods that can be worked with at BSL-121. AAV is a commonly used gene therapy vector; however, because of the dilution effect, AAV-transduced T cells have a gradual reduction in transgene expression, making an AAV-only system not ideal for delivery of CAR transgenes into T cells. Electroporation of DNA transposon/transposase and of CAR-encoding mRNA construct are two non-viral gene-transfer strategies, but key limitations include low viability and high toxicity for the former and transient transgene expression for the latter.
The notable success of immune cell-based cell therapies has invited the introduction of other technologies to enhance immune cell engineering. CRISPR is one such technology that is being rapidly and broadly applied to immune cell editing29,30,64. Such strategies rely on Cas9, Cas12a/Cpf1 or other DNA-targeting endonucleases to generate double-strand breaks (DSBs), which are then repaired with a donor template via homology-directed repair (HDR). The efficiency of these knock-in/knock-out systems is thus dependent on two steps: (1) the efficiency of enzymatic gene knockout and (2) the rate of incorporation of the homology template. As to (1), knockout efficiency depends on the availability of an optimal guide because poorly designed guides may not cut efficiently and may cause undesired off-target effects65–70. In addition, CRISPR knockout generates exposed DSBs that may trigger mutagenic non-homologous end-joining pathways rather than HDR, which is especially hazardous for off-target editing, when no repair template is available for HDR to occur. As to (2), HDR is limited to the late S and G2 phases of the cell cycle71, restricting the interval in which the second step can occur. In CRISPR-based gene editing approaches, DSB occurs and brings two types of risk—the triggering of the p53 pathway and the possibility of chromosome alterations, which increases with the number of DSBs62, for example, the observed chromosomal aberrations following Cas9-mediated genome editing in recent studies31–33. MAJESTIC itself does not involve CRISPR-based gene editing.
Unlike γ-retroviruses, SB reduces the likelihood of genotoxicity as studies have shown that this class of transposons has close to a random genomic integration profile72. However, introduction of the SB system into cells by DNA transfection or electroporation can lead to higher cellular toxicity38,73. During remobilization, SB can leave behind a tri-nucleotide footprint; thus, continuous remobilization of the transposon is a potential limitation of an all-in-one AAV-SB system44, where both the transposon and transposase are delivered by AAV. Our system addresses this issue by separating the transposase into a transient delivery component (mRNA). A previous study used a hyperactive transposase SB100X to improve CAR T-cell transduction of DNA transposon74. The SB system has also been engineered in the form of combinations or hybrid vectors, for example, dCas9–SB100X to re-target SB transposition75, and an adenovirus–SB hybrid system to achieve higher transduction efficiency76. The MAJESTIC system differs from all such efforts by combining the advantages of all three delivery vehicles (AAV, transposon and mRNA) in an organic manner: transducing cells with the hybrid AAV-transposon vector with electroporation of transposase mRNA. AAV-SB transduction retains the benefits of high cell viability and stable transgene expression. The process of gene transfer of the MAJESTIC system is similar to conventional SB nucleofection, with mRNA electroporation instead of plasmid or MC electroporation and an extra AAV transduction step in which the virus is added directly into the media. From our data, it appears that approaches involving DNA electroporation including plasmid transposon, MC/MC and/or MC/mRNA naturally have an associated impact on cell viability and yield. MAJESTIC avoids introducing circular DNA into cells and instead uses AAV and mRNA, both of which have reasonably low cellular toxicity.
The MAJESTIC system has a number of limitations. By virtue of relying on AAVs for gene transfer, the MAJESTIC system will be limited by the packaging size of the AAV, ~4.75 kb (~4.3 kb without SB arms; Supplementary Table 1). This is usually sufficient to include the CAR construct and additional elements (such as iCasp9) but will face challenges with significantly larger transgenes, which could be accommodated with DNA transposon plasmid/MC systems, as transposons can in principle carry large transgene cargos, although the efficiency may drop as the size increases77. In addition, given that MAJESTIC is a composite system, the generation of therapeutic immune cells is a two-step process including electroporation/nucleofection + viral transduction, although they can be streamlined to be performed at the same period (as demonstrated in our 0 h transduction/electroporation experiments); lentivirus and plasmid electroporation are both one-step methods. Also, compared with lentivirus or plasmid production, the good manufacturing practice (GMP) production cost of MAJESTIC will be higher because of the requirement for both AAV and mRNA. Without considering yield, MAJESTIC, knock-in and knock-out, AAV and lentiviral/retroviral approaches all have higher GMP cost compared with non-viral approaches such as transposon/MC, which is more economic to manufacture per today’s GMP landscape (Supplementary Table 1). MAJESTIC itself cannot achieve precisely targeted gene editing as CRISPR; rather, its advantage is being a high-efficiency gene-editing-free delivery approach. We present MAJESTIC as an alternative cargo delivery and therapeutic cell generation strategy with the strength of producing CAR+ cells with high viability at high yield. We thus propose that the MAJESTIC system may be worth considering when viability or yield are particularly important.
In Supplementary Table 1 we summarized the differences, advantages and limitations of the MAJESTIC system and of other approaches for therapeutic cell engineering. It should be noted that MAJESTIC does not replace nor diminish other methods; instead, it provides a recently developed alternative for gene-delivery technology that is superior and advantageous in certain feature areas, such as high viability, efficiency and yield. However, MAJESTIC is associated with limitations of cargo size and with additional procedures and costs. The versatility of the MAJESTIC system is not limited to cell therapy for cancer; any therapy or research effort using engineered immune cells could, in principle, benefit from this system. Future research could examine the efficacy of MAJESTIC in generating CAR T cells from T cells derived from patients with cancer in translational studies to validate the utility in a more clinically relevant scenario. Further improvements in the safety of the system can be achieved, using other components, including different AAV serotypes, other viral vectors or different transposon systems such as a high-soluble SB transposase54. Because of the modular nature of the MAJESTIC system, other cargos and cell types can be tested.
Methods
Institutional approval
This study has received institutional regulatory approval. All recombinant DNA and biosafety work were performed under the guidelines of Yale Environment, Health and Safety Committee with approved protocols (Chen-rDNA-15–45; Chen-rDNA-18–45). All human sample work was performed under the guidelines of Yale University Institutional Review Board with an approved protocol (HIC#2000020784). All animal work was performed under the guidelines of Yale University Institutional Animal Care and Use Committee with approved protocols (Chen-2018–20068; Chen-2021–20068).
Mouse model
Mice were housed in standard conditions in Yale vivarium, maintained on a 12 h light and dark cycle (07:00 to 19:00 light on). Mice, both females and males, aged 8–12 weeks were used for experiments. NOD-scid IL2Rgammanull mice were purchased from JAX and bred in-house for T cell-based anti-tumour therapeutic efficacy testing experiments. Mouse health was monitored daily after tumour induction.
Construction of AAV-SB-CAR vector
To create hybrid AAV-SB-CAR vectors, we began with our previously established AAV-SB-CRISPR vector as a backbone, which has an sgRNA/SB100X expression cassette nested between SB arms and AAV ITRs44. We replaced this expression cassette between the U6 promoter and the short polyA sequence with a CAR or other expression cassettes. As this study utilized multiple types of CAR (such as CD22 and BCMA), we obtained each CAR sequence via either (1) PCR amplification of CAR sequences from existing CAR constructs78 or (2) Integrated DNA Technologies gene synthesis. The SB100X transposase was subcloned from44 and cloned into the Nco I and Hind III restriction endonuclease sites of the empty vector pcDNA3.1, which was used for in vitro transcription of mRNA.
Preparation of MC DNA
Genes of interest such as SB-CAR and SB100X constructs were first cloned into a parental plasmid (System Biosciences). Then MC DNA was produced and purified by using an MC-Easy Kit (System Biosciences), without the optional dNTP removal step.
