ABSTRACT
Interbacterial antagonism involves all major phyla, occurs across the full range of ecological niches, and has great significance for the environment, clinical arena, and agricultural and industrial sectors. Though the earliest insight into interbacterial antagonism traces back to the discovery of antibiotics, a paradigm shift happened when it was learned that protein secretion systems (e.g., types VI and IV secretion systems) deliver toxic “effectors” against competitors. However, a link between interbacterial antagonism and the Gram-negative type II secretion system (T2SS), which exists in many pathogens and environmental species, is not evident in prior reviews on bacterial competition or T2SS function. A current examination of the literature revealed four examples of a T2SS or one of its known substrates having a bactericidal activity against a Gram-positive target or another Gram-negative. When further studied, the T2SS effectors proved to be peptidases that target the peptidoglycan of the competitor. There are also reports of various bacteriolytic enzymes occurring in the culture supernatants of some other Gram-negative species, and a link between these bactericidal activities and T2SS is suggested. Thus, a T2SS can be a mediator of interbacterial antagonism, and it is possible that many T2SSs have antibacterial outputs. Yet, at present, the T2SS remains relatively understudied for its role in interbacterial competition. Arguably, there is a need to analyze the T2SSs of a broader range of species for their role in interbacterial antagonism. Such investigation offers, among other things, a possible pathway toward developing new antimicrobials for treating disease.
KEYWORDS: type II secretion, T2SS, bacterial protein secretion, interbacterial competition, antibacterial effectors, bactericidal activity, bacteriolytic enzymes
INTERBACTERIAL ANTAGONISM AND THE EMERGENT ROLE OF PROTEIN SECRETION SYSTEMS
Recently, there has been a renewed and expanded interest in interbacterial antagonism, that is, when one bacterium compromises another’s viability (1 – 6). Interbacterial antagonism occurs across the full range of ecological niches and includes all major phyla. Thus, understanding its mechanisms has much significance for clinical, environmental, agricultural, and industrial arenas. The earliest insight into interbacterial antagonism traces back to the time when antibiotics were revealed, and since then, various metabolites, peptidic bacteriocins, colicins, and perforin-like proteins have demonstrated antibacterial activity (1 – 3, 7 – 13). In these cases, which encompass Gram-negative bacteria and Gram-positive bacteria, the antibacterial factors are released from the producer by cell lysis or via the action of the Sec-translocon, ABC-type transporters, efflux pumps, or outer membrane (OM) vesicles and thereafter diffuse toward the target bacterium (1, 2, 14 – 16). However, some of these antibacterial proteins, for example, WapA of Bacillus subtilis, are exported by the Sec-translocon not to the extracellular milieu but to the cognate bacterial surface and once there mediates a form of contact-dependent growth inhibition (3, 16 – 18). As a variation on this theme, SitA is transferred from the surface of Myxococcus xanthus to the target’s OM in a process called OM exchange (2, 19, 20).
A paradigm shift occurred when it was realized that protein secretion systems, which are multi-component, membrane-spanning apparatuses, can also mediate the delivery of toxic “effectors” into competitors (1 – 3, 14, 21, 22). The most widely studied of this type of secretion system is the type VI secretion system (T6SS). Present in various Gram-negative bacteria, the T6SS is a spear-like apparatus (i.e., a repurposed phage fiber) that directly contacts the competing bacteria and then injects lipases, nucleases, peptidoglycan hydrolases, and other effectors (23 – 30). We and others have shown that type IV secretion systems (T4SSs), which are present in a subset of Gram-negative bacteria and evolutionarily related to conjugation systems, can also promote contact-dependent, interbacterial killing by delivering multiple protein effectors (22, 31 – 40). Some conjugative T4SSs mediate interbacterial antagonism independently of protein or DNA-based cargo (5). Contact-dependent interbacterial killing has also been linked to the type VII secretion system of some Gram-positive bacteria (41 – 45) and a subclass of the Gram-negative type V secretion system known as contact-dependent inhibition (CDI) (14, 46 – 50). In yet another example, variants of the type I secretion system of some Gram-negative bacteria secrete bacteriocins into the extracellular milieu or deliver other toxic proteins to the producer’s surface for cell-to-cell antagonism (3, 21, 51, 52). Finally, a version of the Gram-negative type III secretion system helps M. xanthus degrade bacterial prey (19, 53), and there is speculation that effectors of the type IX secretion system of Bacteroidota hinder competitors (54, 55). Not surprisingly, many bacteria use multiple methods for antagonizing competitors, including contact-dependent and contact-independent mechanisms and the utilization of more than one secretion apparatus (2, 3, 22, 56). Yet, there is another type of protein secretion system, the type II secretion system (T2SS), which, though well studied for other reasons, has been largely overlooked for its role in interbacterial competition.
THE T2SS
Evolutionarily related to the type IV pilus apparatus, T2SSs mediate a multi-step form of protein secretion (57 – 65). Proteins to be secreted by this system (substrates) are first transported across the inner membrane by the Sec or Tat translocon. Once in the periplasm, the substrates assume their tertiary conformation and, in some cases, oligomerize. Finally, the folded substrates are transited across the OM by the T2SS apparatus. In this last step, the T2SS “pseudopilus” behaves like a piston or Archimedes screw to propel the substrates through the T2SS’s OM secretin and deliver them into the extracellular space. The T2SS apparatus is typically composed of 12 core proteins, although there are instances of some bacteria having fewer constituent parts (62). Finally, in some cases, additional chaperones aid with the stabilizing and secreting of the substrates (66, 67). What ultimately causes a substrate to be recognized by the T2SS apparatus is not clear but likely involves the protein’s tertiary structure (61, 68).
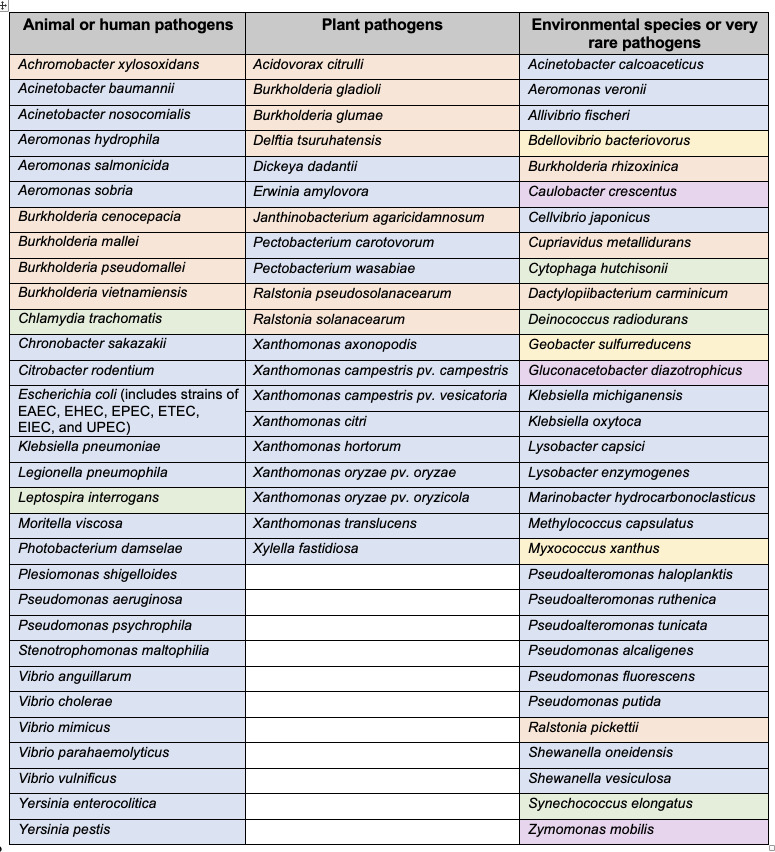
Although, at one time, referred to as the main terminal branch of the general secretory pathway (57, 69, 70), the T2SS is not universal in Gram-negative bacteria (71). Indeed, in its canonical form, the T2SS is mainly present in the Proteobacteria and, even there, is not 100% conserved (62, 72, 73). Hence, the T2SS is rightly considered as a specialized system that (only) a subset of Gram-negative bacteria has evolved for growth in the environment and/or infection of host organisms (62, 74). However, many human and animal pathogens are known to express a T2SS, including, among others, Acinetobacter baumannii, Burkholderia cenocepacia, Escherichia coli, Klebsiella pneumoniae, Legionella pneumophila, Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Vibrio cholerae, and Yersinia enterocolitica (Fig. 1) (62, 73, 75 – 85). T2SS-expressing plant pathogens include Dickeya dadantii, Erwinia amylovora, Pectobacterium carotovorum, Xanthomonas campestris, and Xylella fastidiosa, among others (Fig. 1) (62, 86 – 93). Just as the number of pathogenic species that have been shown to express a functional T2SS has increased in recent years, as reflected in Fig. 1, the number of processes ascribed to T2SSs during infection has steadily increased and currently encompasses tissue degradation in a range of body sites, plant cell wall degradation, and subversion of host defense factors, including complement, neutrophils, reactive oxygen species and mucus layers, suppression of immune signaling and cytokine destruction, adherence to surfaces, biofilm formation, invasion of and growth within host cells, host cell death and lysis, alterations in ion flux, reductions in blood coagulation, and nutrient assimilation after the breakdown of proteins, lipids, and carbohydrates (62, 94 – 113). T2SSs are also active in many environmental, non-pathogenic species (Fig. 1), expediting an expanding list of metabolic processes and symbioses, for example, iron, manganese, and iodate reduction, hydrocarbon degradation, and nutrient trafficking (62, 114 – 127). For many of the genera in Fig. 1, there are additional species in the genus that also carry the genes for a T2SS but functional analyses have not yet been reported (66, 73, 76, 128 – 144). In addition to the pathogenic and non-pathogenic genera listed in Fig. 1, there are many other genera within the Proteobacteria that encode the genes for a T2SS (that are distinguishable from the genes for a type IV pilus) and likely express T2SS-dependent proteins (Fig. 2) (73, 135, 145 – 156). The genes for T2SSs are typically present within the bacterial chromosome; however, there are examples of the system being encoded within a plasmid (108, 146, 157).
Fig 1.
Species in which a secreted protein/activity or phenotype is linked to the T2SS. Species belonging to the α-Proteobacteria are shaded in purple, β-Proteobacteria in orange, γ-Proteobacteria in blue, δ-Proteobacteria in yellow, and non-Proteobacteria in green. This is not necessarily an exhaustive list.
Fig 2.
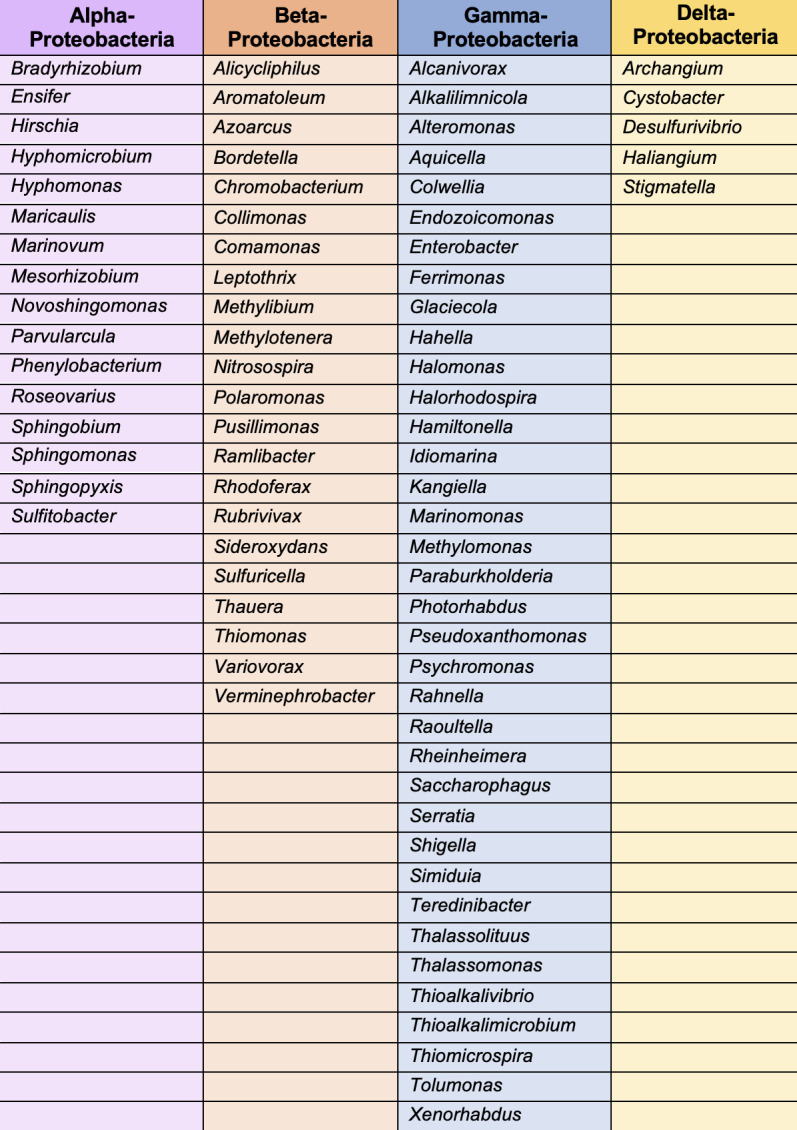
Additional genera within the Proteobacteria that carry genes for a T2SS. This is not necessarily an exhaustive list.
