Abstract
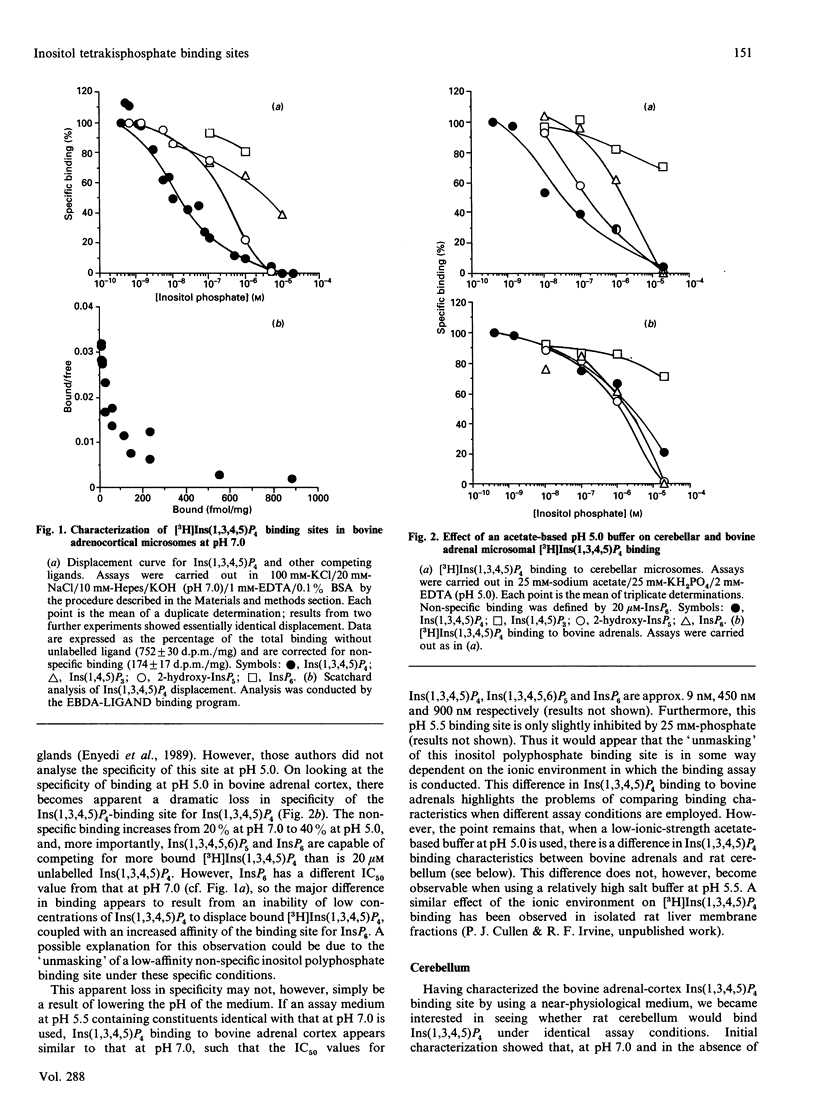
1. Ins(1,3,4,5)P4 binding sites were studied in cerebellar and hepatic microsomes from rat, and in bovine adrenal-cortical microsomes. 2. At pH 7.0, all three tissues showed specific binding, with Ins(1,3,4,5)P4 being the most potent competing ligand of those tested [which included Ins(1,4,5)P3, Ins(1,3,4,5,6)P5 and InsP6] and Scatchard analysis suggested two sites; a site with high affinity and high specificity [Kd (1-6) x 10(-9) M] and a site with low affinity and low specificity [Kd (2-6) x 10(-7) M]. 3. At pH 5.5, cerebellar and bovine adrenal microsomes showed similar binding properties: two affinities with a similar specificity for Ins(1,3,4,5)P4 as at pH 7.0. 4. However, when assayed in a low-ionic strength acetate-based buffer at pH 5.0, cerebellar microsomes retain specific Ins(1,3,4,5)P4 binding sites, whereas bovine adrenal and hepatic microsomal binding sites lose much of their specificity, as InsP6 and Ins(1,3,4,5,6)P5 are equally as potent as Ins(1,3,4,5)P4. 5. Pi (25 mM), which is frequently included in Ins(1,3,4,5)P4 binding assays, had a small inhibitory effect on binding of cerebellar and adrenal microsomes at pH 5.5, but a large effect at pH 7.0, so that a considerable decrease occurs in the amount of specific binding at pH 5.5 compared with that at pH 7.0, if Pi is omitted from the binding assay. 6. Cerebellar and adrenal microsomes were used in a ligand-displacement mass assay (conducted under near-physiological conditions, at pH 7.0) on extracts of cerebral-cortex slices stimulated with agonists, and both preparations faithfully detected the increases in Ins(1,3,4,5)P4 that occurred, implying that Ins(1,3,4,5)P4 is the principal ligand on these binding sites in intact cells. 7. Apparent contradictions in the literature with regard to Ins(1,3,4,5)P4 binding sites in neuronal and peripheral tissues can be largely accounted for by the data, and the properties of the binding sites detected at physiological pH are consistent with the possibility that they are putative receptors for the proposed second-messenger role for Ins(1,3,4,5)P4.