Cell culture
HEK293T, NALM6, MM.1R, MCF7, NK-92, THP-1, human CD14+ monocytes, human peripheral blood mononuclear cells (PBMCs) and human iPSCs were purchased from commercial sources (ThermoFisher, American Type Culture Collection and StemCell). HEK293T and MCF7 cells were cultured in DMEM (Gibco) media supplemented with 10% FBS (Corning) and 200 U ml−1 penicillin–streptomycin (Gibco), hereafter referred to as D10. NALM6 and MM.1R cells were cultured in RPMI-1640 (Gibco) media supplemented with 10% FBS and 200 U ml−1 penicillin–streptomycin.
NK-92 cells were cultured in Minimum Essential Medium α (Gibco) supplemented with 12.5% horse serum, 12.5% FBS, 0.2 mM inositol, 0.1 mM 2-mercaptoethanol, 0.02 mM folic acid, and 200 U ml−1 human IL-2 (BioLegend).
THP-1 and CD14+ monocytes were cultured in RPMI-1640 media supplemented with 10% FBS, 1% Glutamax, 1% HEPES, and 1% penicillin–streptomycin. About 20 ng ml−1 of human granulocyte-macrophage colony-stimulating factor (BioLegend) was used to differentiate monocytes into macrophages for 7 days, then macrophages were collected for electroporation and/or viral transduction, CAR-MA cells were maintained in RPMI complete media supplied with 20 ng ml−1 of human granulocyte-macrophage colony-stimulating factor.
Human PBMCs, CD4 and CD8 T cells were purchased from the StemCell and cultured in X-VIVO 15 media (Lonza) supplied with 5% human AB serum (MP Biomedicals) and 400 U ml−1 human IL-2 (BioLegend). T cells were activated with Dynabeads Human T-Activator CD3/CD28 (ThermoFisher) with a T cell:beads ratio at 1:1. In this study, multiple T-cell donors were involved in various experiments; the donors for each experiment are clarified in figure legends.
Human iPSCs were cultured in StemFlex Medium (Gibco).
Lentivirus production and titration
Low-passage (less than 15 passages) HEK293T cells were used for lentiviral packaging. One day before transfection, 2 × 107 HEK293T cells were seeded per 150 mm dish. D10 media was replaced with 13 ml pre-warmed Opti-MEM medium (Invitrogen) before transfection. For each plate, 20 μg transgene plasmid, 15 μg psPAX2 (Addgene), 10 μg pMD2.G (Addgene) and 90 μl lipofectamine 2000 (Thermo Fisher) were mixed in 450 μl Opti-MEM. The mixture was vortexed briefly and incubated for 10–15 min at room temperature, then added dropwise to cells. To minimize the toxicity of lipofectamine, Opti-MEM media was replaced with pre-warmed 20 ml D10 media 5–6 h after transfection. Viral supernatant was collected 48 h post-transfection and then concentrated using the Amicon Ultra-15 centrifugal filter unit (Millipore) or purified with Lenti-X Concentrator (Takara). All virus were titrated with Lenti-X GoStix Plus (Takara) before being aliquoted and stored at −80 °C.
AAV production, purification and titration
HEK293T cells were prepared in 150 mm dishes as above. D10 media was replaced with 13 ml pre-warmed DMEM (FBS-free). For each 150 mm dish, HEK293T cells were transiently transfected with 5.2 μg transfer, 8.9 μg AAV6 serotype and 10.4 μg pDF6 plasmids, which was pre-mixed with 130 μl of polyethylenimine (1 mg ml−1) in 450 μl Opti-MEM medium. After 6 h of transfection, DMEM was replaced with 20 ml pre-warmed D10 media. Transfected cells were dislodged and collected in 50 ml Falcon tubes 72 h post-transfection for AAV purification. AAV purification was performed as previously reported44. Viral titre was measured via reverse transcription qPCR with a Taqman probe targeting the elongation factor 1α short sequence in the AAV vector.
Copy number determination
T cells were collected on day 21 after electroporation and washed with PBS twice to remove the media. Cells were incubated with CD22-Fc protein (R&D Systems) in PBS for 30 min on ice and washed with PBS twice to remove the unbonded protein. The cells were then stained with anti-human IgG Fc-APC (BioLegend, catalogue number 366906) on ice for 30 min and washed with PBS twice. The CAR-positive T cells were purified by Anti-APC MicroBeads (Miltenyi). After that, gDNA was extracted using the Qiagen Blood Mini Kit. To minimize episomal AAV contamination, the extracted gDNA samples were separated on a 1% agarose gel, and DNA bands over 10 kbp were gel isolated and purified with QIAquick Gel Extraction Kit (Qiagen). We conducted qPCR to determine copy number, using primers that specifically target SB transposon:
IRDR-left (forward: 5′-CTCGTTTTTCAACTACTCCACAAATTTCT-3′; reverse: 5′- GTGTCATGCACAAAGTAGATGTCCTA-3′)
IRDR-right (forward: 5′- GCTGAAATGAATCATTCTCTCTACTATTA TTCTGA-3′; reverse: 5′- AATTCCCTGTCTTAGGTCAGTTAGGA-3′).
As an internal control, we amplified samples with primers for RPPH1, a housekeeping gene known to have two copies per cell50 (forward: 5′-AGCTGAGTGCGTCCTGTCACT-3′; reverse: 5′-TCTGGCCCTAGTCTCAGACCTT-3′).
The qPCR reactions were set up with 30 ng of gDNA (using three technical replicates), forward and reverse primers at a final concentration of 250 nM, and SYBR Green PowerUp Master Mix (ThermoFisher). Reactions were run in standard mode: a 2 min hold at 95 °C followed by 40 cycles of 15 s at 95 °C to denature and 60 s at 60 °C to anneal and extend.
Excision efficiency determination
T cells were collected at different time points after SB100X mRNA and viral transduction for transposase excision efficiency evaluation. Primers 5′-ccgcacgcgttctagact-3′ targeting AAV backbone and 5′-acaa agtagatgtcctaactgacttgcc-3′ targeting SB left arm were designed to evaluate SB left arm excision efficiency. Primers 5′-gccgctcggtccgcacgtg-3′ targeting AAV backbone and 5′-agtgagtttaaatgtatttggctaaggtgtatg-3′ targeting SB right arm were designed to evaluate SB right arm excision efficiency. The SYBR Green Master Mix (ThermoFisher) was applied for qPCR quantification as previously described. For the excision efficiency calculation, the AAV-SB-CAR only group (only transduced with AAV) was determined as baseline level of viral copy number that existed in the T cells, then viral copy number in the AAV-SB-CAR + SB100X mRNA group was divided by the baseline viral copy number, which was determined as excision efficiency.
Flow cytometry
T cells (or other immune cells) were collected and spun down to remove media. For CAR constructs lacking a Flag tag (for example, for CD22 and BCMA CARs), cells were incubated with CD22-Fc or BCMA-Fc protein (R&D Systems) in PBS for 30 min on ice, then stained with anti-human IgG Fc-PE and other immune markers antibodies and incubated on ice for 30 min. For CAR constructs containing a Flag tag, Flag was stained directly with an anti-Flag antibody. For the CD19.20.CAR detection, cells were incubated with biotinylated protein L (R&D) on ice for 30 min, then stained with APC streptavidin for 30 min on ice. General T-cell marker staining such as exhaustion and memory followed a previous study78. In brief, T cells or CAR T cells were collected and washed with MACS buffer (0.5% BSA and 2 mM EDTA in PBS) then stained with specific antibodies for 30 min on ice. Cells were washed with MACS buffer after staining and then analysed on a BD FACS Aria cytometer. Data analysis was performed using FlowJo software 9.9.4 (Threestar). All flow cytometry antibodies were purchased from BioLegend. In this study, CD4+ cells refer to cell populations that were gated CD3 positive and CD8 negative. CD8+ cells refer to cell populations that were gated CD3 positive and CD8 negative.