The output of a T2SS can range from one to dozens of proteins, encompassing a diverse array of peptidases, proteases, phosphatases, carbohydrate-degrading enzymes (e.g., cellulases, chitinases, and mucinases), lipolytic enzymes (lipases and phospholipases), nucleases (DNase and RNase), reductases, pore-forming proteins, ADP-ribosylating toxins, and novel proteins (62, 99, 102, 158 – 168). Although most T2SS substrates ultimately exist (only) in the extracellular milieu, some also locate to the surface of the expressing cell (62, 107, 111, 169 – 174). Some bacteria encode two or three T2SSs that might mediate the release of different sets of substrates (62, 153, 175). Despite the vast amount of work done on T2SSs, a significant role for these systems in interbacterial antagonism has not been described or posited in the many reviews on the T2SS that extend from 1990 to the present (2, 3, 14, 18, 21, 22, 57, 58, 60 – 62, 69 – 71, 73, 79, 114, 174, 176 – 222). Consequently, the impression has been that T2SSs are not important for interbacterial competition but are devoted to virulence or nutrient assimilation.
CONNECTIONS BETWEEN T2SSs AND INTERBACTERIAL ANTAGONISM
From the mid-1960s to the present, bacteriolytic enzymes have been detected in the culture supernatants of different environmental, Gram-negative bacteria (223 – 228). Such enzymes that have been characterized include the peptidoglycan-targeting α-lytic proteases, β-lytic proteases, and CwhA amidases of Achromobacter lyticus and Lysobacter sp. and the lysozyme-like enzymes and lipases from M. xanthus and other myxobacteria (229 – 240). The documented presence of a signal sequence in the N-terminus of many of these enzymes suggests that at least some of them are substrates of the T2SS. Compatible with such a scenario, the T2SS apparatus genes are upregulated at the time when Lysobacter capsici produces its bacteriolytic proteases and M. xanthus preys on other bacteria (125, 241). However, a formal linkage to the T2SS, for example, documenting the loss of the secreted protein in a T2SS mutant’s supernatant, has not occurred yet. The first clear connection of a secreted, bacteriolytic enzyme to a T2SS began in 1993 when the LasA elastase of P. aeruginosa was shown to be equivalent to a previously defined staphylolytic enzyme in P. aeruginosa supernatants (242 – 247). In 1998, LasA was confirmed as being a substrate of the P. aeruginosa T2SS, when it proved to be undetected in supernatants of an xcp T2SS mutant (248 – 250). A member of the M23 family of peptidases (251, 252), LasA lyses Staphylococcus aureus by cleaving the pentaglycine within the peptidoglycan of that target cell (242, 247). Despite these data, the antibacterial function of LasA was not featured in the many reviews on T2SSs and interbacterial antagonism that later appeared (as noted above), although attention was frequently directed toward the role of the protein’s elastase activity in infection. Incidentally, another staphylolytic enzyme dependent on the P. aeruginosa T2SS has been suggested, but the identity of that factor remains undefined (253 – 255). The next connection between an antibacterial activity and a T2SS occurred in 2020 and involved a marine species of Pseudoalteromonas. Specifically, an M23-peptidase known as pseudoalterin was found to be secreted via the T2SS and to promote the killing of S. aureus and various other marine Gram-positive bacteria (122, 256). As is the case for LasA, pseudoalterin acts on the peptide chain within the peptidoglycan of its target bacterium (122). The third link between antibacterial activity and a T2SS came in 2021, when the NlpC/P60 endopeptidase (PnpA) secreted via the T2SS of Photobacterium damselae was shown to degrade in vitro purified Vibrio peptidoglycan (257 – 259). Yet, an outstanding question from this study is how PnpA naturally bypasses the Gram-negative target’s OM in order to reach the peptidoglycan. One possibility is that a T2SS-dependent lipase or an effect of another secretion system first disrupts the lipid bilayer creating a pathway for PnpA to access the periplasm. A final study linking a T2SS to interbacterial antagonism occurred in 2022, when a T2SS mutant of Plesiomonas shigelloides was found to be impaired for killing E. coli upon co-incubation on solid media (81). The secreted bactericidal protein(s) of P. shigelloides remains unknown, however. Based on these data, the T2SS can, in fact, be a mediator of interbacterial antagonism, and it is conceivable that many T2SSs have antibacterial output. Yet, the T2SS still remains understudied for its role in interbacterial competition, especially when compared to other protein secretion systems.
CONCLUDING THOUGHTS AND FUTURE QUESTIONS
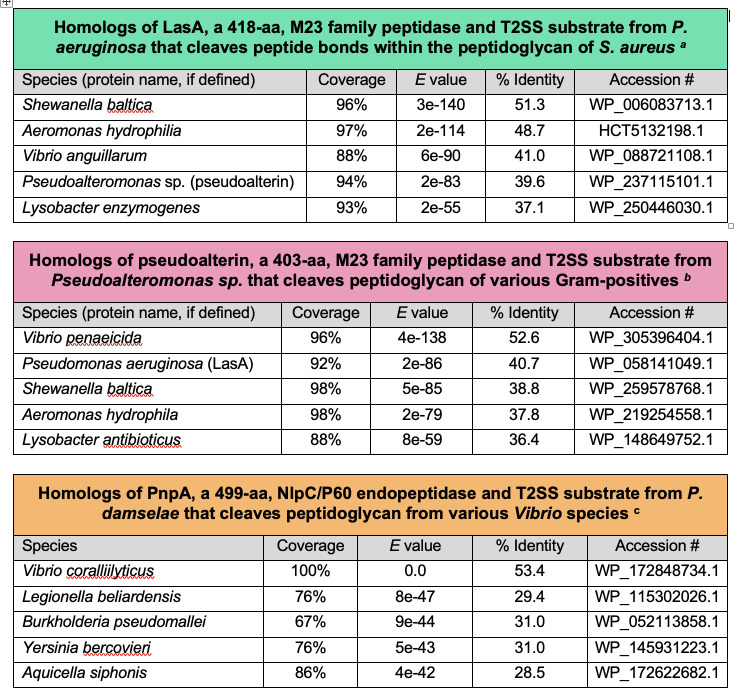
Despite what has been the prevailing impression, it is logical that T2SSs would be another means for interbacterial antagonism. For example, the different proteases/peptidases, lipases, and carbohydrate-degrading enzymes that are secreted by T2SSs could theoretically alter many moieties on the surface or in the envelope of a competitor leading to a loss of function or cell death (while not necessarily harming the producer). Based on the examples above, peptidoglycan appears to be a common target for antibacterial T2SSs. When the competitor is a Gram-positive bacterium, an enzyme acting on peptidoglycan might alone suffice. But, when the competitor is another Gram-negative bacterium, enzymes that act on the target’s OM would seem to be also necessary for effective competition. On the other hand, some T2SS substrates might act indirectly, for example, by processing foodstuffs in the extracellular milieu in a way that makes them less accessible or useful to competitors. Since some T2SS substrates (also) reside on the producer’s surface, T2SSs might even facilitate a novel form of contact-dependent killing. Finally, it is possible that some T2SS substrates potentiate the action of another antibacterial secretion system, just as some T2SSs act to enhance the effects of those other systems on eukaryotic hosts (222, 260). Overall, T2SSs likely contribute to a multi-pronged strategy of interbacterial antagonism, especially for those Gram-negative bacteria that do not have one or more of the other systems. Aside from these types of mechanistic questions, it will be beneficial for future investigations to discern what other T2SS-encoding bacteria (Fig. 1 and 2) use their T2SS for antibacterial antagonism, to what degree, and with what types of effectors. Current Basic Local Alignment Search Tool (BLASTP) searches indicated that proteins with significant amino acid sequence similarity to LasA, pseudoalterin, or PnpA are encoded within the genomes of many of these other species (Fig. 3), further suggesting that these organisms might similarly employ their T2SS for interbacterial competition. Yet, given the ecological diversity of the >100 genera in Fig. 1 and 2, it is likely that new types of effectors and new forms of competition will also be revealed. For such an endeavor, it will be valuable to assess the role of the T2SS in models that simulate natural niches, whether that be an aquatic or terrestrial habitat, the rhizosphere, or infection of an animal or human host. Another interesting question will be if any known or yet-to-be-defined T2SS substrates that target bacteria also confer activity against fungi or protists. Further investigation of T2SS substrates as agents of antibacterial antagonism also offers a possible pathway toward identifying new antimicrobials that could be used to treat infectious diseases. Along those lines, LasA has been used as a treatment for experimental staphylococcal eye infections (261 – 263). In sum, an expanded appreciation for T2SSs is likely to yield important new insight into the mechanisms of interbacterial antagonism, pathogenesis and potential disease therapies, and diverse ecological niches.
Fig 3.
Known antibacterial effectors of T2SSs and some of their homologs encoded within the genomes of other T2SS-encoding species. (a) BLASTP results using the LasA sequence from P. aeruginosa strain PA01 (accession no. NP_250562) as the query. Top hits were proteins from other species of Pseudomonas; however, they were not presented in order to focus on related proteins that occur in other genera. The five examples given are proteins that show some of the greatest levels of similarity to LasA and are from diverse species that are known to encode a T2SS. (b) BLASTP results using the pseudoalterin sequence from Pseudoalteromonas sp. strain CF6-2 (accession no. WP_237115101) as the query. The examples listed are five that show some of the greatest levels of similarity to pseudoalterin and are from a range of non-Pseudoalteromonas species that are known to encode a T2SS. (c) BLASTP results using the PnpA sequence from P. damselae strain MT1415 (accession no. 6SQX_B) as the query. The proteins listed are five that showed some of the greatest levels of similarity to PnpA and are from non-Photobacterium species that are known to encode a T2SS.
ACKNOWLEDGMENTS
The author thanks the members of the lab past and present for their research into T2SSs and interbacterial competition.
This work was supported by grants AI175460 and AI171325.
Contributor Information
Nicholas P. Cianciotto, Email: n-cianciotto@northwestern.edu.
