Abstract
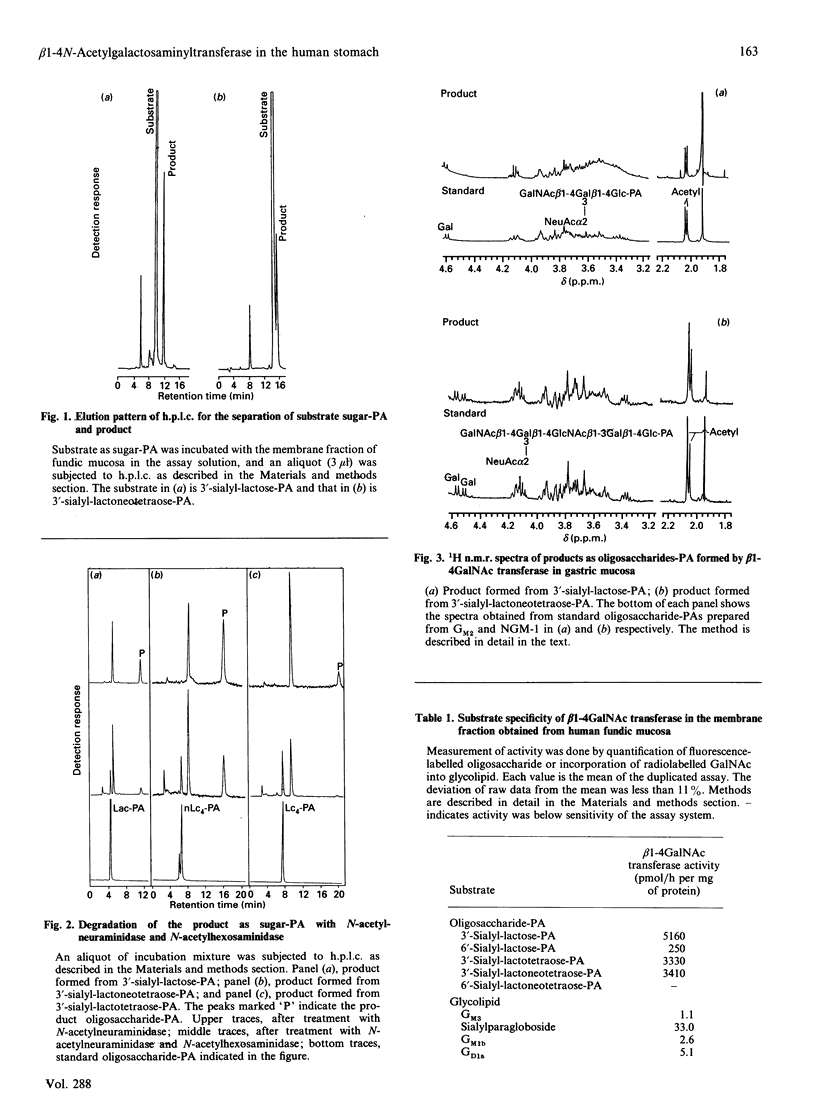
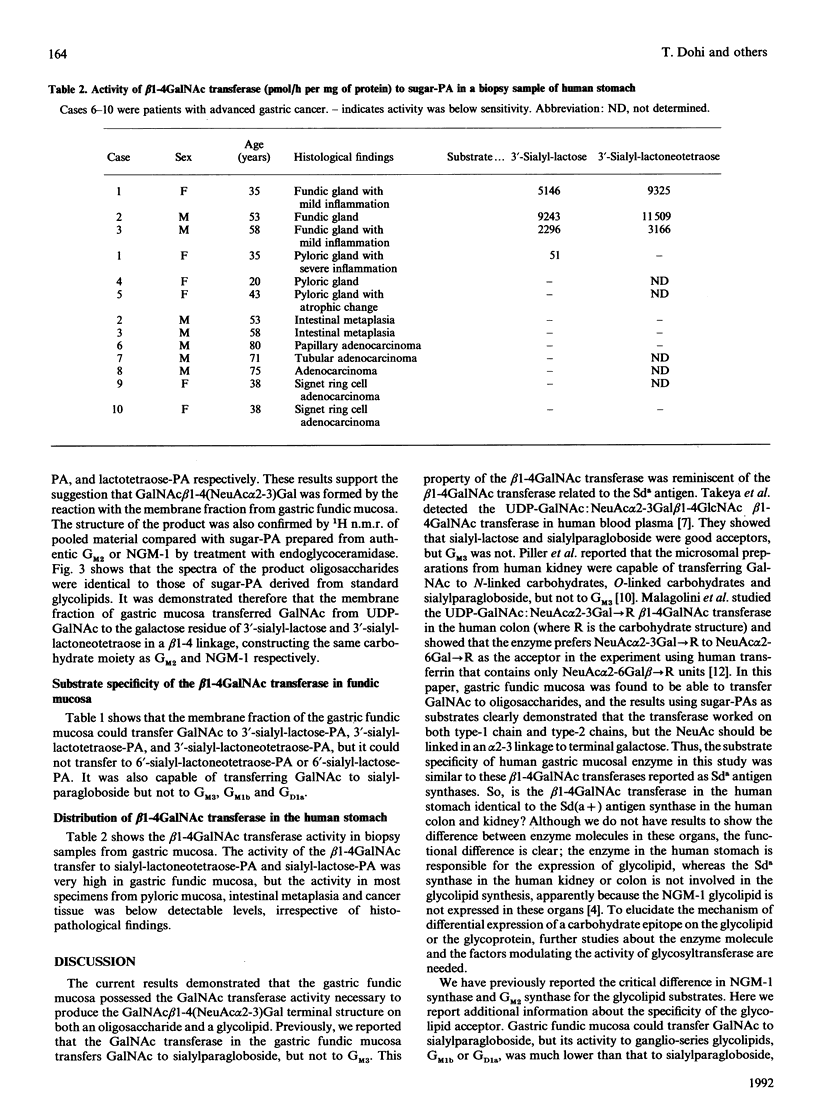
The detailed substrate specificity of the UDP-GalNAc:sialylparagloboside N-acetylgalactosaminyltransferase to form the Sd(a+) blood group active carbohydrate determinant GalNAc beta 1-4(NeuAc alpha 2-3)Gal was studied using a membrane fraction prepared from human gastric fundic mucosa. Various sialosylated oligosaccharides and gangliosides were examined as acceptor substrates. Oligosaccharide substrates were fluorescence-labelled with 2-aminopyridine, and the transferase activity was quantified by h.p.l.c. using a reversed-phase column. The structures of the products were determined by glycosidase degradation and proton n.m.r. 3'-Sialyl-lactose (II3NeuAcLac), 3'-sialyl-lactotetraose (IV3NeuAcLc4), and 3'-sialyl-lactoneotetraose (IV3NeuAcnLc4) were good substrates for the beta 1-4GalNAc transferase in gastric fundic mucosa, but 6'-sialyl-lactoneotetraose (IV6NeuAcnLc4) or 6'-sialyl-lactose (II6NeuAcLac) were not. Gangliosides with a terminal NeuAc alpha 2-3Gal residue such as GM3, sialylparagloboside, GM1b and GD1a were also studied. The activity of beta 1-4GalNAc transfer to sialylparagloboside was much higher than that to GM2, GM1b or GD1a in spite of them having the same terminal residue. Measurement of the activity of the beta 1-4GalNAc transferase in biopsy specimens demonstrated that the activity was localized in gastric fundic mucosa and was absent in pyloric mucosa, intestinal metaplasia and gastric cancer tissue. Thus the beta 1-4GalNAc transferase present specifically in fundic mucosa required a NeuAc alpha 2-3Gal residue connected to either type-1-chain or type-2-chain oligosaccharides. In glycolipids, the acceptor specificity was restricted to NeuAc alpha 2-3Gal beta 1-4GlcNAc because the NeuAc alpha 2-3Gal beta 1-3GalNAc structure in ganglio-series glycolipids was not a good acceptor substrate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dohi T., Hanai N., Yamaguchi K., Oshima M. Localization of UDP-GalNAc:NeuAc alpha 2,3Gal-R beta 1,4(GalNAc to Gal)N-acetylgalactosaminyltransferase in human stomach. Enzymatic synthesis of a fundic gland-specific ganglioside and GM2. J Biol Chem. 1991 Dec 15;266(35):24038–24043. [PubMed] [Google Scholar]