Kill assay (co-culture cytotoxicity)
To interrogate AAV-SB-CAR T-cell killing efficacy, 1 × 105 of NALM6-GL (GFP-Luciferase), 1 × 105 of MM.1R-GL and 5 × 104 of MCF7-PL (Puromycin-Luciferase) cancer cell lines per well per 100 μl T-cell media were seeded in 96-well plates. Corresponding CAR T cells or CAR NK cells were then added according to various effector-to-target (T/NK cell: cancer cell) ratios. All input CAR T cells or CAR NK cells were normalized by CAR-positive percentage to make sure each well received a consistent number of CAR-positive cells. Cytolysis was measured through luciferase assays. About 150 μg ml−1 D-luciferin (PerkinElmer) was added to the plate using a multi-channel pipette. Following an ~5 min incubation at room temperature, luciferin intensity was measured by a plate reader (PerkinElmer).
In vivo CAR T-cell functionality testing
NOD-scid IL2Rgammanull mice were intravenously injected with 5 × 105
NALM6-GL cancer cells. After 4 days of cancer inoculation, 5 × 106 CD22. CAR T cells were injected in the tail vein as treatments. Bioluminescent imaging was performed via IVIS system to monitor leukaemia progression. Animal survival study followed an approved death-as-endpoint protocol.
In vitro mRNA transcription
There were two sources for the mRNA used in this study: (1) commercial synthesis by TriLink Biotechnologies (TriLink mRNA was used in Figs. 2a and 4, Extended Data Figs. 2, 3, 4 and 6 and Supplementary Fig. 1) and (2) in vitro transcription (used in experiments in figures otherwise) from the SB100X plasmid using the HiScribe T7 ARCA mRNA (with tailing) Kit (NEB). Following RNA transcription in vitro, DNase treatment and poly-A tailing, RNA purification was conducted using the Monarch RNA Cleanup Kit (50 μg) (NEB). After the concentration of the product was measured via Nanodrop (with default RNA settings), the RNA was aliquoted and stored at −80 °C. RNA was thawed on ice shortly before use in electroporation.
Gene transfer/CAR delivery into human immune cells
The vast majority of electroporation experiments were done using a Neon System (ThermoFisher). Before electroporation, cells were collected, washed and counted. About 5 × 105 to 3 × 106 cells were used per reaction, depending on the specific experiment. Per 1 million cells, 1 μg of SB100X mRNA was used. about 100 μl Buffer R with the cell and SB100X mRNA mixture was loaded into the Neon pipette, carefully avoiding the production of bubbles. The electroporation parameter was set at 1,600 V, 10 ms and 3 pulses for T cells, THP-1 cells and NK-92 cells, and 1,900 V, 30 ms and 1 pulse for macrophages. Cells were immediately transferred to a 24-well plate with pre-warmed media after electroporation. Depending on the experiment, specific quantities of AAV were then added to the cells at defined time points versus electroporation (details in each figure panel and the panel’s legend, mostly 0 h if not specified otherwise). Electroporation using Maxcyte system follows a similar procedure except with the manufacturer’s suggested electroporation presets. Lentiviral transduction followed standard protocols, mostly following a previous study78, with the conditions specified in the figure legends.
Insertion site library preparation and sequencing
To conduct integration site analysis for the MAJESTIC method, we created a custom protocol to prepare libraries of insertion sites from a CAR T-cell generation experiment: CD8+ donor 4003 T cells (collected on day 14 after electroporation). The custom procedure was created by combining elements of protocols from Illumina’s NEBNext Ultra II FS DNA Library Prep Kit and Friedrich et al.79, with oligos obtained from the latter. From the non-sorted pool of T cells, roughly 106 CAR T cells were collected. Then, gDNA was isolated using the Qiagen Blood Mini Kit. DNA concentrations were quantified via Nanodrop. About 500 ng of gDNA were distributed into three separate tubes to serve as three technical replicates for further library preparations. Next, DNA was fragmented for 20 min using the Ultra II FS Enzyme Mix from NEB. About 100 μM splinkerette V1.2TS and V1.2BS oligos from Integrated DNA Technologies were annealed in an Eppendorf tube by heating the mixture to 98 °C for 10 min in a heat block and then unplugging the heat block to allow the reaction to cool to room temperature. The final 15 μM annealed splinkerette adaptor was ligated to the fragmented DNA reactions for 15 min at 20 °C using NEBNext Ultra II Ligation Master Mix and Ligation Enhancer. Size selection was performed to achieve an insert size distribution of roughly 200–350 bp using NEBNext Sample Purification beads: 30 μl for the first bead selection and 15 μl for the second selection.
Then a two-step PCR was performed, using NEBNext Ultra II Q5 Master Mix as the reaction buffer. The first PCR (98 °C for 30 s for 1 cycle, 98 °C for 10 s and 65 °C for 75 s for 18 cycles, and 65 °C for 5 min for 1 cycle) was used to amplify genomic fragments containing the SB left arm using two oligos: one specific to the splinkerette adaptor and another specific to the SB left arm. The second PCR (98 °C for 30 s for 1 cycle; 98 °C for 10 s and 65 °C for 75 s for 12 cycles, and 65 °C for 5 min for 1 cycle) was used to attach i7 index to the library. After each PCR, PCR clean-up using SPRIselect Purification Beads was performed. Quality control of each key step was performed by running 1 μl of sample on a Tapestation. To quantify each library, aliquots were first diluted to 1:10,000. A 1 nM to 0.01 pM dilution series of Illumina PhiX library was then prepared to serve as a standard. The qPCR reaction was prepared using 2× PowerUp SYBR Green, qPCR2.1 and qPCR 2.2 primers at a final concentration of 250 nM, and 5 μl of the diluted libraries in 20 μl total volume. Quantified libraries were diluted to 2 nM and then pooled in equal volumes and denatured according to the Miseq System Denature and Dilute libraries Guide. PhiX was spiked in at 50%, and the denatured pool was diluted to 8 pM and sequenced on Miseq system. A 300-cycle Miseq v2 kit was used to sequence the library with a single-end setting (150 cycles for R1 and 8 cycles for the index1). Two custom sequencing primers (Spl_tag_seq for the index tag and SB_R_pr_seq for the forward read of SleepyBeauty left-end libraries) were spiked into the illumine sequencing primers according to Illumina’s bulletin on ‘Spiking custom primers into the Illumina sequencing primers’ (https://support.illumina.com/bulletins/2016/04/spiking-custom-primers-into-the-illumina-sequencing-primers-.html)
Splinkerette data processing, analysis and visualization
Single-end FASTQ reads were quality trimmed with BBDuk80 using the settings trimq = 27 minlen = 80 maq = 30 qtrim = rl. Then, non-integrated AAV-SB sequences were removed by using a sequence specific to the AAV ITR and by using Cutadapt81 with the following settings: -g TATAGTCTAGAACGCGTGCG -e 0.1–overlap 15–discard-trimmed. Then, to trim out the transposon arms and keep only the sequences that were trimmed, we used Cutadapt with the settings -g ^ACTTCAACTG -e 0.1 -m 15–overlap 10–discard-untrimmed. Ten bases were removed from the 5′ end (cutadapt -u 10), and all reads were trimmed to a fixed length of 30 (cutadapt -l 30). Reads were mapped using hisat282 onto the HISAT2 indexed GRCh38 genome (obtained from http://daehwankimlab.github.io/hisat2/download/). SAMtools view was used to filter out mapped reads with a quality score of less than 30, and the files were subsequently converted to the .bed format using SAMtools view83 and bedtools bamtobed84. Genomic coordinate files in .bed format were loaded into R, keeping only the starting genomic coordinate. They were further processed and formatted into GRanges objects for data visualization. Key packages used for R processing and visualization include GenomicRanges85, genomation86, ggbio87, BRGenomics (https://mdeber.github.io), and pheatmap (https://cran.r-project.org/web/packages/pheatmap/pheatmap.pdf).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Extended Data
Extended Data Fig. 1 |. MAJESTIC CAR generation and optimization.