Anthony R. Richardson, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
REFERENCES
- 1. Stubbendieck RM, Straight PD. 2016. Multifaceted interfaces of bacterial competition. J Bacteriol 198:2145–2155. doi: 10.1128/JB.00275-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Granato ET, Meiller-Legrand TA, Foster KR. 2019. The evolution and ecology of bacterial warfare. Curr Biol 29:R521–R537. doi: 10.1016/j.cub.2019.04.024 [DOI] [PubMed] [Google Scholar]
- 3. Peterson SB, Bertolli SK, Mougous JD. 2020. The central role of interbacterial antagonism in bacterial life. Curr Biol 30:R1203–R1214. doi: 10.1016/j.cub.2020.06.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao Q, Bertolli S, Park Y-J, Tan Y, Cutler KJ, Srinivas P, Asfahl KL, Fonesca-García C, Gallagher LA, Li Y, Wang Y, Coleman-Derr D, DiMaio F, Zhang D, Peterson SB, Veesler D, Mougous JD. 2024. Streptomyces umbrella toxin particles block hyphal growth of competing species. Nature 629:165–173. doi: 10.1038/s41586-024-07298-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gordils-Valentin L, Ouyang H, Qian L, Hong J, Zhu X. 2024. Conjugative type IV secretion systems enable bacterial antagonism that operates independently of plasmid transfer. Commun Biol 7:499. doi: 10.1038/s42003-024-06192-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kastrat E, Cheng HP. 2024. Escherichia coli has an undiscovered ability to inhibit the growth of both Gram-negative and Gram-positive bacteria. Sci Rep 14:7420. doi: 10.1038/s41598-024-57996-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waksman SA. 1941. Antagonistic relations of microorganisms. Bacteriol Rev 5:231–291. doi: 10.1128/br.5.3.231-291.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soltani S, Hammami R, Cotter PD, Rebuffat S, Said LB, Gaudreau H, Bédard F, Biron E, Drider D, Fliss I. 2021. Bacteriocins as a new generation of antimicrobials: toxicity aspects and regulations. FEMS Microbiol Rev 45:fuaa039. doi: 10.1093/femsre/fuaa039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans JC, McEneany VL, Coyne MJ, Caldwell EP, Sheahan ML, Von SS, Coyne EM, Tweten RK, Comstock LE. 2022. A proteolytically activated antimicrobial toxin encoded on a mobile plasmid of Bacteroidales induces a protective response. Nat Commun 13:4258. doi: 10.1038/s41467-022-31925-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paškevičius Š, Gleba Y, Ražanskienė A. 2022. Stenocins: novel modular bacteriocins from opportunistic pathogen Stenotrophomonas maltophilia. J Biotechnol 351:9–12. doi: 10.1016/j.jbiotec.2022.04.006 [DOI] [PubMed] [Google Scholar]
- 11. Holland M, Farinella DN, Cruz-Lorenzo E, Laubscher MI, Doakes DA, Ramos MA, Kubota N, Levin TC. 2023. L. pneumophila resists its self-harming metabolite HGA via secreted factors and collective peroxide scavenging. mBio 14:e0120723. doi: 10.1128/mbio.01207-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hagh Ranjbar H, Hosseini-Abari A, Ghasemi SM, Hafezi Birgani Z. 2023. Antibacterial activity of epsilon-poly-l-lysine produced by Stenotrophomonas maltophilia HS4 and Paenibacillus polymyxa HS5, alone and in combination with bacteriophages. Microbiology (Reading) 169:001363. doi: 10.1099/mic.0.001363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang H, Han Y, Wang X, Jia Y, Zhang Y, Müller R, Huo L. 2023. Genome mining of myxopeptins reveals a class of lanthipeptide-derived linear dehydroamino acid-containing peptides from Myxococcus sp. MCy9171. ACS Chem Biol 18:2163–2169. doi: 10.1021/acschembio.3c00265 [DOI] [PubMed] [Google Scholar]
- 14. Ruhe ZC, Low DA, Hayes CS. 2020. Polymorphic toxins and their immunity proteins: diversity, evolution, and mechanisms of delivery. Annu Rev Microbiol 74:497–520. doi: 10.1146/annurev-micro-020518-115638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lahiri D, Nag M, Dutta B, Sarkar T, Pati S, Basu D, Abdul Kari Z, Wei LS, Smaoui S, Wen Goh K, Ray RR. 2022. Bacteriocin: a natural approach for food safety and food security. Front Bioeng Biotechnol 10:1005918. doi: 10.3389/fbioe.2022.1005918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jamet A, Nassif X. 2015. New players in the toxin field: polymorphic toxin systems in bacteria. mBio 6:e00285-15. doi: 10.1128/mBio.00285-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kobayashi K. 2021. Diverse LXG toxin and antitoxin systems specifically mediate intraspecies competition in Bacillus subtilis biofilms. PLoS Genet 17:e1009682. doi: 10.1371/journal.pgen.1009682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kostakioti M, Newman CL, Thanassi DG, Stathopoulos C. 2005. Mechanisms of protein export across the bacterial outer membrane. J Bacteriol 187:4306–4314. doi: 10.1128/JB.187.13.4306-4314.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaimer C, Weltzer ML, Wall D. 2023. Two reasons to kill: predation and kin discrimination in myxobacteria. Microbiology (Reading) 169:001372. doi: 10.1099/mic.0.001372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vassallo CN, Cao P, Conklin A, Finkelstein H, Hayes CS, Wall D. 2017. Infectious polymorphic toxins delivered by outer membrane exchange discriminate kin in myxobacteria. Elife 6:e29397. doi: 10.7554/eLife.29397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klein TA, Ahmad S, Whitney JC. 2020. Contact-dependent interbacterial antagonism mediated by protein secretion machines. Trends Microbiol 28:387–400. doi: 10.1016/j.tim.2020.01.003 [DOI] [PubMed] [Google Scholar]
- 22. Crisan CV, Goldberg JB. 2022. Antibacterial contact-dependent proteins secreted by Gram-negative cystic fibrosis respiratory pathogens. Trends Microbiol 30:986–996. doi: 10.1016/j.tim.2022.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allsopp LP, Bernal P. 2023. Killing in the name of: T6SS structure and effector diversity. Microbiology (Reading) 169:001367. doi: 10.1099/mic.0.001367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Unni R, Pintor KL, Diepold A, Unterweger D. 2022. Presence and absence of type VI secretion systems in bacteria. Microbiology (Reading) 168. doi: 10.1099/mic.0.001151 [DOI] [PubMed] [Google Scholar]
- 25. Kanarek K, Fridman CM, Bosis E, Salomon D. 2023. The RIX domain defines a class of polymorphic T6SS effectors and secreted adaptors. Nat Commun 14:4983. doi: 10.1038/s41467-023-40659-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jensen SJ, Ruhe ZC, Williams AF, Nhan DQ, Garza-Sánchez F, Low DA, Hayes CS. 2023. Paradoxical activation of a type VI secretion system phospholipase effector by its cognate immunity protein. J Bacteriol 205:e0011323. doi: 10.1128/jb.00113-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bosch DE, Abbasian R, Parajuli B, Peterson SB, Mougous JD. 2023. Structural disruption of Ntox15 nuclease effector domains by immunity proteins protects against type VI secretion system intoxication in Bacteroidales. mBio 14:e0103923. doi: 10.1128/mbio.01039-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rudzite M, Subramoni S, Endres RG, Filloux A. 2023. Effectiveness of Pseudomonas aeruginosa type VI secretion system relies on toxin potency and type IV pili-dependent interaction. PLoS Pathog 19:e1011428. doi: 10.1371/journal.ppat.1011428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. García-Bayona L, Coyne MJ, Hantman N, Montero-Llopis P, Von SS, Ito T, Malamy MH, Basler M, Barquera B, Comstock LE. 2020. Nanaerobic growth enables direct visualization of dynamic cellular processes in human gut symbionts. Proc Natl Acad Sci U S A 117:24484–24493. doi: 10.1073/pnas.2009556117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oscarsson J, Bao K, Shiratsuchi A, Grossmann J, Wolski W, Aung KM, Lindholm M, Johansson A, Mowsumi FR, Wai SN, Belibasakis GN, Bostanci N. 2024. Bacterial symbionts in oral niche use type VI secretion nanomachinery for fitness increase against pathobionts. iScience 27:109650. doi: 10.1016/j.isci.2024.109650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sheedlo MJ, Ohi MD, Lacy DB, Cover TL. 2022. Molecular architecture of bacterial type IV secretion systems. PLoS Pathog 18:e1010720. doi: 10.1371/journal.ppat.1010720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nas MY, White RC, DuMont AL, Lopez AE, Cianciotto NP. 2019. Stenotrophomonas maltophilia encodes a VirB/VirD4 type IV secretion system that modulates apoptosis in human cells and promotes competition against heterologous bacteria, including Pseudomonas aeruginosa. Infect Immun 87:e00457-19. doi: 10.1128/IAI.00457-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nas MY, Gabell J, Cianciotto NP. 2021. Effectors of the Stenotrophomonas maltophilia type IV secretion system mediate killing of clinical isolates of Pseudomonas aeruginosa. mBio 12:e0150221. doi: 10.1128/mBio.01502-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cobe BL, Dey S, Minasov G, Inniss N, Satchell KJF, Cianciotto NP. 2024. Bactericidal effectors of the Stenotrophomonas maltophilia type IV secretion system: functional definition of the nuclease TfdA and structural determination of TfcB. mBio:e0119824. doi: 10.1128/mbio.01198-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bayer-Santos E, Cenens W, Matsuyama BY, Oka GU, Di Sessa G, Mininel IDV, Alves TL, Farah CS. 2019. The opportunistic pathogen Stenotrophomonas maltophilia utilizes a type IV secretion system for interbacterial killing. PLoS Pathog 15:e1007651. doi: 10.1371/journal.ppat.1007651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Souza DP, Oka GU, Alvarez-Martinez CE, Bisson-Filho AW, Dunger G, Hobeika L, Cavalcante NS, Alegria MC, Barbosa LRS, Salinas RK, Guzzo CR, Farah CS. 2015. Bacterial killing via a type IV secretion system. Nat Commun 6:6453. doi: 10.1038/ncomms7453 [DOI] [PubMed] [Google Scholar]
- 37. Shen X, Wang B, Yang N, Zhang L, Shen D, Wu H, Dong Y, Niu B, Chou SH, Puopolo G, Fan J, Qian G. 2021. Lysobacter enzymogenes antagonizes soilborne bacteria using the type IV secretion system. Environ Microbiol 23:4673–4688. doi: 10.1111/1462-2920.15662 [DOI] [PubMed] [Google Scholar]
- 38. Purtschert-Montenegro G, Cárcamo-Oyarce G, Pinto-Carbó M, Agnoli K, Bailly A, Eberl L. 2022. Pseudomonas putida mediates bacterial killing, biofilm invasion and biocontrol with a type IVB secretion system. Nat Microbiol 7:1547–1557. doi: 10.1038/s41564-022-01209-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Drehkopf S, Scheibner F, Büttner D. 2023. Functional characterization of VirB/VirD4 and Icm/Dot type IV secretion systems from the plant-pathogenic bacterium Xanthomonas euvesicatoria Front Cell Infect Microbiol 13:1203159. doi: 10.3389/fcimb.2023.1203159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liao J, Li Z, Xiong D, Shen D, Wang L, Lin L, Shao X, Liao L, Li P, Zhang LQ, Wang HH, Qian G. 2023. Quorum quenching by a type IVA secretion system effector. ISME J 17:1564–1577. doi: 10.1038/s41396-023-01457-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tran H-KR, Grebenc DW, Klein TA, Whitney JC. 2021. Bacterial type VII secretion: an important player in host-microbe and microbe-microbe interactions. Mol Microbiol 115:478–489. doi: 10.1111/mmi.14680 [DOI] [PubMed] [Google Scholar]