- Dohi T., Ohta S., Hanai N., Yamaguchi K., Oshima M. Sialylpentaosylceramide detected with anti-GM2 monoclonal antibody. Structural characterization and complementary expression with GM2 in gastric cancer and normal gastric mucosa. J Biol Chem. 1990 May 15;265(14):7880–7885. [PubMed] [Google Scholar]
- Donald A. S., Soh C. P., Yates A. D., Feeney J., Morgan W. T., Watkins W. M. Structure, biosynthesis and genetics of the Sda antigen. Biochem Soc Trans. 1987 Aug;15(4):606–608. doi: 10.1042/bst0150606. [DOI] [PubMed] [Google Scholar]
- Gillard B. K., Blanchard D., Bouhours J. F., Cartron J. P., van Kuik J. A., Kamerling J. P., Vliegenthart J. F., Marcus D. M. Structure of a ganglioside with Cad blood group antigen activity. Biochemistry. 1988 Jun 28;27(13):4601–4606. doi: 10.1021/bi00413a003. [DOI] [PubMed] [Google Scholar]
- Hase S., Ibuki T., Ikenaka T. Reexamination of the pyridylamination used for fluorescence labeling of oligosaccharides and its application to glycoproteins. J Biochem. 1984 Jan;95(1):197–203. doi: 10.1093/oxfordjournals.jbchem.a134585. [DOI] [PubMed] [Google Scholar]
- Hiraiwa N., Tsuyuoka K., Li Y. T., Tanaka M., Seno T., Okubo Y., Fukuda Y., Imura H., Kannagi R. Gangliosides and sialoglycoproteins carrying a rare blood group antigen determinant, Cad, associated with human cancers as detected by specific monoclonal antibodies. Cancer Res. 1990 Sep 1;50(17):5497–5503. [PubMed] [Google Scholar]
- Ilyas A. A., Li S. C., Chou D. K., Li Y. T., Jungalwala F. B., Dalakas M. C., Quarles R. H. Gangliosides GM2, IV4GalNAcGM1b, and IV4GalNAcGC1a as antigens for monoclonal immunoglobulin M in neuropathy associated with gammopathy. J Biol Chem. 1988 Mar 25;263(9):4369–4373. [PubMed] [Google Scholar]
- Itoh T., Li Y. T., Li S. C., Yu R. K. Isolation and characterization of a novel monosialosylpentahexosyl ceramide from Tay-Sachs brain. J Biol Chem. 1981 Jan 10;256(1):165–169. [PubMed] [Google Scholar]
- Kawano M., Honke K., Tachi M., Gasa S., Makita A. An assay method for ganglioside synthase using anion-exchange chromatography. Anal Biochem. 1989 Oct;182(1):9–15. doi: 10.1016/0003-2697(89)90709-4. [DOI] [PubMed] [Google Scholar]