a, Schematic of AAV-SB-CD22.CAR, AAV-SB-BCMA.CAR, and SB100X constructs and key procedures: mRNA in vitro transcription, AAV production, mRNA electroporation, flow cytometry, and kill assay. b, Representative flow cytometry plots of AAV-SB-BCMA.CAR CD4 (left) and CD8 (right) T cells show the percentage of CAR-expressing cells. CD4 cells are defined as CD3+ and CD8− cells. c, Quantification of CD22.CAR T cell ratio of (Fig. 1b, c). d, Quantification of BCMA.CAR T ratio in human CD4 and CD8 T cells. In this figure, for optimization of conditions, each assay was done with one donor with three technical replicates. Donor 2 T cells were used in this figure.
Extended Data Fig. 2 |. Timepoint optimization, SB100X transposase mRNA titration, and MAJESTIC CAR T yield.
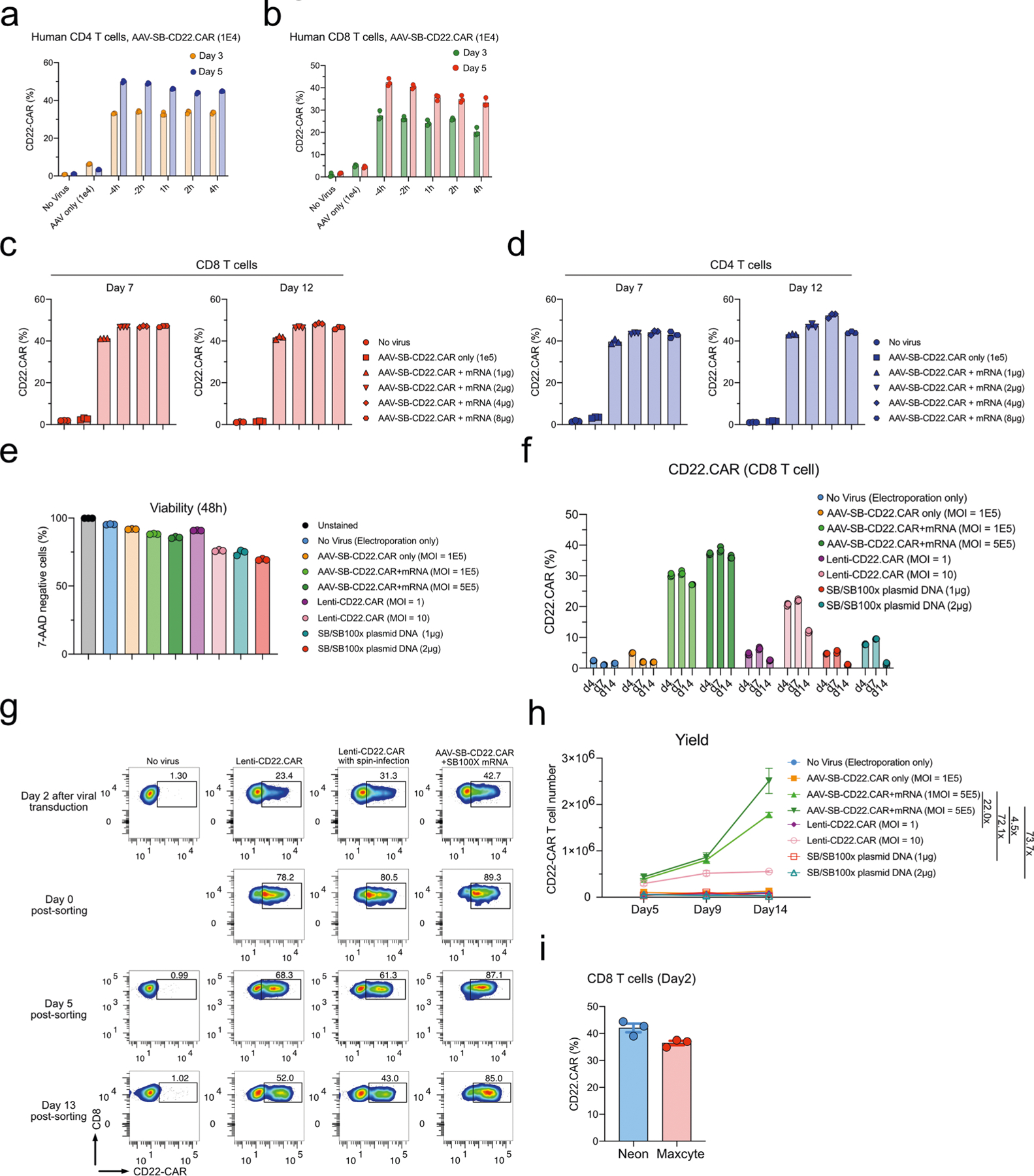
a, Quantification of CD22.CAR T cell ratio for CD4 T cells that were transduced with AAV-SB-CD22.CAR virus at various time points. b, Quantification of CD22.CAR T cell ratio for CD8 T cells that were transduced with AAV-SB-CD22.CAR virus at various time points. c-d, Representative flow cytometry plots of CD22.CAR T cells produced via AAV-SB and a titrated serial of SB100X mRNA. (c) CD8 T cells. (d) CD4 T cells. e, Quantification of the cell viability of CD8 T cells. f, Quantification of CAR T cells. g, Flow cytometry plots of CAR T cell ratios before and after sorting. h, CD22.CAR T cell yield quantification (yield = total viable cell count × CAR-positive percentage). Cells were split into 3 technical replicates after electroporation. Yield is calculated for each technical replicate separately. i, CAR+ T cell generation efficiency (CAR+%) of MAJESTIC using Neon and Maxcyte approaches. In this figure, for optimization of conditions, each assay was done with one donor with three technical replicates. Donor 2 and donor 0286 T cells were used in this figure.
Extended Data Fig. 3 |. Vector-copy-number quantification, immune-marker profiling, and functionality testing of MAJESTIC-produced CD8 CAR T cells.