- 42. Cao Z, Casabona MG, Kneuper H, Chalmers JD, Palmer T. 2016. The type VII secretion system of Staphylococcus aureus secretes a nuclease toxin that targets competitor bacteria. Nat Microbiol 2:16183. doi: 10.1038/nmicrobiol.2016.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Whitney JC, Peterson SB, Kim J, Pazos M, Verster AJ, Radey MC, Kulasekara HD, Ching MQ, Bullen NP, Bryant D, Goo YA, Surette MG, Borenstein E, Vollmer W, Mougous JD. 2017. A broadly distributed toxin family mediates contact-dependent antagonism between gram-positive bacteria. Elife 6:e26938. doi: 10.7554/eLife.26938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ulhuq FR, Gomes MC, Duggan GM, Guo M, Mendonca C, Buchanan G, Chalmers JD, Cao Z, Kneuper H, Murdoch S, Thomson S, Strahl H, Trost M, Mostowy S, Palmer T. 2020. A membrane-depolarizing toxin substrate of the Staphylococcus aureus type VII secretion system mediates intraspecies competition. Proc Natl Acad Sci U S A 117:20836–20847. doi: 10.1073/pnas.2006110117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tassinari M, Doan T, Bellinzoni M, Chabalier M, Ben-Assaya M, Martinez M, Gaday Q, Alzari PM, Cascales E, Fronzes R, Gubellini F. 2022. The antibacterial type VII secretion system of Bacillus subtilis: structure and interactions of the pseudokinase YukC/EssB. mBio 13:e0013422. doi: 10.1128/mbio.00134-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aoki SK, Pamma R, Hernday AD, Bickham JE, Braaten BA, Low DA. 2005. Contact-dependent inhibition of growth in Escherichia coli. Science 309:1245–1248. doi: 10.1126/science.1115109 [DOI] [PubMed] [Google Scholar]
- 47. Lin HH, Filloux A, Lai EM. 2020. Role of recipient susceptibility factors during contact-dependent interbacterial competition. Front Microbiol 11:603652. doi: 10.3389/fmicb.2020.603652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Allen JP, Ozer EA, Minasov G, Shuvalova L, Kiryukhina O, Satchell KJF, Hauser AR. 2020. A comparative genomics approach identifies contact-dependent growth inhibition as a virulence determinant. Proc Natl Acad Sci U S A 117:6811–6821. doi: 10.1073/pnas.1919198117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ikryannikova LN, Kurbatov LK, Gorokhovets NV, Zamyatnin Jr AA. 2020. Contact-dependent growth inhibition in bacteria: do not get too close! Int J Mol Sci 21:7990. doi: 10.3390/ijms21217990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cuthbert BJ, Hayes CS, Goulding CW. 2022. Functional and structural diversity of bacterial contact-dependent growth inhibition effectors. Front Mol Biosci 9:866854. doi: 10.3389/fmolb.2022.866854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. García-Bayona L, Guo MS, Laub MT. 2017. Contact-dependent killing by Caulobacter crescentus via cell surface-associated, glycine zipper proteins. Elife 6:e24869. doi: 10.7554/eLife.24869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Linhartová I, Bumba L, Mašín J, Basler M, Osička R, Kamanová J, Procházková K, Adkins I, Hejnová-Holubová J, Sadílková L, Morová J, Sebo P. 2010. RTX proteins: a highly diverse family secreted by a common mechanism. FEMS Microbiol Rev 34:1076–1112. doi: 10.1111/j.1574-6976.2010.00231.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thiery S, Turowski P, Berleman JE, Kaimer C. 2022. The predatory soil bacterium Myxococcus xanthus combines a Tad- and an atypical type 3-like protein secretion system to kill bacterial cells. Cell Rep 40:111340. doi: 10.1016/j.celrep.2022.111340 [DOI] [PubMed] [Google Scholar]
- 54. Veith PD, Glew MD, Gorasia DG, Cascales E, Reynolds EC. 2022. The type IX secretion system and its role in bacterial function and pathogenesis. J Dent Res 101:374–383. doi: 10.1177/00220345211051599 [DOI] [PubMed] [Google Scholar]
- 55. Paillat M, Lunar Silva I, Cascales E, Doan T. 2023. A journey with type IX secretion system effectors: selection, transport, processing and activities. Microbiology (Reading) 169:001320. doi: 10.1099/mic.0.001320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Idosa AW, Wozniak DJ, Hall-Stoodley L. 2022. Surface dependent inhibition of Mycobacterium abscessus by diverse Pseudomonas aeruginosa strains. Microbiol Spectr 10:e0247122. doi: 10.1128/spectrum.02471-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shaliutina-Loginova A, Francetic O, Doležal P. 2023. Bacterial type II secretion system and its mitochondrial counterpart. mBio 14:e0314522. doi: 10.1128/mbio.03145-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Naskar S, Hohl M, Tassinari M, Low HH. 2021. The structure and mechanism of the bacterial type II secretion system. Mol Microbiol 115:412–424. doi: 10.1111/mmi.14664 [DOI] [PubMed] [Google Scholar]
- 59. Ghosal D, Kim KW, Zheng H, Kaplan M, Truchan HK, Lopez AE, McIntire IE, Vogel JP, Cianciotto NP, Jensen GJ. 2019. In vivo structure of the Legionella type II secretion system by electron cryotomography. Nat Microbiol 4:2101–2108. doi: 10.1038/s41564-019-0603-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Christie PJ. 2019. The rich tapestry of bacterial protein translocation systems. Protein J 38:389–408. doi: 10.1007/s10930-019-09862-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Korotkov KV, Sandkvist M. 2019. Architecture, function, and substrates of the type II secretion system. EcoSal Plus 8. doi: 10.1128/ecosalplus.ESP-0034-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cianciotto NP, White RC. 2017. The expanding role of type II secretion in bacterial pathogenesis and beyond. Infect Immun 85:e00014-17. doi: 10.1128/IAI.00014-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dazzoni R, Li Y, López-Castilla A, Brier S, Mechaly A, Cordier F, Haouz A, Nilges M, Francetic O, Bardiaux B, Izadi-Pruneyre N. 2023. Structure and dynamic association of an assembly platform subcomplex of the bacterial type II secretion system. Structure 31:152–165. doi: 10.1016/j.str.2022.12.003 [DOI] [PubMed] [Google Scholar]
- 64. Yu Z, Wu Y, Chen M, Huo T, Zheng W, Ludtke SJ, Shi X, Wang Z. 2023. Membrane translocation process revealed by in situ structures of type II secretion system secretins. Nat Commun 14:4025. doi: 10.1038/s41467-023-39583-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Escobar CA, Douzi B, Ball G, Barbat B, Alphonse S, Quinton L, Voulhoux R, Forest KT. 2021. Structural interactions define assembly adapter function of a type II secretion system pseudopilin. Structure 29:1116–1127. doi: 10.1016/j.str.2021.05.015 [DOI] [PubMed] [Google Scholar]
- 66. Kinsella RL, Lopez J, Palmer LD, Salinas ND, Skaar EP, Tolia NH, Feldman MF. 2017. Defining the interaction of the protease CpaA with its type II secretion chaperone CpaB and its contribution to virulence in Acinetobacter species. J Biol Chem 292:19628–19638. doi: 10.1074/jbc.M117.808394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Urusova DV, Kinsella RL, Salinas ND, Haurat MF, Feldman MF, Tolia NH. 2019. The structure of Acinetobacter-secreted protease CpaA complexed with its chaperone CpaB reveals a novel mode of a T2SS chaperone-substrate interaction. J Biol Chem 294:13344–13354. doi: 10.1074/jbc.RA119.009805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pineau C, Guschinskaya N, Gonçalves IR, Ruaudel F, Robert X, Gouet P, Ballut L, Shevchik VE. 2021. Structure-function analysis of pectate lyase Pel3 reveals essential facets of protein recognition by the bacterial type 2 secretion system. J Biol Chem 296:100305. doi: 10.1016/j.jbc.2021.100305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dalbey RE, Kuhn A. 2012. Protein traffic in Gram-negative bacteria--how exported and secreted proteins find their way. FEMS Microbiol Rev 36:1023–1045. doi: 10.1111/j.1574-6976.2012.00327.x [DOI] [PubMed] [Google Scholar]
- 70. Desvaux M, Hébraud M, Talon R, Henderson IR. 2009. Secretion and subcellular localizations of bacterial proteins: a semantic awareness issue. Trends Microbiol 17:139–145. doi: 10.1016/j.tim.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 71. Cianciotto NP. 2005. Type II secretion: a protein secretion system for all seasons. Trends Microbiol 13:581–588. doi: 10.1016/j.tim.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 72. Abby SS, Cury J, Guglielmini J, Néron B, Touchon M, Rocha EPC. 2016. Identification of protein secretion systems in bacterial genomes. Sci Rep 6:23080. doi: 10.1038/srep23080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. White RC, Cianciotto NP. 2019. Assessing the impact, genomics, and evolution of type II secretion across a large, medically-important genus: the Legionella type II secretion paradigm. Microb Genom 5:e000273. doi: 10.1099/mgen.0.000273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Denise R, Abby SS, Rocha EPC. 2019. Diversification of the type IV filament superfamily into machines for adhesion, protein secretion, DNA uptake, and motility. PLoS Biol 17:e3000390. doi: 10.1371/journal.pbio.3000390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Andersson JA, Sha J, Erova TE, Fitts EC, Ponnusamy D, Kozlova EV, Kirtley ML, Chopra AK. 2017. Identification of new virulence factors and vaccine candidates for Yersinia pestis. Front Cell Infect Microbiol 7:448. doi: 10.3389/fcimb.2017.00448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jang H, Gopinath GR, Eshwar A, Srikumar S, Nguyen S, Gangiredla J, Patel IR, Finkelstein SB, Negrete F, Woo J, Lee Y, Fanning S, Stephan R, Tall BD, Lehner A. 2020. The secretion of toxins and other exoproteins of cronobacter: role in virulence, adaption, and persistence. Microorganisms 8:229. doi: 10.3390/microorganisms8020229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Llanos Salinas SP, Castillo Sánchez LO, Castañeda Miranda G, Rodríguez Reyes EA, Ordoñez López L, Mena Bañuelos R, Alcaraz Sosa LE, Núñez Carrera MG, José Manuel RO, Carmona Gasca CA, Matsunaga J, Haake DA, Candanosa Aranda IE, de la Peña-Moctezuma A. 2020. Gspd, the type II secretion system secretin of Leptospira, protects hamsters against lethal infection with a virulent L. interrogans isolate. Vaccines (Basel) 8:759. doi: 10.3390/vaccines8040759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Barger PC, Liles MR, Newton JC. 2020. Type II secretion is essential for virulence of the emerging fish pathogen, hypervirulent Aeromonas hydrophila. Front Vet Sci 7:574113. doi: 10.3389/fvets.2020.574113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mekasha S, Linke D. 2021. Secretion systems in Gram-negative bacterial fish pathogens. Front Microbiol 12:782673. doi: 10.3389/fmicb.2021.782673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tomaś N, Myszka K, Wolko Ł. 2022. Black pepper and tarragon essential oils suppress the lipolytic potential and the type II secretion system of P. psychrophila KM02. Sci Rep 12:5487. doi: 10.1038/s41598-022-09311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yan J, Guo X, Li J, Li Y, Sun H, Li A, Cao B. 2022. RpoN is required for the motility and contributes to the killing ability of Plesiomonas shigelloides. BMC Microbiol 22:299. doi: 10.1186/s12866-022-02722-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Veschetti L, Boaretti M, Saitta GM, Passarelli Mantovani R, Lleò MM, Sandri A, Malerba G. 2022. Achromobacter spp. prevalence and adaptation in cystic fibrosis lung infection. Microbiol Res 263:127140. doi: 10.1016/j.micres.2022.127140 [DOI] [PubMed] [Google Scholar]