Full text
PDF

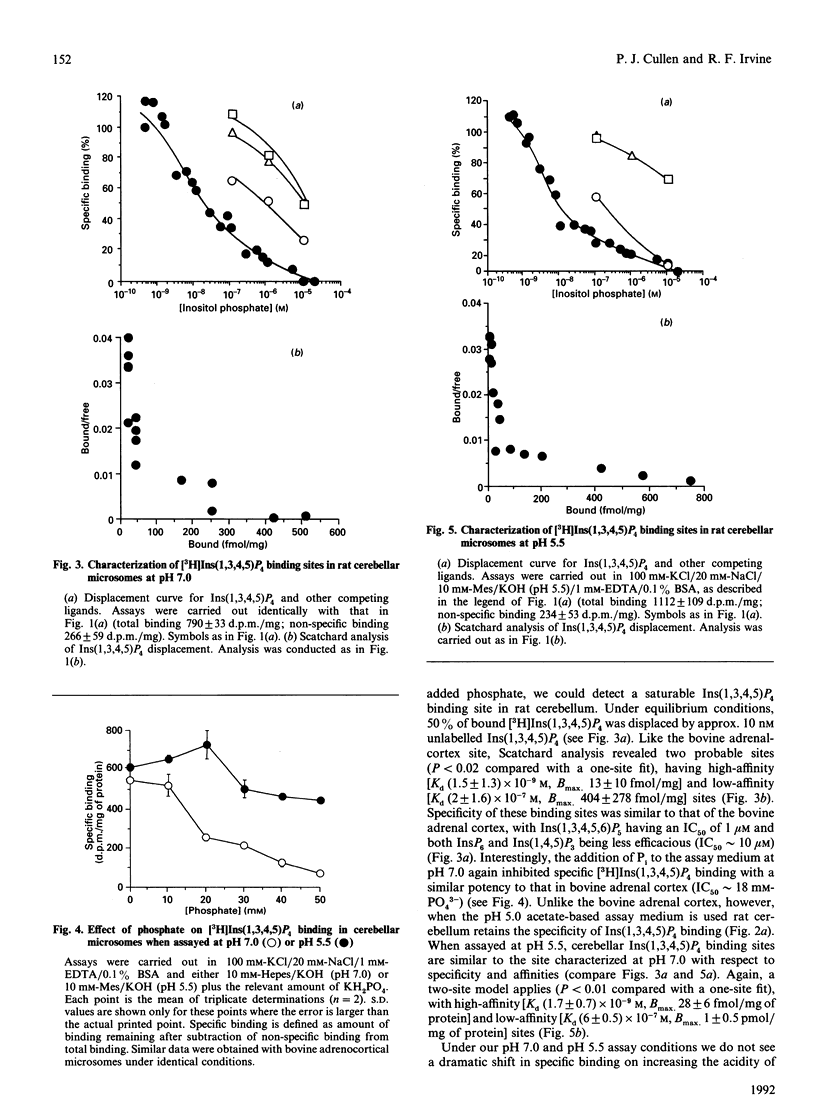


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Irvine R. F. Specific binding sites for [3H]inositol(1,3,4,5)tetrakisphosphate on membranes of HL-60 cells. Biochem Biophys Res Commun. 1987 Dec 16;149(2):680–685. doi: 10.1016/0006-291x(87)90421-9. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Nahorski S. R. Neurotransmitter and depolarization-stimulated accumulation of inositol 1,3,4,5-tetrakisphosphate mass in rat cerebral cortex slices. J Neurochem. 1990 Jun;54(6):2138–2141. doi: 10.1111/j.1471-4159.1990.tb04920.x. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Willcocks A. L., Mulloy B., Potter B. V., Nahorski S. R. Characterization of inositol 1,4,5-trisphosphate- and inositol 1,3,4,5-tetrakisphosphate-binding sites in rat cerebellum. Biochem J. 1991 Mar 15;274(Pt 3):861–867. doi: 10.1042/bj2740861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P. J., Irvine R. F., Drøbak B. K., Dawson A. P. Inositol 1,3,4,5-tetrakisphosphate causes release of Ca2+ from permeabilized mouse lymphoma L1210 cells by its conversion into inositol 1,4,5-trisphosphate. Biochem J. 1989 May 1;259(3):931–933. doi: 10.1042/bj2590931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donié F., Reiser G. A novel, specific binding protein assay for quantitation of intracellular inositol 1,3,4,5-tetrakisphosphate (InsP4) using a high-affinity InsP4 receptor from cerebellum. FEBS Lett. 1989 Aug 28;254(1-2):155–158. doi: 10.1016/0014-5793(89)81029-4. [DOI] [PubMed] [Google Scholar]
- Donié F., Reiser G. Purification of a high-affinity inositol 1,3,4,5-tetrakisphosphate receptor from brain. Biochem J. 1991 Apr 15;275(Pt 2):453–457. doi: 10.1042/bj2750453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi P., Brown E., Williams G. Distinct binding sites for Ins(1,4,5)P3 and Ins(1,3,4,5)P4 in bovine parathyroid glands. Biochem Biophys Res Commun. 1989 Feb 28;159(1):200–208. doi: 10.1016/0006-291x(89)92423-6. [DOI] [PubMed] [Google Scholar]