- Malagolini N., Dall'Olio F., Di Stefano G., Minni F., Marrano D., Serafini-Cessi F. Expression of UDP-GalNAc:NeuAc alpha 2,3Gal beta-R beta 1,4(GalNAc to Gal) N-acetylgalactosaminyltransferase involved in the synthesis of Sda antigen in human large intestine and colorectal carcinomas. Cancer Res. 1989 Dec 1;49(23):6466–6470. [PubMed] [Google Scholar]
- Morton J. A., Pickles M. M., Terry A. M. The Sda blood group antigen in tissues and body fluids. Vox Sang. 1970 Nov-Dec;19(5):472–482. doi: 10.1111/j.1423-0410.1970.tb01779.x. [DOI] [PubMed] [Google Scholar]
- Morton J. A., Pickles M. M., Vanhegan R. I. The Sda antigen in the human kidney and colon. Immunol Invest. 1988 May;17(3):217–224. doi: 10.3109/08820138809052961. [DOI] [PubMed] [Google Scholar]
- Piller F., Blanchard D., Huet M., Cartron J. P. Identification of a alpha-NeuAc-(2----3)-beta-D-galactopyranosyl N-acetyl-beta-D-galactosaminyltransferase in human kidney. Carbohydr Res. 1986 Jun 1;149(1):171–184. doi: 10.1016/s0008-6215(00)90376-8. [DOI] [PubMed] [Google Scholar]
- Pohlentz G., Klein D., Schwarzmann G., Schmitz D., Sandhoff K. Both GA2, GM2, and GD2 synthases and GM1b, GD1a, and GT1b synthases are single enzymes in Golgi vesicles from rat liver. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7044–7048. doi: 10.1073/pnas.85.19.7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Månsson J. E., Li Y. T. Isolation and structural determination of a novel ganglioside, a disialosylpentahexosylceramide from human brain. J Biol Chem. 1973 Jan 25;248(2):740–742. [PubMed] [Google Scholar]
- Takeya A., Hosomi O., Kogure T. Identification and characterization of UDP-GalNAc: NeuAc alpha 2-3Gal beta 1-4Glc(NAc) beta 1-4(GalNAc to Gal)N-acetylgalactosaminyltransferase in human blood plasma. J Biochem. 1987 Jan;101(1):251–259. doi: 10.1093/oxfordjournals.jbchem.a121898. [DOI] [PubMed] [Google Scholar]
- Taniguchi N., Nishikawa A., Fujii S., Gu J. G. Glycosyltransferase assays using pyridylaminated acceptors: N-acetylglucosaminyltransferase III, IV, and V. Methods Enzymol. 1989;179:397–408. doi: 10.1016/0076-6879(89)79139-4. [DOI] [PubMed] [Google Scholar]
- Vrionis F. D., Wikstrand C. J., Fredman P., Månsson J. E., Svennerholm L., Bigner D. D. Five new epitope-defined monoclonal antibodies reactive with GM2 and human glioma and medulloblastoma cell lines. Cancer Res. 1989 Dec 1;49(23):6645–6651. [PubMed] [Google Scholar]