- 83. Feng Y, Yu Z, Zhao R, Qin Z, Geng Y, Chen D, Huang X, Ouyang P, Zuo Z, Guo H, Deng H, Huang C, Lai W. 2023. Unraveling extracellular protein signatures to enhance live attenuated vaccine development through type II secretion system disruption in Vibrio mimicus. Microb Pathog 181:106215. doi: 10.1016/j.micpath.2023.106215 [DOI] [PubMed] [Google Scholar]
- 84. Paauw A, Scholz HC, Mars-Groenendijk RH, Dekker LJM, Luider TM, van Leeuwen HC. 2023. Expression of virulence and antimicrobial related proteins in Burkholderia mallei and Burkholderia pseudomallei. PLoS Negl Trop Dis 17:e0011006. doi: 10.1371/journal.pntd.0011006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Krekhno Z, Woodward SE, Serapio-Palacios A, Peña-Díaz J, Moon KM, Foster LJ, Finlay BB. 2024. Citrobacter rodentium possesses a functional type II secretion system necessary for successful host infection. Gut Microbes 16:2308049. doi: 10.1080/19490976.2024.2308049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang Y, Teper D, Xu J, Wang N. 2019. Stringent response regulators (p)ppGpp and DksA positively regulate virulence and host adaptation of Xanthomonas citri. Mol Plant Pathol 20:1550–1565. doi: 10.1111/mpp.12865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yin Z, Liu X, Qian C, Sun L, Pang S, Liu J, Li W, Huang W, Cui S, Zhang C, Song W, Wang D, Xie Z. 2022. Pan-genome analysis of Delftia tsuruhatensis reveals important traits concerning the genetic diversity, pathogenicity, and biotechnological properties of the species. Microbiol Spectr 10:e0207221. doi: 10.1128/spectrum.02072-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rosenberg T, Jiménez-Guerrero I, Tamir-Ariel D, Yarnitzky T, Burdman S. 2022. The GDSL-lipolytic enzyme Lip1 is required for full virulence of the cucurbit pathogenic bacterium Acidovorax citrulli. Microorganisms 10:1016. doi: 10.3390/microorganisms10051016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Morinière L, Mirabel L, Gueguen E, Bertolla F. 2022. A comprehensive overview of the genes and functions required for lettuce infection by the hemibiotrophic phytopathogen Xanthomonas hortorum pv. vitians. mSystems 7:e0129021. doi: 10.1128/msystems.01290-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wein P, Dornblut K, Herkersdorf S, Krüger T, Molloy EM, Brakhage AA, Hoffmeister D, Hertweck C. 2023. Bacterial secretion systems contribute to rapid tissue decay in button mushroom soft rot disease. mBio 14:e0078723. doi: 10.1128/mbio.00787-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shah SMA, Khojasteh M, Wang Q, Haq F, Xu X, Li Y, Zou L, Osdaghi E, Chen G. 2023. Comparative transcriptome analysis of wheat cultivars in response to Xanthomonas translucens pv. cerealis and its T2SS, T3SS and TALEs deficient strains. Phytopathology 113:2073–2082. doi: 10.1094/PHYTO-02-23-0049-SA [DOI] [PubMed] [Google Scholar]
- 92. Inoue K, Takemura C, Senuma W, Maeda H, Kai K, Kiba A, Ohnishi K, Tsuzuki M, Hikichi Y. 2023. The behavior of Ralstonia pseudosolanacearum strain OE1-1 and morphological changes of cells in tomato roots. J Plant Res 136:19–31. doi: 10.1007/s10265-022-01427-3 [DOI] [PubMed] [Google Scholar]
- 93. Ingel B, Castro C, Burbank L, Her N, De Anda NI, Way H, Wang P, Roper MC. 2023. Xylella fastidiosa requires the type II secretion system for pathogenicity and survival in grapevine. Mol Plant Microbe Interact 36:636–646. doi: 10.1094/MPMI-03-23-0027-R [DOI] [PubMed] [Google Scholar]
- 94. Passmore IJ, Nishikawa K, Lilley KS, Bowden SD, Chung JCS, Welch M. 2015. Mep72, a metzincin protease that is preferentially secreted by biofilms of Pseudomonas aeruginosa. J Bacteriol 197:762–773. doi: 10.1128/JB.02404-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. DuMont AL, Cianciotto NP. 2017. Stenotrophomonas maltophilia serine protease StmPr1 induces matrilysis, anoikis, and protease-activated receptor-2 activation in human lung epithelial cells. Infect Immun 85:e00544-17. doi: 10.1128/IAI.00544-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jang KK, Lee ZW, Kim B, Jung YH, Han HJ, Kim MH, Kim BS, Choi SH. 2017. Identification and characterization of Vibrio vulnificus plpA encoding a phospholipase A2 essential for pathogenesis. J Biol Chem 292:17129–17143. doi: 10.1074/jbc.M117.791657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. do Vale A, Pereira C, Osorio CR, dos Santos NMS. 2017. The apoptogenic toxin AIP56 is secreted by the type II secretion system of Photobacterium damselae subsp. piscicida. Toxins (Basel) 9:368. doi: 10.3390/toxins9110368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Saint-Criq V, Villeret B, Bastaert F, Kheir S, Hatton A, Cazes A, Xing Z, Sermet-Gaudelus I, Garcia-Verdugo I, Edelman A, Sallenave JM. 2018. Pseudomonas aeruginosa LasB protease impairs innate immunity in mice and humans by targeting a lung epithelial cystic fibrosis transmembrane regulator-IL-6-antimicrobial-repair pathway. Thorax 73:49–61. doi: 10.1136/thoraxjnl-2017-210298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Waack U, Warnock M, Yee A, Huttinger Z, Smith S, Kumar A, Deroux A, Ginsburg D, Mobley HLT, Lawrence DA, Sandkvist M. 2018. CpaA is a glycan-specific adamalysin-like protease secreted by Acinetobacter baumannii that Inactivates coagulation factor XII. mBio 9:e01606-18. doi: 10.1128/mBio.01606-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Carda-Diéguez M, Silva-Hernández FX, Hubbard TP, Chao MC, Waldor MK, Amaro C. 2018. Comprehensive identification of Vibrio vulnificus genes required for growth in human serum. Virulence 9:981–993. doi: 10.1080/21505594.2018.1455464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bastaert F, Kheir S, Saint-Criq V, Villeret B, Dang PM-C, El-Benna J, Sirard J-C, Voulhoux R, Sallenave J-M. 2018. Pseudomonas aeruginosa LasB subverts alveolar macrophage activity by interfering with bacterial killing through downregulation of innate immune defense, reactive oxygen species generation, and complement activation. Front Immunol 9:1675. doi: 10.3389/fimmu.2018.01675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wilton M, Halverson TWR, Charron-Mazenod L, Parkins MD, Lewenza S. 2018. Secreted phosphatase and deoxyribonuclease are required by Pseudomonas aeruginosa to defend against neutrophil extracellular traps. Infect Immun 86:e00403-18. doi: 10.1128/IAI.00403-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. White RC, Truchan HK, Zheng H, Tyson JY, Cianciotto NP. 2019. Type II secretion promotes bacterial growth within the Legionella-containing vacuole in infected amoebae. Infect Immun 87:e00374-19. doi: 10.1128/IAI.00374-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liu L, Gueguen-Chaignon V, Gonçalves IR, Rascle C, Rigault M, Dellagi A, Loisel E, Poussereau N, Rodrigue A, Terradot L, Condemine G. 2019. A secreted metal-binding protein protects necrotrophic phytopathogens from reactive oxygen species. Nat Commun 10:4853. doi: 10.1038/s41467-019-12826-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Elhosseiny NM, Elhezawy NB, Sayed RM, Khattab MS, El Far MY, Attia AS. 2020. Gamma-glutamyltransferase as a novel virulence factor of Acinetobacter baumannii inducing alveolar wall destruction and renal damage in systemic disease. J Infect Dis 222:871–879. doi: 10.1093/infdis/jiaa262 [DOI] [PubMed] [Google Scholar]
- 106. Callaghan JD, Stella NA, Lehner KM, Treat BR, Brothers KM, St Leger AJ, Shanks RMQ. 2020. Xylose-inducible promoter tools for Pseudomonas species and their use in implicating a role for the type II secretion system protein XcpQ in the inhibition of corneal epithelial wound closure. Appl Environ Microbiol 86:e00250-20. doi: 10.1128/AEM.00250-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rehman S, Grigoryeva LS, Richardson KH, Corsini P, White RC, Shaw R, Portlock TJ, Dorgan B, Zanjani ZS, Fornili A, Cianciotto NP, Garnett JA. 2020. Structure and functional analysis of the Legionella chitinase ChiA reveals a novel mechanism of metal-dependent mucin degradation. PLoS Pathog 16:e1008342. doi: 10.1371/journal.ppat.1008342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Holmes A, Pritchard L, Hedley P, Morris J, McAteer SP, Gally DL, Holden NJ. 2020. A high-throughput genomic screen identifies a role for the plasmid-borne type II secretion system of Escherichia coli O157:H7 (Sakai) in plant-microbe interactions. Genomics 112:4242–4253. doi: 10.1016/j.ygeno.2020.07.021 [DOI] [PubMed] [Google Scholar]
- 109. Scheithauer L, Thiem S, Schmelz S, Dellmann A, Büssow K, Brouwer RMHJ, Ünal CM, Blankenfeldt W, Steinert M. 2021. Zinc metalloprotease ProA of Legionella pneumophila increases alveolar septal thickness in human lung tissue explants by collagen IV degradation. Cell Microbiol 23:e13313. doi: 10.1111/cmi.13313 [DOI] [PubMed] [Google Scholar]
- 110. Scheithauer Lina, Thiem S, Ünal CM, Dellmann A, Steinert M. 2022. Zinc metalloprotease ProA from Legionella pneumophila inhibits the pro-inflammatory host response by degradation of bacterial flagellin. Biomolecules 12:624. doi: 10.3390/biom12050624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jackson-Litteken CD, Di Venanzio G, Le N-H, Scott NE, Djahanschiri B, Distel JS, Pardue EJ, Ebersberger I, Feldman MF. 2022. InvL, an Invasin-like adhesin, is a type II secretion system substrate required for Acinetobacter baumannii uropathogenesis. mBio 13:e0025822. doi: 10.1128/mbio.00258-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Banerjee B, Zeng Q, Yu M, Hsueh BY, Waters CM, Yang CH. 2022. Quorum-sensing master regulator VfmE is a C-Di-GMP effector that controls pectate lyase production in the phytopathogen Dickeya dadantii. Microbiol Spectr 10:e0180521. doi: 10.1128/spectrum.01805-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pfeilmeier S, Werz A, Ote M, Bortfeld-Miller M, Kirner P, Keppler A, Hemmerle L, Gäbelein CG, Petti GC, Wolf S, Pestalozzi CM, Vorholt JA. 2024. Leaf microbiome dysbiosis triggered by T2SS-dependent enzyme secretion from opportunistic Xanthomonas pathogens. Nat Microbiol 9:136–149. doi: 10.1038/s41564-023-01555-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Evans FF, Egan S, Kjelleberg S. 2008. Ecology of type II secretion in marine gammaproteobacteria. Environ Microbiol 10:1101–1107. doi: 10.1111/j.1462-2920.2007.01545.x [DOI] [PubMed] [Google Scholar]
- 115. Moebius N, Üzüm Z, Dijksterhuis J, Lackner G, Hertweck C. 2014. Active invasion of bacteria into living fungal cells. Elife 3:e03007. doi: 10.7554/eLife.03007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Nagar E, Zilberman S, Sendersky E, Simkovsky R, Shimoni E, Gershtein D, Herzberg M, Golden SS, Schwarz R. 2017. Type 4 pili are dispensable for biofilm development in the cyanobacterium Synechococcus elongatus. Environ Microbiol 19:2862–2872. doi: 10.1111/1462-2920.13814 [DOI] [PubMed] [Google Scholar]