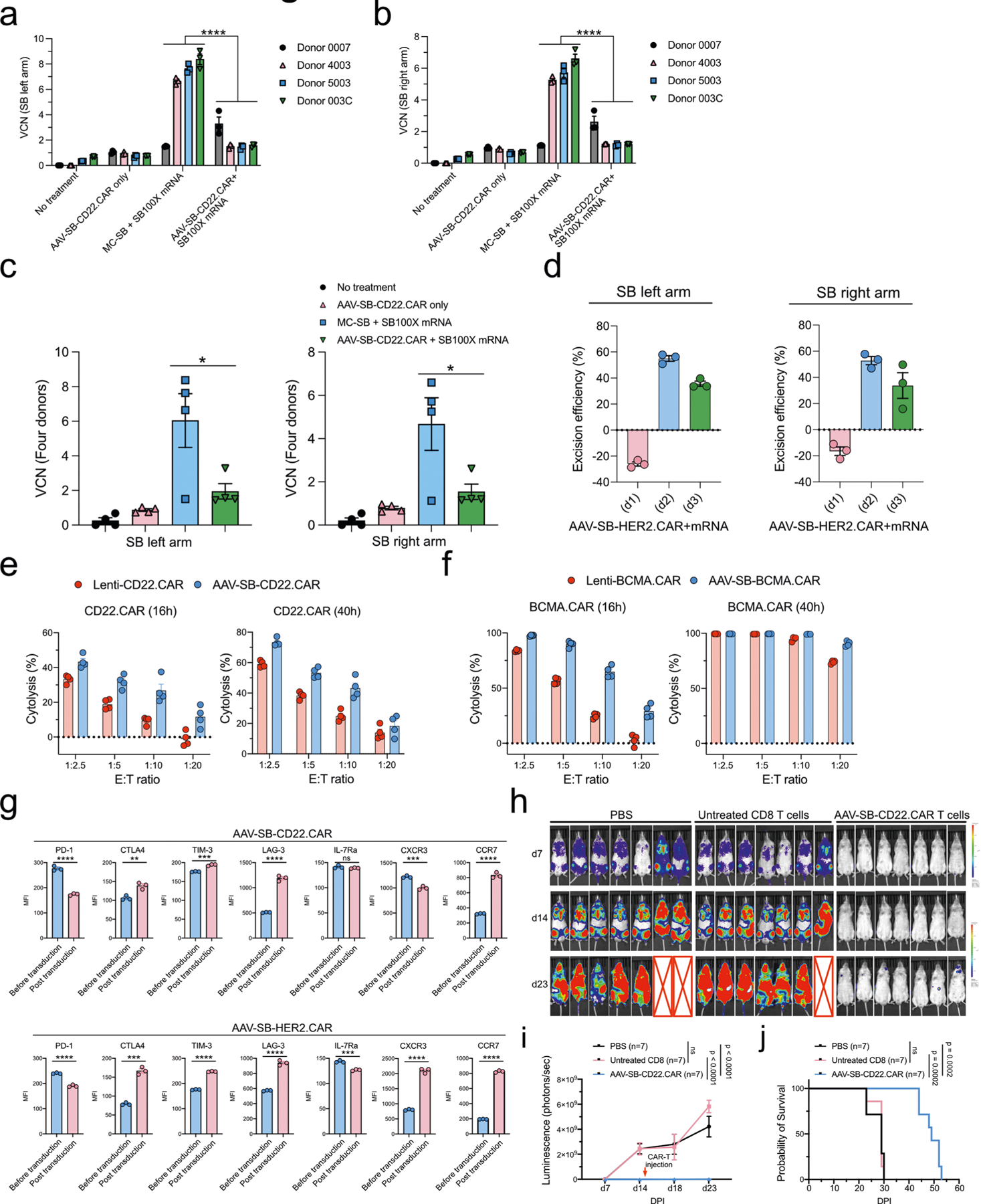
a-c, Vector copy number (VCN) quantification of MAJESTIC-manufactured CAR-T cells. Purified CAR T cells were collected for DNA extraction after three weeks of mRNA electroporation and viral transduction. (a) left arm probe, (b) right arm probe, (c) left panel: left arm probe, right panel: right arm probe. d, SB100X transposase excision efficiency evaluation. Left panel: left arm probe, right panel: right arm probe. e, Cytolysis analysis of NAML6-GL (NAML6 with GFP and luciferase reporters) cancer cells that were co-cultured with Lenti-CD22.CAR and AAV-SB-CD22.CAR T cells. CAR-Ts were seeded at various effector:target (E:T) ratios, and luciferase imaging was performed at two time points (16h and 40h). f, Cytolysis analysis of MM.1R-GL (MM.1R with GFP and luciferase reporters) cancer cells that were co-cultured with Lenti-BCMA.CAR and AAV-SB-BCMA.CAR T cells. CAR-Ts were seeded at various effector : target (E:T) ratios, and luciferase imaging was performed at two time points (16h and 40h). g, Exhaustion and memory marker expression in CD22-CAR and HER2-CAR T cells before and post transfection. Unpaired t tests were performed to evaluate statistical significance. h, Bioluminescent density of NSG mice that were injected with NALM6-GL cancer cells and with CD22-CAR therapy (n = 7 mice per group). i, Quantification of total luminescence for (h). n = 7 mice. Two-way ANOVA with multiple comparisons tests was performed to evaluate statistical significance. j, Survival curve of NALM6-GL-induced leukemia-bearing NSG mice that treated with PBS, untreated CD8 T cells, and AAV-SB-CD22.CAR T cells. Log-rank (Mantel-Cox) tests were performed to evaluate statistical significance. Donor 0007, 4003, 5003, 003C, 0286 T cells were used in this figure. Significance notes: ns - not significant; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Extended Data Fig. 4 |. Generation of various therapeutic immune cells by MAJESTIC.
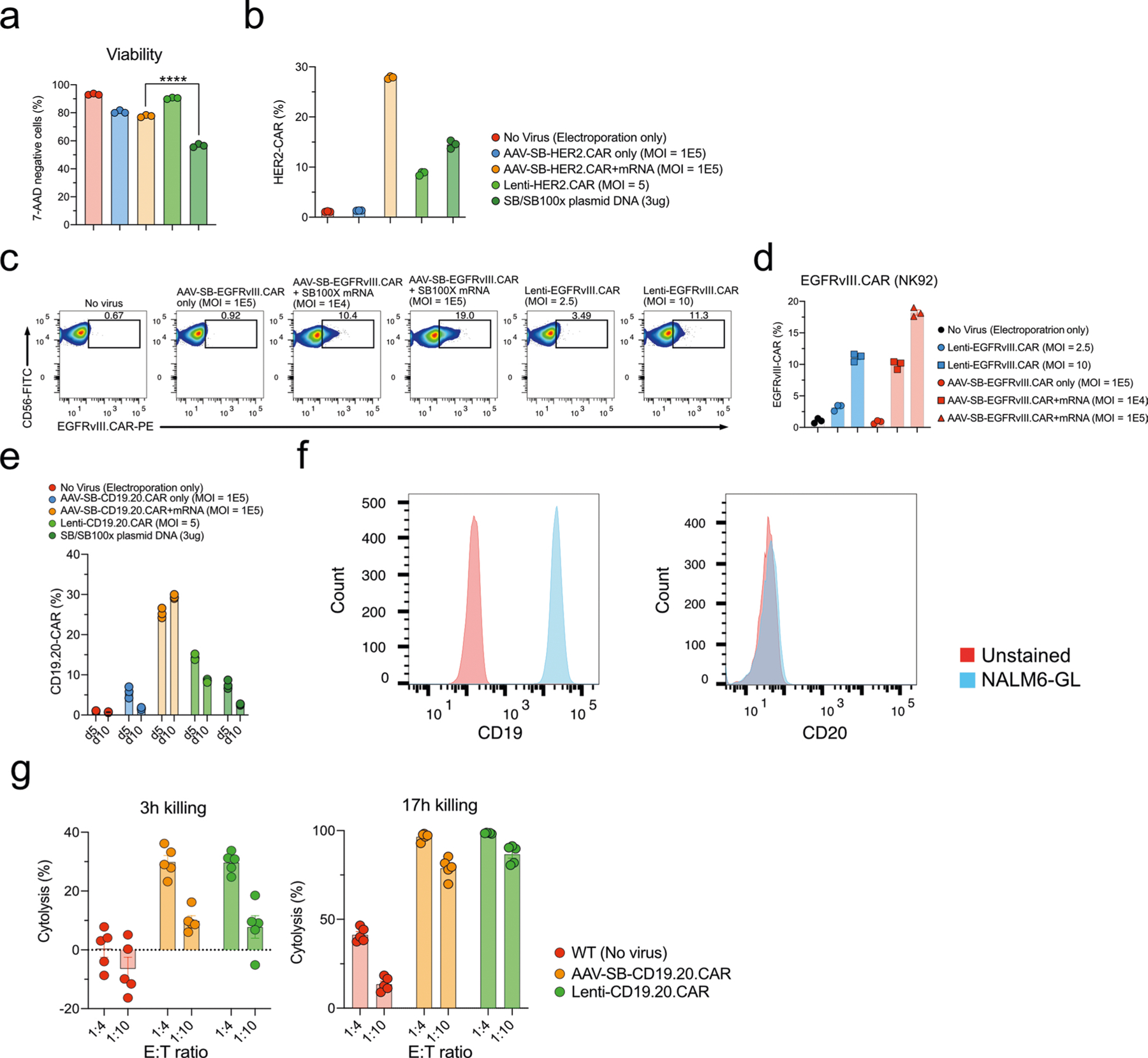
a, Quantification of the HER2.CAR T cell viability. b, Quantification of HER2.CAR-positive CD8 T cells. c, Representative flow-cytometry plots of EGFRvIII.CAR-NK92 cells were produced via either the SB system or lentiviral transduction. d, Quantification of (c). e, Quantification of CD19.20.CAR T cells. f, Flow cytometry detection of CD19 and CD20 expression in NALM6-GL cells. g, Cytolysis analysis of NALM6-GL cancer cells that were co-cultured with lenti-CD19.20.CAR and AAV-SB-CD19.20.CAR T cells. Donor 2 and donor VP2 T cells were used in this figure.