- Enyedi P., Williams G. H. Heterogenous inositol tetrakisphosphate binding sites in the adrenal cortex. J Biol Chem. 1988 Jun 15;263(17):7940–7942. [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. Inositol tetrakisphosphate as a second messenger: confusions, contradictions, and a potential resolution. Bioessays. 1991 Aug;13(8):419–427. doi: 10.1002/bies.950130810. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Berridge M. J. Specificity of inositol phosphate-stimulated Ca2+ mobilization from Swiss-mouse 3T3 cells. Biochem J. 1986 Nov 15;240(1):301–304. doi: 10.1042/bj2400301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S., Wakelam M. J. The Ins(1,4,5)P3 binding site of bovine adrenocortical microsomes: function and regulation. Biochem J. 1989 Jun 1;260(2):593–596. doi: 10.1042/bj2600593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Reiser G., Schäfer R., Donié F., Hülser E., Nehls-Sahabandu M., Mayr G. W. A high-affinity inositol 1,3,4,5-tetrakisphosphate receptor protein from brain is specifically labelled by a newly synthesized photoaffinity analogue, N-(4-azidosalicyl)aminoethanol(1)-1-phospho-D-myo-inositol 3,4,5-trisphosphate. Biochem J. 1991 Dec 1;280(Pt 2):533–539. doi: 10.1042/bj2800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W., Richardson A. Structure and function of inositol trisphosphate receptors. Pharmacol Ther. 1991;51(1):97–137. doi: 10.1016/0163-7258(91)90043-l. [DOI] [PubMed] [Google Scholar]
- Theibert A. B., Estevez V. A., Ferris C. D., Danoff S. K., Barrow R. K., Prestwich G. D., Snyder S. H. Inositol 1,3,4,5-tetrakisphosphate and inositol hexakisphosphate receptor proteins: isolation and characterization from rat brain. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3165–3169. doi: 10.1073/pnas.88.8.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theibert A. B., Supattapone S., Worley P. F., Baraban J. M., Meek J. L., Snyder S. H. Demonstration of inositol 1,3,4,5-tetrakisphosphate receptor binding. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1283–1289. doi: 10.1016/s0006-291x(87)80272-3. [DOI] [PubMed] [Google Scholar]