- 117. Wang X, Han Q, Chen G, Zhang W, Liu W. 2017. A putative type II secretion system is involved in cellulose utilization in Cytophaga hutchisonii. Front Microbiol 8:1482. doi: 10.3389/fmicb.2017.01482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Todhanakasem T, Sowatad A, Kanokratana P, Havanapan PO, Champreda V. 2019. Expression and extracellular secretion of ENDO-glucanase and xylanase by Zymomonas mobilis. Appl Biochem Biotechnol 187:239–252. doi: 10.1007/s12010-018-2821-4 [DOI] [PubMed] [Google Scholar]
- 119. Toporek YJ, Mok JK, Shin HD, Lee BD, Lee MH, DiChristina TJ. 2019. Metal reduction and protein secretion genes required for Iodate reduction by Shewanella oneidensis. Appl Environ Microbiol 85:e02115-18. doi: 10.1128/AEM.02115-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bustamante-Brito R, Vera-Ponce de León A, Rosenblueth M, Martínez-Romero JC, Martínez-Romero E. 2019. Metatranscriptomic analysis of the bacterial symbiont Dactylopiibacterium carminicum from the carmine cochineal Dactylopius coccus (Hemiptera: Coccoidea: Dactylopiidae). Life (Basel) 9:4. doi: 10.3390/life9010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chen C, Kawamoto J, Kawai S, Tame A, Kato C, Imai T, Kurihara T. 2020. Isolation of a novel bacterial strain capable of producing abundant extracellular membrane vesicles carrying a single major cargo protein and analysis of its transport mechanism. Front Microbiol 10:3001. doi: 10.3389/fmicb.2019.03001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tang BL, Yang J, Chen XL, Wang P, Zhao HL, Su HN, Li CY, Yu Y, Zhong S, Wang L, Lidbury I, Ding H, Wang M, McMinn A, Zhang XY, Chen Y, Zhang YZ. 2020. A predator-prey interaction between a marine Pseudoalteromonas sp. and Gram-positive bacteria. Nat Commun 11:285. doi: 10.1038/s41467-019-14133-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yan X, Yang J, Wang Q, Lin S. 2021. Transcriptomic analysis reveals resistance mechanisms of Klebsiella michiganensis to copper toxicity under acidic conditions. Ecotoxicol Environ Saf 211:111919. doi: 10.1016/j.ecoenv.2021.111919 [DOI] [PubMed] [Google Scholar]
- 124. Aharon E, Mookherjee A, Pérez-Montaño F, Mateus da Silva G, Sathyamoorthy R, Burdman S, Jurkevitch E. 2021. Secretion systems play a critical role in resistance to predation by Bdellovibrio bacteriovorus. Res Microbiol 172:103878. doi: 10.1016/j.resmic.2021.103878 [DOI] [PubMed] [Google Scholar]
- 125. Afoshin A, Kudryakova I, Tarlachkov S, Leontyevskaya E, Zelenov D, Rudenko P, Leontyevskaya Vasilyeva N. 2023. Transcriptomic analysis followed by the isolation of extracellular bacteriolytic proteases from Lysobacter capsici VKM B-2533T . Int J Mol Sci 24:11652. doi: 10.3390/ijms241411652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Liu H, Xu G, Guo B, Liu F. 2024. Old role with new feature: T2SS ATPase as a cyclic-di-GMP receptor to regulate antibiotic production. Appl Environ Microbiol 90:e0041824. doi: 10.1128/aem.00418-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Farci D, Milenkovic S, Iesu L, Tanas M, Ceccarelli M, Piano D. 2024. Structural characterization and functional insights into the type II secretion system of the poly-extremophile Deinococcus radiodurans. J Biol Chem 300:105537. doi: 10.1016/j.jbc.2023.105537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kimes NE, Grim CJ, Johnson WR, Hasan NA, Tall BD, Kothary MH, Kiss H, Munk AC, Tapia R, Green L, Detter C, Bruce DC, Brettin TS, Colwell RR, Morris PJ. 2012. Temperature regulation of virulence factors in the pathogen Vibrio coralliilyticus. ISME J 6:835–846. doi: 10.1038/ismej.2011.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Smits THM, Rezzonico F, López MM, Blom J, Goesmann A, Frey JE, Duffy B. 2013. Phylogenetic position and virulence apparatus of the pear flower necrosis pathogen Erwinia piriflorinigrans CFBP 5888T as assessed by comparative genomics. Syst Appl Microbiol 36:449–456. doi: 10.1016/j.syapm.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 130. Zuleta LFG, Cunha C de O, de Carvalho FM, Ciapina LP, Souza RC, Mercante FM, de Faria SM, Baldani JI, Straliotto R, Hungria M, de Vasconcelos ATR. 2014. The complete genome of Burkholderia phenoliruptrix strain BR3459a, a symbiont of Mimosa flocculosa: highlighting the coexistence of symbiotic and pathogenic genes. BMC Genomics 15:535. doi: 10.1186/1471-2164-15-535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Choudhury JD, Pramanik A, Webster NS, Llewellyn LE, Gachhui R, Mukherjee J. 2015. The pathogen of the great barrier reef sponge Rhopaloeides odorabile is a new strain of Pseudoalteromonas agarivorans containing abundant and diverse virulence-related genes. Mar Biotechnol (NY) 17:463–478. doi: 10.1007/s10126-015-9627-y [DOI] [PubMed] [Google Scholar]
- 132. de Bruijn I, Cheng X, de Jager V, Expósito RG, Watrous J, Patel N, Postma J, Dorrestein PC, Kobayashi D, Raaijmakers JM. 2015. Comparative genomics and metabolic profiling of the genus Lysobacter. BMC Genomics 16:991. doi: 10.1186/s12864-015-2191-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chen W-J, Kuo T-Y, Hsieh F-C, Chen P-Y, Wang C-S, Shih Y-L, Lai Y-M, Liu J-R, Yang Y-L, Shih M-C. 2016. Involvement of type VI secretion system in secretion of iron chelator pyoverdine in Pseudomonas taiwanensis. Sci Rep 6:32950. doi: 10.1038/srep32950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Saffarian A, Touchon M, Mulet C, Tournebize R, Passet V, Brisse S, Rocha EPC, Sansonetti PJ, Pédron T. 2017. Comparative genomic analysis of Acinetobacter strains isolated from murine colonic crypts. BMC Genomics 18:525. doi: 10.1186/s12864-017-3925-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. McQuade R, Stock SP. 2018. Secretion systems and secreted proteins in Gram-negative entomopathogenic bacteria: their roles in insect virulence and beyond. Insects 9:68. doi: 10.3390/insects9020068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bansal K, Kumar S, Patil PB. 2020. Phylogenomic insights into diversity and evolution of nonpathogenic Xanthomonas strains associated with citrus. mSphere 5:e00087-20. doi: 10.1128/mSphere.00087-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhang Z, Yu YX, Wang YG, Liu X, Wang LF, Zhang H, Liao MJ, Li B. 2020. Complete genome analysis of a virulent Vibrio scophthalmi strain VSc190401 isolated from diseased marine fish half-smooth tongue sole, Cynoglossus semilaevis. BMC Microbiol 20:341. doi: 10.1186/s12866-020-02028-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kloska A, Cech GM, Sadowska M, Krause K, Szalewska-Pałasz A, Olszewski P. 2020. Adaptation of the marine bacterium Shewanella baltica to low temperature stress. Int J Mol Sci 21:4338. doi: 10.3390/ijms21124338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Tan XJ, Zhang ZW, Xiao JJ, Wang W, He F, Gao X, Jiang B, Shen L, Wang X, Sun Y, Zhu GP. 2022. Genomic and phenotypic biology of a novel Dickeya zeae WH1 isolated from rice in China: insights into pathogenicity and virulence factors. Front Microbiol 13:997486. doi: 10.3389/fmicb.2022.997486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ramnarine SDBJ, Jayaraman J, Ramsubhag A. 2022. Comparative genomics of the black rot pathogen Xanthomonas campestris pv. campestris and non-pathogenic co-inhabitant Xanthomonas melonis from Trinidad reveal unique pathogenicity determinants and secretion system profiles. PeerJ 9:e12632. doi: 10.7717/peerj.12632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ragab W, Kawato S, Nozaki R, Kondo H, Hirono I. 2022. Comparative genome analyses of five Vibrio penaeicida strains provide insights into their virulence-related factors. Microb Genom 8:000766. doi: 10.1099/mgen.0.000766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lee HJ, Storesund JE, Lunestad BT, Hoel S, Lerfall J, Jakobsen AN. 2023. Whole genome sequence analysis of Aeromonas spp. isolated from ready-to-eat seafood: antimicrobial resistance and virulence factors. Front Microbiol 14:1175304. doi: 10.3389/fmicb.2023.1175304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lopez AE, Mayoral J, Cianciotto NP. 2023. Complete genome sequence of Legionella cardiaca strain H63T, isolated from a case of native valve endocarditis. Microbiol Resour Announc 12:e0017523. doi: 10.1128/mra.00175-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wan Q, Zhai S, Chen M, Xu M, Guo S. 2024. Comparative phenotype and transcriptome analysis revealed the role of ferric uptake regulator (Fur) in the virulence of Vibrio harveyi isolated from diseased American eel (Anguilla rostrata). J Fish Dis 47:e13931. doi: 10.1111/jfd.13931 [DOI] [PubMed] [Google Scholar]
- 145. Slightom RN, Buchan A. 2009. Surface colonization by marine roseobacters: integrating genotype and phenotype. Appl Environ Microbiol 75:6027–6037. doi: 10.1128/AEM.01508-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Frank O, Göker M, Pradella S, Petersen J. 2015. Ocean's twelve: flagellar and biofilm chromids in the multipartite genome of Marinovum algicola DG898 exemplify functional compartmentalization. Environ Microbiol 17:4019–4034. doi: 10.1111/1462-2920.12947 [DOI] [PubMed] [Google Scholar]
- 147. Osman WAM, van Berkum P, León-Barrios M, Velázquez E, Elia P, Tian R, Ardley J, Gollagher M, Seshadri R, Reddy TBK, Ivanova N, Woyke T, Pati A, Markowitz V, Baeshen MN, Baeshen NN, Kyrpides N, Reeve W. 2017. High-quality draft genome sequence of Ensifer meliloti Mlalz-1, a microsymbiont of Medicago laciniata (L.) miller collected in Lanzarote, Canary Islands, Spain. Stand Genomic Sci 12:58. doi: 10.1186/s40793-017-0270-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Mota FF, Castro DP, Vieira CS, Gumiel M, Albuquerque JP, Carels N, Azambuja P. 2018. In vitro trypanocidal activity, genomic analysis of isolates, and in vivo transcription of type VI secretion system of Serratia marcescens belonging to the microbiota of Rhodnius prolixus digestive tract. Front Microbiol 9:3205. doi: 10.3389/fmicb.2018.03205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Boncan DAT, David AME, Lluisma AO. 2018. A CAZyme-rich genome of a taxonomically novel rhodophyte-associated Carrageenolytic marine bacterium. Mar Biotechnol 20:685–705. doi: 10.1007/s10126-018-9840-6 [DOI] [PubMed] [Google Scholar]
- 150. Ku C, Barak-Gavish N, Maienschein-Cline M, Green SJ, Vardi A. 2018. Complete genome sequence of Sulfitobacter sp. strain D7, a virulent bacterium isolated from an Emiliania huxleyi algal bloom in the North Atlantic. Microbiol Resour Announc 7:e01379-18. doi: 10.1128/MRA.01379-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Pratama AA, Jiménez DJ, Chen Q, Bunk B, Spröer C, Overmann J, van Elsas JD. 2020. Delineation of a subgroup of the genus Paraburkholderia, including P. terrae DSM 17804T, P. hospita DSM 17164T, and four soil-isolated fungiphiles, reveals remarkable genomic and ecological features-proposal for the definition of a P. hospita species cluster. Genome Biol Evol 12:325–344. doi: 10.1093/gbe/evaa031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ali S, Jenkins B, Cheng J, Lobb B, Wei X, Egan S, Charles TC, McConkey BJ, Austin J, Doxey AC. 2020. Slr4, a newly identified S-layer protein from Marine gammaproteobacteria, is a major biofilm matrix component. Mol Microbiol 114:979–990. doi: 10.1111/mmi.14588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zhu Z, Wang L, Qian H, Gu F, Li Y, Zhang H, Chen Y, Shi J, Ma P, Bao C, Gu B. 2021. Comparative genome analysis of 12 Shigella sonnei strains: virulence, resistance, and their interactions. Int Microbiol 24:83–91. doi: 10.1007/s10123-020-00145-x [DOI] [PubMed] [Google Scholar]