Extended Data Fig. 5 |. Generation of various therapeutic immune cells by MAJESTIC.
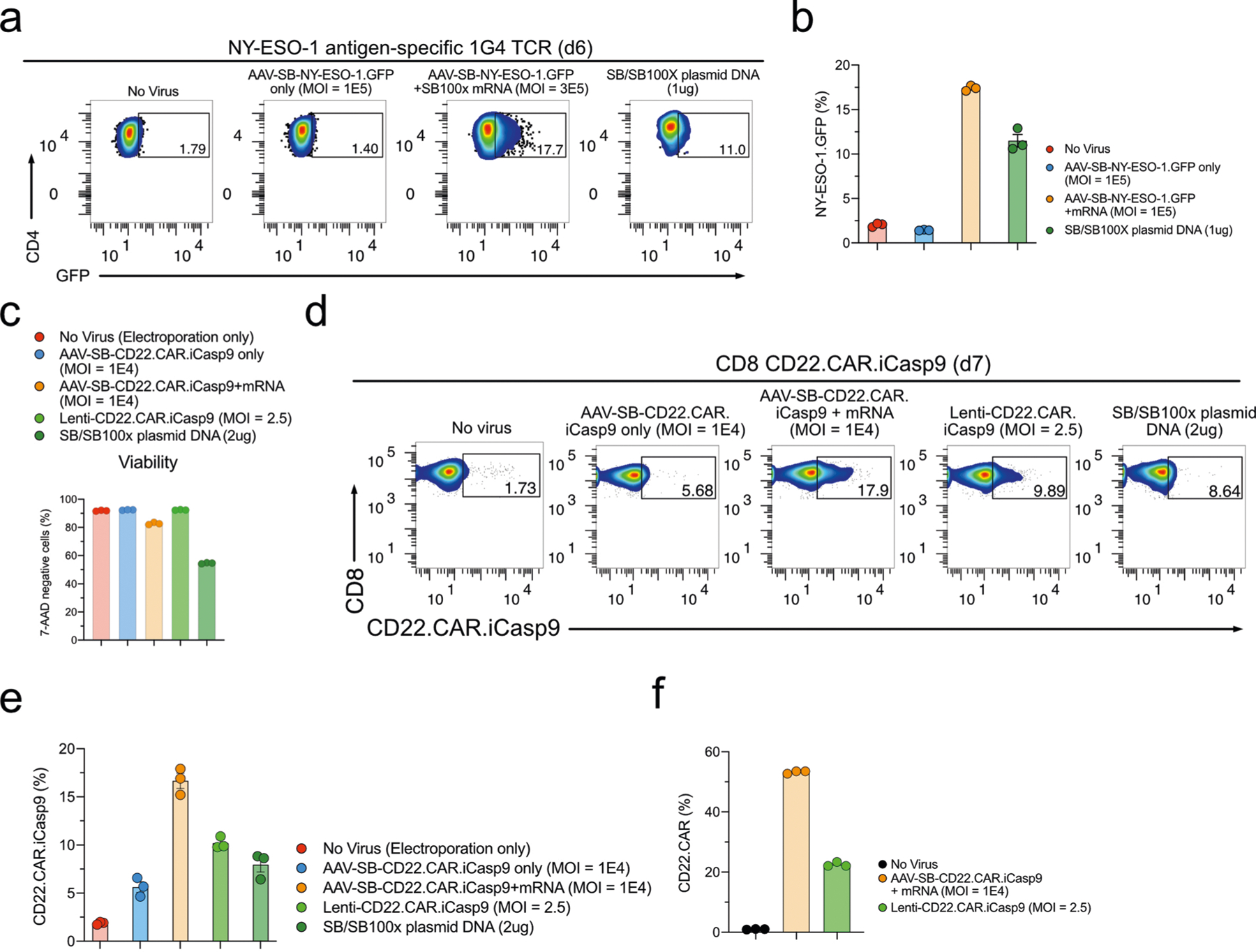
a, Representative flow cytometry plots of NY-ESO-1 T cells. b, Quantification of (a). c, Quantification of the CD22.CAR.iCasp T cell viability. d, Representative flow cytometry plots of CD22.CAR.iCasp9 T cells. e, Quantification of (d). f, Quantification of the CD22.CAR.iCasp9 T cells post antigen-specific cancer cells stimulation. In this figure, each assay has three technical replicates, donor 601c and donor 02 T cells were used in this figure.
Extended Data Fig. 6 |. Gene-delivery efficiency comparison of MC-SB/ SB100X mRNA with the MAJESTIC system.