- 154. Takizawa S, Soga E, Hayashi W, Sakaguchi K, Koide S, Tanabe M, Denda T, Sugawara Y, Yu L, Kayama S, Sugai M, Nagano Y, Nagano N. 2022. Genomic landscape of blaGES-5- and blaGES-24-harboring Gram-negative bacteria from hospital wastewater: emergence of class 3 integron-associated blaGES-24 genes. J Glob Antimicrob Resist 31:196–206. doi: 10.1016/j.jgar.2022.09.005 [DOI] [PubMed] [Google Scholar]
- 155. Basak C, Chakraborty R. 2023. A novel strain of Shigella isolated from the gut of Lepidocephalichthys guntea has in its genome a complete gene package for Type ll secretion system, and elaborate repertoire of genes responsible for multiple antibiotic-resistance and metal resistance via specific efflux channels. Lett Appl Microbiol 76:ovac049. doi: 10.1093/lambio/ovac049 [DOI] [PubMed] [Google Scholar]
- 156. Maire J, Tandon K, Collingro A, van de Meene A, Damjanovic K, Gotze CR, Stephenson S, Philip GK, Horn M, Cantin NE, Blackall LL, van Oppen MJH. 2023. Colocalization and potential interactions of Endozoicomonas and chlamydiae in microbial aggregates of the coral Pocillopora acuta. Sci Adv 9:eadg0773. doi: 10.1126/sciadv.adg0773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lathem WW, Grys TE, Witowski SE, Torres AG, Kaper JB, Tarr PI, Welch RA. 2002. StcE, a metalloprotease secreted by Escherichia coli O157:H7, specifically cleaves C1 esterase inhibitor. Mol Microbiol 45:277–288. doi: 10.1046/j.1365-2958.2002.02997.x [DOI] [PubMed] [Google Scholar]
- 158. Zhang N, Yin S, Liu S, Sun A, Zhou M, Gong X, Ge H. 2017. Crystal structure of lpg1832, a VirK family protein from Legionella pneumophila, reveals a novel fold for bacterial VirK proteins. FEBS Lett 591:2929–2935. doi: 10.1002/1873-3468.12773 [DOI] [PubMed] [Google Scholar]
- 159. Vences A, Rivas AJ, Lemos ML, Husmann M, Osorio CR. 2017. Chromosome-encoded hemolysin, phospholipase, and collagenase in plasmidless isolates of Photobacterium damselae subsp. damselae contribute to virulence for fish. Appl Environ Microbiol 83:e00401-17. doi: 10.1128/AEM.00401-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gong X, Zhao X, Zhang W, Wang J, Chen X, Hameed MF, Zhang N, Ge H. 2018. Structural characterization of the hypothetical protein Lpg2622, a new member of the C1 family peptidases from Legionella pneumophila. FEBS Lett 592:2798–2810. doi: 10.1002/1873-3468.13210 [DOI] [PubMed] [Google Scholar]
- 161. Portlock TJ, Tyson JY, Dantu SC, Rehman S, White RC, McIntire IE, Sewell L, Richardson KH, Shaw R, Pandini A, Cianciotto NP, Garnett JA. 2020. Structure, dynamics and cellular insight into novel substrates of the Legionella pneumophila type II secretion system. Front Mol Biosci 7:112. doi: 10.3389/fmolb.2020.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Haurat MF, Scott NE, Di Venanzio G, Lopez J, Pluvinage B, Boraston AB, Ferracane MJ, Feldman MF. 2020. The glycoprotease CpaA secreted by medically relevant Acinetobacter species targets multiple O-linked host glycoproteins. mBio 11:e02033-20. doi: 10.1128/mBio.02033-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Chen X, Liu S, Jiang S, Zhang X, Zhang N, Ma J, Ge H. 2020. Crystal structure of a hypothetical T2SS effector Lpg0189 from Legionella pneumophila reveals a novel protein fold. Biochem Biophys Res Commun 521:799–805. doi: 10.1016/j.bbrc.2019.10.195 [DOI] [PubMed] [Google Scholar]
- 164. Rule CS, Park Y-J, Delarosa JR, Turley S, Hol WGJ, McColm S, Gura C, DiMaio F, Korotkov KV, Sandkvist M. 2020. Suppressor mutations in type II secretion mutants of Vibrio cholerae: inactivation of the VesC protease. mSphere 5:e01125-20. doi: 10.1128/mSphere.01125-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Condemine G, Le Derout B. 2022. Identification of new Dickeya dadantii virulence factors secreted by the type 2 secretion system. PLoS One 17:e0265075. doi: 10.1371/journal.pone.0265075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Martínez E, Orihuela CJ, Campos-Gomez J. 2022. Pseudomonas aeruginosa secretes the oxylipin autoinducer synthases OdsA and OdsB via the Xcp type 2 secretion system. J Bacteriol 204:e0011422. doi: 10.1128/jb.00114-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Nathawat R, Maku RV, Patel HK, Sankaranarayanan R, Sonti RV. 2022. Role of the FnIII domain associated with a cell wall-degrading enzyme cellobiosidase of Xanthomonas oryzae pv. oryzae. Mol Plant Pathol 23:1011–1021. doi: 10.1111/mpp.13205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hiller M, Diwo M, Wamp S, Gutsmann T, Lang C, Blankenfeldt W, Flieger A. 2024. Structure-function relationships underpin disulfide loop cleavage-dependent activation of Legionella pneumophila lysophospholipase A PlaA. Mol Microbiol 121:497–512. doi: 10.1111/mmi.15201 [DOI] [PubMed] [Google Scholar]
- 169. Sah GP, Cao P, Wall D. 2020. MYXO-CTERM sorting tag directs proteins to the cell surface via the type II secretion system. Mol Microbiol 113:1038–1051. doi: 10.1111/mmi.14473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Kamasaka K, Kawamoto J, Chen C, Yokoyama F, Imai T, Ogawa T, Kurihara T. 2020. Genetic characterization and functional implications of the gene cluster for selective protein transport to extracellular membrane vesicles of Shewanella vesiculosa HM13. Biochem Biophys Res Commun 526:525–531. doi: 10.1016/j.bbrc.2020.03.125 [DOI] [PubMed] [Google Scholar]
- 171. Gadwal S, Johnson TL, Remmer H, Sandkvist M. 2018. C-terminal processing of GlyGly-CTERM containing proteins by rhombosortase in Vibrio cholerae. PLoS Pathog 14:e1007341. doi: 10.1371/journal.ppat.1007341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. East A, Mechaly AE, Huysmans GHM, Bernarde C, Tello-Manigne D, Nadeau N, Pugsley AP, Buschiazzo A, Alzari PM, Bond PJ, Francetic O. 2016. Structural basis of pullulanase membrane binding and secretion revealed by X-ray crystallography, molecular dynamics and biochemical analysis. Structure 24:92–104. doi: 10.1016/j.str.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 173. Abdel-Nour M, Duncan C, Prashar A, Rao C, Ginevra C, Jarraud S, Low DE, Ensminger AW, Terebiznik MR, Guyard C. 2014. The Legionella pneumophila collagen-like protein mediates sedimentation, autoaggregation, and pathogen-phagocyte interactions. Appl Environ Microbiol 80:1441–1454. doi: 10.1128/AEM.03254-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Rondelet A, Condemine G. 2013. Type II secretion: the substrates that won't go away. Res Microbiol 164:556–561. doi: 10.1016/j.resmic.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 175. Paliwal D, Rabiey M, Mauchline TH, Hassani-Pak K, Nauen R, Wagstaff C, Andrews S, Bass C, Jackson RW. 2024. Multiple toxins and a protease contribute to the aphid-killing ability of Pseudomonas fluorescens PpR24. Environ Microbiol 26:e16604. doi: 10.1111/1462-2920.16604 [DOI] [PubMed] [Google Scholar]
- 176. Lazdunski A, Guzzo J, Filloux A, Bally M, Murgier M. 1990. Secretion of extracellular proteins by Pseudomonas aeruginosa. Biochimie 72:147–156. doi: 10.1016/0300-9084(90)90140-c [DOI] [PubMed] [Google Scholar]
- 177. Tommassen J, Filloux A, Bally M, Murgier M, Lazdunski A. 1992. Protein secretion in Pseudomonas aeruginosa. FEMS Microbiol Rev 9:73–90. doi: 10.1016/0378-1097(92)90336-m [DOI] [PubMed] [Google Scholar]
- 178. Lory S. 1992. Determinants of extracellular protein secretion in Gram-negative bacteria. J Bacteriol 174:3423–3428. doi: 10.1128/jb.174.11.3423-3428.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Wandersman C. 1992. Secretion across the bacterial outer membrane. Trends Genet 8:317–322. doi: 10.1016/0168-9525(92)90264-5 [DOI] [PubMed] [Google Scholar]
- 180. Salmond GP, Reeves PJ. 1993. Membrane traffic wardens and protein secretion in Gram-negative bacteria. Trends Biochem Sci 18:7–12. doi: 10.1016/0968-0004(93)90080-7 [DOI] [PubMed] [Google Scholar]
- 181. Pugsley AP. 1993. The complete general secretory pathway in Gram-negative bacteria. Microbiol Rev 57:50–108. doi: 10.1128/mr.57.1.50-108.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Hobbs M, Mattick JS. 1993. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol Microbiol 10:233–243. doi: 10.1111/j.1365-2958.1993.tb01949.x [DOI] [PubMed] [Google Scholar]
- 183. Pugsley AP, Francetic O, Possot OM, Sauvonnet N, Hardie KR. 1997. Recent progress and future directions in studies of the main terminal branch of the general secretory pathway in Gram-negative bacteria--a review. Gene 192:13–19. doi: 10.1016/s0378-1119(96)00803-7 [DOI] [PubMed] [Google Scholar]
- 184. Pugsley AP, Francetic O, Hardie K, Possot OM, Sauvonnet N, Seydel A. 1997. Pullulanase: model protein substrate for the general secretory pathway of Gram-negative bacteria. Folia Microbiol (Praha) 42:184–192. doi: 10.1007/BF02818976 [DOI] [PubMed] [Google Scholar]
- 185. Filloux A, Michel G, Bally M. 1998. GSP-dependent protein secretion in Gram-negative bacteria: the Xcp system of Pseudomonas aeruginosa. FEMS Microbiol Rev 22:177–198. doi: 10.1111/j.1574-6976.1998.tb00366.x [DOI] [PubMed] [Google Scholar]
- 186. Lory S. 1998. Secretion of proteins and assembly of bacterial surface organelles: shared pathways of extracellular protein targeting. Curr Opin Microbiol 1:27–35. doi: 10.1016/s1369-5274(98)80139-2 [DOI] [PubMed] [Google Scholar]
- 187. Russel M. 1998. Macromolecular assembly and secretion across the bacterial cell envelope: type II protein secretion systems. J Mol Biol 279:485–499. doi: 10.1006/jmbi.1998.1791 [DOI] [PubMed] [Google Scholar]
- 188. Thanassi DG, Hultgren SJ. 2000. Multiple pathways allow protein secretion across the bacterial outer membrane. Curr Opin Cell Biol 12:420–430. doi: 10.1016/s0955-0674(00)00111-3 [DOI] [PubMed] [Google Scholar]
- 189. Koster M, Bitter W, Tommassen J. 2000. Protein secretion mechanisms in Gram-negative bacteria. Int J Med Microbiol 290:325–331. doi: 10.1016/S1438-4221(00)80033-8 [DOI] [PubMed] [Google Scholar]
- 190. Stathopoulos C, Hendrixson DR, Thanassi DG, Hultgren SJ, St Geme JW, Curtiss III R. 2000. Secretion of virulence determinants by the general secretory pathway in Gram-negative pathogens: an evolving story. Microbes Infect 2:1061–1072. doi: 10.1016/s1286-4579(00)01260-0 [DOI] [PubMed] [Google Scholar]
- 191. Sandkvist M. 2001. Type II secretion and pathogenesis. Infect Immun 69:3523–3535. doi: 10.1128/IAI.69.6.3523-3535.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Sandkvist M. 2001. Biology of type II secretion. Mol Microbiol 40:271–283. doi: 10.1046/j.1365-2958.2001.02403.x [DOI] [PubMed] [Google Scholar]