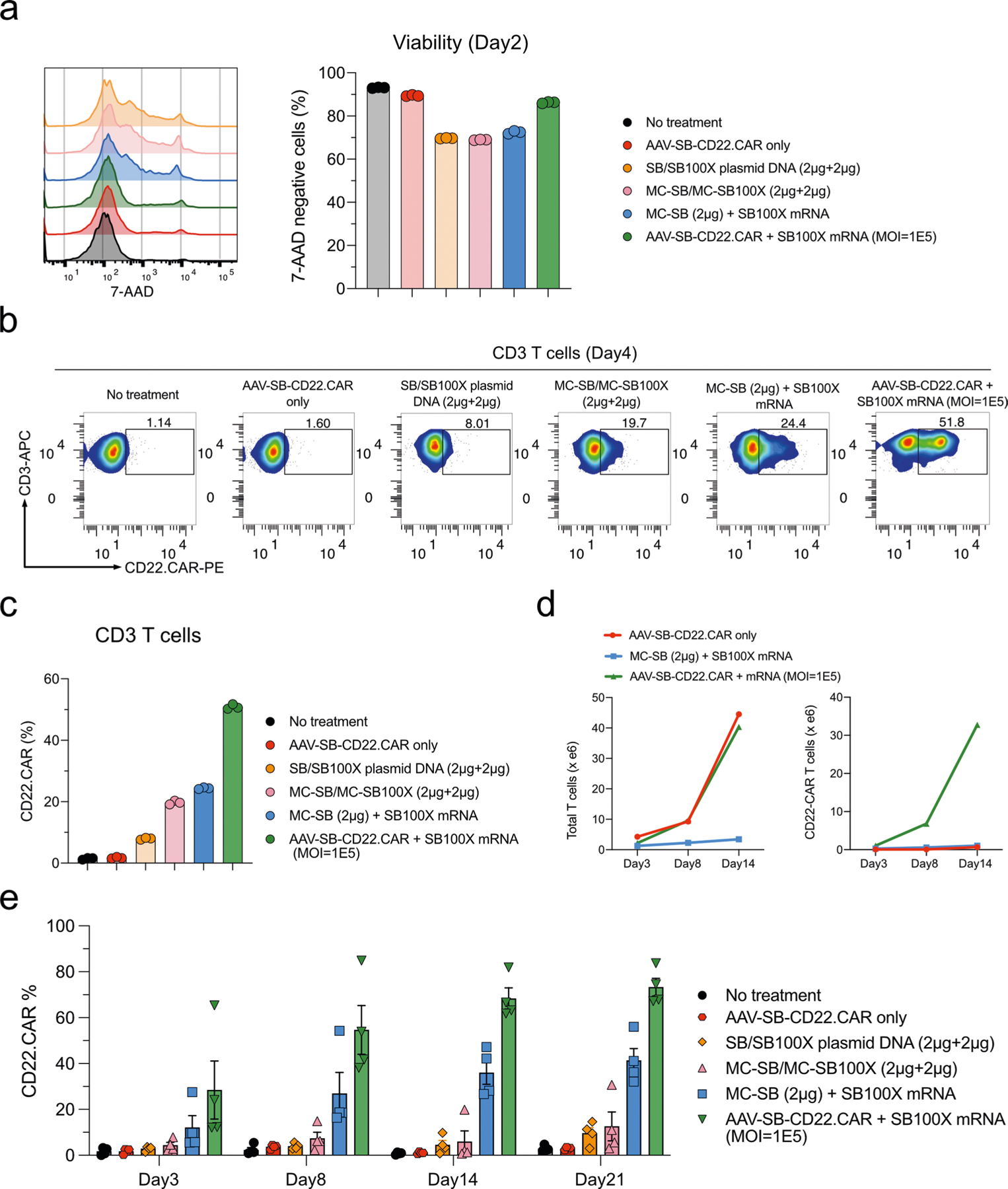
a, Flow-cytometry histogram overlays and bar plots of cell viability post-electroporation as measured with 7-AAD staining. b, Flow-cytometry data of CD22.CAR T cells from human primary CD3 T cells produced by plasmid transposon plasmid, transposon MC, transposon MC with mRNA-transposase, and MAJESTIC. c, Quantification of (b). d, Yield calculations (yield = CAR% * total viable cell count). All conditions started with an equal amount of primary T cells per replicate. Three CAR% replicates were averaged and then multiplied by the average of 2 cell count replicates. Left panel, total T cell count; Right panel, total CAR+ T cell count. e, Quantification of flow-cytometry data of CD22.CAR T cells from four human PBMCs (same data as Fig. 4e–f, plotted in dot-whisker plots).
Supplementary Material
Acknowledgements
We thank all members of the Chen Laboratory and of various entities at the University of Yale for discussions. We thank various Yale core facilities for technical support. In particular, we thank K. Tang and P. Renauer for technical assistance on Illumina sequencing and data analysis. We also thank C. Miskey for sharing processed sequence files from ref. 54. S.C. is supported by a Yale SBI/Genetics Startup Fund and by grants from NIH/NCI/NIDA (DP2CA238295, R01CA231112, R33CA225498, RF1DA048811), DoD (W81XWH-20-1-0072, W81XWH-21-10514), Alliance for Cancer Gene Therapy, Sontag Foundation (DSA), Pershing Square Sohn Cancer Research Alliance, Yale Cancer Center Pilot Award, Dexter Lu Gift, Ludwig Family Foundation, and Chenevert Family Foundation. S.Z.L. is supported by Yale College Fellowships.
Footnotes
Code availability
The scripts used to process the insertion site-mapping data are available at https://github.com/stanleyzlam/SB-CAR.
Competing interests
A patent related to this study was filed by Yale University (inventors: S.C., L.Y. and S.Z.L.) and licensed to Cellinfinity Bio, a Yale biotech start-up founded by S.C. S.C. is also a (co)founder of EvolveImmune Tx, Chen Consulting, Chen Tech and NumericGlobal, all unrelated to this study. The other authors declare no competing interests.
Extended data is available for this paper at https://doi.org/10.1038/s41551-023-01058-6.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41551-023-01058-6.
Data availability
The main data supporting the results in this study are available within the paper and its supplementary information. The raw and processed genome-sequence data from the splinkerette experiments are available from the NIH Sequence Read Archive/Gene Expression Omnibus under the accession number GSE220202 (token mtqrayyonvwfzmd). The raw and analysed datasets generated during the study are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
- 1.Hayes C Cellular immunotherapies for cancer. Ir. J. Med. Sci 190, 41–57 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laskowski T & Rezvani K Adoptive cell therapy: living drugs against cancer. J. Exp. Med 217, e20200377 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.June CH, O’Connor RS, Kawalekar OU, Ghassemi S & Milone MC CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Majzner RG & Mackall CL Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med 25, 1341–1355 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Upadhaya S et al. The clinical pipeline for cancer cell therapies. Nat. Rev. Drug Discov 20, 503–504 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Verdegaal EM et al. Neoantigen landscape dynamics during human melanoma–T cell interactions. Nature 536, 91–95 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Johnson LA et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114, 535–546 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas R et al. NY-ESO-1 based immunotherapy of cancer: current perspectives. Front. Immunol 9, 947 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu J et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 28, 917–927 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genssler S et al. Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. Oncoimmunology 5, e1119354 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q et al. Synergistic effects of cabozantinib and EGFR-specific CAR-NK-92 cells in renal cell carcinoma. J. Immunol. Res 2017, 6915912 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruschinski A et al. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proc. Natl Acad. Sci. USA 105, 17481–17486 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu E et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 32, 520–531 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu Y et al. Targeting CD20+ aggressive B-cell non-Hodgkin lymphoma by anti-CD20 CAR mRNA-modified expanded natural killer cells in vitro and in NSG mice. Cancer Immunol. Res 3, 333–344 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Hermanson DL, Moriarity BS & Kaufman DS Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 23, 181–192 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klichinsky M et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol 38, 947–953 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J. Hematol. Oncol 13, 153 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Themeli M et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat. Biotechnol 31, 928–933 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Stasi A et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med 365, 1673–1683 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah NN et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat. Med 26, 1569–1575 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Morgan RA & Boyerinas B Genetic modification of T cells. Biomedicines 4, 9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis J Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum. Gene Ther 16, 1241–1246 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Ciuffi A Mechanisms governing lentivirus integration site selection. Curr. Gene Ther 8, 419–429 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Hacein-Bey-Abina S et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302, 415–419 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Hacein-Bey-Abina S et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest 118, 3132–3142 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kustikova O et al. Clonal dominance of hematopoietic stem cells triggered by retroviral gene marking. Science 308, 1171–1174 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Zhou S et al. Evaluating the safety of retroviral vectors based on insertional oncogene activation and blocked differentiation in cultured thymocytes. Mol. Ther 24, 1090–1099 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naso MF, Tomkowicz B, Perry WL 3rd & Strohl WR Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs 31, 317–334 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eyquem J et al. Targeting a CAR to the TRAC locus with CRISPR/ Cas9 enhances tumour rejection. Nature 543, 113–117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai X et al. One-step generation of modular CAR-T cells with AAV-Cpf1. Nat. Methods 16, 247–254 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nahmad AD et al. Frequent aneuploidy in primary human T cells after CRISPR-Cas9 cleavage. Nat. Biotechnol 40, 1807–1813 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papathanasiou S et al. Whole chromosome loss and genomic instability in mouse embryos after CRISPR-Cas9 genome editing. Nat. Commun 12, 5855 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuccaro MV et al. Allele-specific chromosome removal after Cas9 cleavage in human embryos. Cell 183, 1650–1664.e15 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Wilson MH, Coates CJ & George AL Jr PiggyBac transposon-mediated gene transfer in human cells. Mol. Ther 15, 139–145 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Doherty JE et al. Hyperactive piggyBac gene transfer in human cells and in vivo. Hum. Gene Ther 23, 311–320 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kebriaei P, Izsvak Z, Narayanavari SA, Singh H & Ivics Z Gene therapy with the Sleeping Beauty transposon system. Trends Genet 33, 852–870 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Sebastian-Martin A, Barrioluengo V & Menendez-Arias L Transcriptional inaccuracy threshold attenuates differences in RNA-dependent DNA synthesis fidelity between retroviral reverse transcriptases. Sci. Rep 8, 627 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monjezi R et al. Enhanced CAR T-cell engineering using non-viral Sleeping Beauty transposition from minicircle vectors. Leukemia 31, 186–194 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Singh H et al. Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using Sleeping Beauty system and artificial antigen presenting cells. PLoS ONE 8, e64138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magnani CF et al. Sleeping Beauty-engineered CAR T cells achieve antileukemic activity without severe toxicities. J. Clin. Invest 130, 6021–6033 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chicaybam L et al. CAR T cells generated using Sleeping Beauty transposon vectors and expanded with an EBV-transformed lymphoblastoid cell line display antitumor activity in vitro and in vivo. Hum. Gene Ther 30, 511–522 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Geurts AM et al. Gene mutations and genomic rearrangements in the mouse as a result of transposon mobilization from chromosomal concatemers. PLoS Genet 2, e156 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu MA A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines 7, 37 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye L et al. In vivo CRISPR screening in CD8 T cells with AAV–Sleeping Beauty hybrid vectors identifies membrane targets for improving immunotherapy for glioblastoma. Nat. Biotechnol 37, 1302–1313 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mates L et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat. Genet 41, 753–761 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Francois A et al. Accurate titration of infectious AAV particles requires measurement of biologically active vector genomes and suitable controls. Mol. Ther. Methods Clin. Dev 10, 223–236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghorashian S et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat. Med 25, 1408–1414 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Chicaybam L et al. Transposon-mediated generation of CAR-T cells shows efficient anti B-cell leukemia response after ex vivo expansion. Gene Ther 27, 85–95 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Xia X, Zhang Y, Zieth CR & Zhang SC Transgenes delivered by lentiviral vector are suppressed in human embryonic stem cells in a promoter-dependent manner. Stem. Cells Dev 16, 167–176 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolacsek O et al. Reliable transgene-independent method for determining Sleeping Beauty transposon copy numbers. Mob. DNA 2, 5 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider D et al. A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J. Immunother. Cancer 5, 42 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Straathof KC et al. An inducible caspase 9 safety switch for T-cell therapy. Blood 105, 4247–4254 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sadelain M, Papapetrou EP & Bushman FD Safe harbours for the integration of new DNA in the human genome. Nat. Rev. Cancer 12, 51–58 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Querques I et al. A highly soluble Sleeping Beauty transposase improves control of gene insertion. Nat. Biotechnol 37, 1502–1512 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roth SL, Malani N & Bushman FD Gammaretroviral integration into nucleosomal target DNA in vivo. J. Virol 85, 7393–7401 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou AJ, Chen LC & Chen YY Navigating CAR-T cells through the solid-tumour microenvironment. Nat. Rev. Drug Discov 20, 531–550 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Dolgin E Cancer-eating immune cells kitted out with CARs. Nat. Biotechnol 38, 509–511 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Marofi F et al. CAR-NK cell: a new paradigm in tumor immunotherapy. Front. Oncol 11, 673276 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Bailey SR & Maus MV Gene editing for immune cell therapies. Nat. Biotechnol 37, 1425–1434 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Tang X et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am. J. Cancer Res 8, 1083–1089 (2018). [PMC free article] [PubMed] [Google Scholar]
- 61.Auwerx J The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte–macrophage differentiation. Experientia 47, 22–31 (1991). [DOI] [PubMed] [Google Scholar]
- 62.Enache OM et al. Cas9 activates the p53 pathway and selects for p53-inactivating mutations. Nat. Genet 52, 662–668 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miskey C et al. Engineered Sleeping Beauty transposase redirects transposon integration away from genes. Nucleic Acids Res 50, 2807–2825 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roth TL et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 559, 405–409 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu Y et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol 31, 822–826 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson KR et al. CRISPR off-target analysis in genetically engineered rats and mice. Nat. Methods 15, 512–514 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frock RL et al. Genome-wide detection of DNA double-stranded breaks induced by engineered nucleases. Nat. Biotechnol 33, 179–186 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsai SQ et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol 33, 187–197 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cameron P et al. Mapping the genomic landscape of CRISPR-Cas9 cleavage. Nat. Methods 14, 600–606 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Tsai SQ et al. CIRCLE-seq: a highly sensitive in vitro screen for genome-wide CRISPR-Cas9 nuclease off-targets. Nat. Methods 14, 607–614 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takata M et al. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J 17, 5497–5508 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang W et al. Hybrid adeno-associated viral vectors utilizing transposase-mediated somatic integration for stable transgene expression in human cells. PLoS ONE 8, e76771 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clauss J et al. Efficient non-viral T-cell engineering by Sleeping Beauty minicircles diminishing DNA toxicity and miRNAs silencing the endogenous T-cell receptors. Hum. Gene Ther 29, 569–584 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Jin Z et al. The hyperactive Sleeping Beauty transposase SB100X improves the genetic modification of T cells to express a chimeric antigen receptor. Gene Ther 18, 849–856 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kovac A et al. RNA-guided retargeting of Sleeping Beauty transposition in human cells. eLife 9, e53868 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muther N, Noske N & Ehrhardt A Viral hybrid vectors for somatic integration—are they the better solution? Viruses 1, 1295–1324 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balciunas D et al. Harnessing a high cargo-capacity transposon for genetic applications in vertebrates. PLoS Genet 2, e169 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ye L et al. A genome-scale gain-of-function CRISPR screen in CD8 T cells identifies proline metabolism as a means to enhance CAR-T therapy. Cell Metab 34, 595–614.e14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Friedrich MJ et al. Genome-wide transposon screening and quantitative insertion site sequencing for cancer gene discovery in mice. Nat. Protoc 12, 289–309 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Bushnell B 9th Annual Genomics of Energy & Environment Meeting (Walnut Creek, 2014). [Google Scholar]
- 81.Martin M Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17, 1 (2011). [Google Scholar]
- 82.Kim D, Paggi JM, Park C, Bennett C & Salzberg SL Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol 37, 907–915 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li H et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Quinlan AR & Hall IM BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lawrence M et al. Software for computing and annotating genomic ranges. PLoS Comput. Biol 9, e1003118 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Akalin A, Franke V, Vlahovicek K, Mason CE & Schubeler D Genomation: a toolkit to summarize, annotate and visualize genomic intervals. Bioinformatics 31, 1127–1129 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Yin T, Cook D & Lawrence M ggbio: an R package for extending the grammar of graphics for genomic data. Genome Biol 13, R77 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The main data supporting the results in this study are available within the paper and its supplementary information. The raw and processed genome-sequence data from the splinkerette experiments are available from the NIH Sequence Read Archive/Gene Expression Omnibus under the accession number GSE220202 (token mtqrayyonvwfzmd). The raw and analysed datasets generated during the study are available from the corresponding author on reasonable request. Source data are provided with this paper.