- 193. Peabody CR, Chung YJ, Yen MR, Vidal-Ingigliardi D, Pugsley AP, Saier Jr MH. 2003. Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella. Microbiology (Reading) 149:3051–3072. doi: 10.1099/mic.0.26364-0 [DOI] [PubMed] [Google Scholar]
- 194. Desvaux M, Parham NJ, Scott-Tucker A, Henderson IR. 2004. The general secretory pathway: a general misnomer? Trends Microbiol 12:306–309. doi: 10.1016/j.tim.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 195. Filloux A. 2004. The underlying mechanisms of type II protein secretion. Biochim Biophys Acta-Mol Cell Res 1694:163–179. doi: 10.1016/j.bbamcr.2004.05.003 [DOI] [PubMed] [Google Scholar]
- 196. Johnson TL, Abendroth J, Hol WGJ, Sandkvist M. 2006. Type II secretion: from structure to function. FEMS Microbiol Lett 255:175–186. doi: 10.1111/j.1574-6968.2006.00102.x [DOI] [PubMed] [Google Scholar]
- 197. Gerlach RG, Hensel M. 2007. Protein secretion systems and adhesins: the molecular armory of Gram-negative pathogens. Int J Med Microbiol 297:401–415. doi: 10.1016/j.ijmm.2007.03.017 [DOI] [PubMed] [Google Scholar]
- 198. Poueymiro M, Genin S. 2009. Secreted proteins from Ralstonia solanacearum: a hundred tricks to kill a plant. Curr Opin Microbiol 12:44–52. doi: 10.1016/j.mib.2008.11.008 [DOI] [PubMed] [Google Scholar]
- 199. Cianciotto NP. 2009. Many substrates and functions of type II protein secretion: lessons learned from Legionella pneumophila. Future Microbiol 4:797–805. doi: 10.2217/fmb.09.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Büttner D, Bonas U. 2010. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol Rev 34:107–133. doi: 10.1111/j.1574-6976.2009.00192.x [DOI] [PubMed] [Google Scholar]
- 201. Ayers M, Howell PL, Burrows LL. 2010. Architecture of the type II secretion and type IV pilus machineries. Future Microbiol 5:1203–1218. doi: 10.2217/fmb.10.76 [DOI] [PubMed] [Google Scholar]
- 202. Korotkov KV, Gonen T, Hol WGJ. 2011. Secretins: dynamic channels for protein transport across membranes. Trends Biochem Sci 36:433–443. doi: 10.1016/j.tibs.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Filloux A. 2011. Protein secretion systems in Pseudomonas aeruginosa: an essay on diversity, evolution, and function. Front Microbiol 2:155. doi: 10.3389/fmicb.2011.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. McLaughlin LS, Haft RJF, Forest KT. 2012. Structural insights into the type II secretion nanomachine. Curr Opin Struct Biol 22:208–216. doi: 10.1016/j.sbi.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Korotkov KV, Sandkvist M, Hol WGJ. 2012. The type II secretion system: biogenesis, molecular architecture and mechanism. Nat Rev Microbiol 10:336–351. doi: 10.1038/nrmicro2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Douzi B, Filloux A, Voulhoux R. 2012. On the path to uncover the bacterial type II secretion system. Philos Trans R Soc Lond B Biol Sci 367:1059–1072. doi: 10.1098/rstb.2011.0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Tils D, Bladel I, Schmidt MA, Heusipp G. 2012. Type II secretion in Yersinia - A secretion system for pathogenicity and environmental fitness. Front Cell Infect Microbiol 2:160. doi: 10.3389/fcimb.2012.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Campos M, Cisneros DA, Nivaskumar M, Francetic O. 2013. The type II secretion system - a dynamic fiber assembly nanomachine. Res Microbiol 164:545–555. doi: 10.1016/j.resmic.2013.03.013 [DOI] [PubMed] [Google Scholar]
- 209. Peter Howard S. 2013. Assembly of the type II secretion system. Res Microbiol 164:535–544. doi: 10.1016/j.resmic.2013.03.018 [DOI] [PubMed] [Google Scholar]
- 210. Sikora AE. 2013. Proteins secreted via the type II secretion system: smart strategies of Vibrio cholerae to maintain fitness in different ecological niches. PLoS Pathog 9:e1003126. doi: 10.1371/journal.ppat.1003126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Nivaskumar M, Francetic O. 2014. Type II secretion system: a magic beanstalk or a protein escalator. Biochim Biophys Acta 1843:1568–1577. doi: 10.1016/j.bbamcr.2013.12.020 [DOI] [PubMed] [Google Scholar]
- 212. Chang JH, Desveaux D, Creason AL. 2014. The ABCs and 123s of bacterial secretion systems in plant pathogenesis. Annu Rev Phytopathol 52:317–345. doi: 10.1146/annurev-phyto-011014-015624 [DOI] [PubMed] [Google Scholar]
- 213. Costa TRD, Felisberto-Rodrigues C, Meir A, Prevost MS, Redzej A, Trokter M, Waksman G. 2015. Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nat Rev Microbiol 13:343–359. doi: 10.1038/nrmicro3456 [DOI] [PubMed] [Google Scholar]
- 214. Green ER, Mecsas J. 2016. Bacterial secretion systems: an overview. Microbiol Spectr 4. doi: 10.1128/microbiolspec.VMBF-0012-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Gu S, Shevchik VE, Shaw R, Pickersgill RW, Garnett JA. 2017. The role of intrinsic disorder and dynamics in the assembly and function of the type II secretion system. Biochim Biophys Acta 1865:1255–1266. doi: 10.1016/j.bbapap.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 216. Thomassin J-L, Santos Moreno J, Guilvout I, Tran Van Nhieu G, Francetic O. 2017. The trans-envelope architecture and function of the type 2 secretion system: new insights raising new questions. Mol Microbiol 105:211–226. doi: 10.1111/mmi.13704 [DOI] [PubMed] [Google Scholar]
- 217. Weber BS, Kinsella RL, Harding CM, Feldman MF. 2017. The secrets of Acinetobacter secretion. Trends Microbiol 25:532–545. doi: 10.1016/j.tim.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Pena RT, Blasco L, Ambroa A, González-Pedrajo B, Fernández-García L, López M, Bleriot I, Bou G, García-Contreras R, Wood TK, Tomás M. 2019. Relationship between quorum sensing and secretion systems. Front Microbiol 10:1100. doi: 10.3389/fmicb.2019.01100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Denise R, Abby SS, Rocha EPC. 2020. The evolution of protein secretion systems by co-option and tinkering of cellular machineries. Trends Microbiol 28:372–386. doi: 10.1016/j.tim.2020.01.005 [DOI] [PubMed] [Google Scholar]
- 220. Hui X, Chen Z, Zhang J, Lu M, Cai X, Deng Y, Hu Y, Wang Y. 2021. Computational prediction of secreted proteins in Gram-negative bacteria. Comput Struct Biotechnol J 19:1806–1828. doi: 10.1016/j.csbj.2021.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Filloux A. 2022. Bacterial protein secretion systems: game of types. Microbiology (Reading) 168. doi: 10.1099/mic.0.001193 [DOI] [PubMed] [Google Scholar]
- 222. Maphosa S, Moleleki LN, Motaung TE. 2023. Bacterial secretion system functions: evidence of interactions and downstream implications. Microbiology (Reading) 169:001326. doi: 10.1099/mic.0.001326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Whitaker DR. 1965. Lytic enzymes of Sorangium sp. isolation and enzymatic properties of the alpha- and beta-lytic proteases. Can J Biochem 43:1935–1954. doi: 10.1139/o65-217 [DOI] [PubMed] [Google Scholar]
- 224. Strominger JL, Ghuysen JM. 1967. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science 156:213–221. doi: 10.1126/science.156.3772.213 [DOI] [PubMed] [Google Scholar]
- 225. Coles NW, Gilbo CM, Broad AJ. 1969. Purification, properties and mechanism of action of a staphylolytic enzyme produced by Aeromonas hydrophila. Biochem J 111:7–15. doi: 10.1042/bj1110007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Sudo S, Dworkin M. 1972. Bacteriolytic enzymes produced by Myxococcus xanthus. J Bacteriol 110:236–245. doi: 10.1128/jb.110.1.236-245.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Dufourcq R, Chalkiadakis E, Fauchon M, Deslandes E, Kerjean V, Chanteau S, Petit E, Guezennec J, Dupont-Rouzeyrol M. 2014. Isolation and partial characterization of bacteria (Pseudoalteromonas sp.) with potential antibacterial activity from a marine costal environment from New Caledonia. Lett Appl Microbiol 58:102–108. doi: 10.1111/lam.12162 [DOI] [PubMed] [Google Scholar]
- 228. Yan Z, Fu M, Mir SH, Zhang L. 2023. Diversity and characterization of antagonistic bacteria against Pseudomonas syringae pv. actinidiae isolated from kiwifruit rhizosphere. FEMS Microbiol Lett 370:fnad078. doi: 10.1093/femsle/fnad078 [DOI] [PubMed] [Google Scholar]
- 229. Li SL, Norioka S, Sakiyama F. 1990. Molecular cloning and nucleotide sequence of the beta-lytic protease gene from Achromobacter lyticus. J Bacteriol 172:6506–6511. doi: 10.1128/jb.172.11.6506-6511.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Li S, Norioka S, Sakiyama F. 1997. Purification, staphylolytic activity, and cleavage sites of alpha-lytic protease from Achromobacter lyticus. J Biochem 122:772–778. doi: 10.1093/oxfordjournals.jbchem.a021822 [DOI] [PubMed] [Google Scholar]
- 4. Li S, Norioka S, Sakiyama F. 1998. Bacteriolytic activity and specificity of Achromobacter beta-lytic protease. J Biochem 124:332–339. doi: 10.1093/oxfordjournals.jbchem.a022116 [DOI] [PubMed] [Google Scholar]
- 5. Epstein DM, Wensink PC. 1988. The alpha-lytic protease gene of Lysobacter enzymogenes. The nucleotide sequence predicts a large prepro-peptide with homology to pro-peptides of other chymotrypsin-like enzymes. J Biol Chem 263:16586–16590. doi: 10.1016/S0021-9258(18)37430-1 [DOI] [PubMed] [Google Scholar]
- 233. Li S, Norioka S, Sakiyama F. 2000. Purification, characterization, and primary structure of a novel cell wall hydrolytic amidase, CwhA, from Achromobacter lyticus. J Biochem 127:1033–1039. doi: 10.1093/oxfordjournals.jbchem.a022694 [DOI] [PubMed] [Google Scholar]
- 234. Ahmed K, Chohnan S, Ohashi H, Hirata T, Masaki T, Sakiyama F. 2003. Purification, bacteriolytic activity, and specificity of beta-lytic protease from Lysobacter sp. IB-9374. J Biosci Bioeng 95:27–34. doi: 10.1016/S1389-1723(03)80144-5 [DOI] [PubMed] [Google Scholar]
- 235. Afoshin AS, Kudryakova IV, Borovikova AO, Suzina NE, Toropygin IY, Shishkova NA, Vasilyeva NV. 2020. Lytic potential of Lysobacter capsici VKM B-2533T: bacteriolytic enzymes and outer membrane vesicles. Sci Rep 10:9944. doi: 10.1038/s41598-020-67122-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Kudryakova I, Afoshin A, Tarlachkov S, Leontyevskaya E, Suzina N, Leontyevskaya Vasilyeva N. 2023. Lysobacter gummosus 10.1.1, a producer of antimicrobial agents. Microorganisms 11:2853. doi: 10.3390/microorganisms11122853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Arend KI, Schmidt JJ, Bentler T, Lüchtefeld C, Eggerichs D, Hexamer HM, Kaimer C. 2021. Myxococcus xanthus predation of Gram-positive or Gram-negative bacteria is mediated by different bacteriolytic mechanisms. Appl Environ Microbiol 87:e02382-20. doi: 10.1128/AEM.02382-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Zhou Y, Yi S, Zang Y, Yao Q, Zhu H. 2021. The predatory myxobacterium Citreicoccus inhibens gen. nov. sp. nov. showed antifungal activity and bacteriolytic property against phytopathogens. Microorganisms 9:2137. doi: 10.3390/microorganisms9102137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Li Y, Zhou X, Zhang X, Xu Z, Dong H, Yu G, Cheng P, Yao Q, Zhu H. 2022. A myxobacterial GH19 lysozyme with bacteriolytic activity on both Gram-positive and negative phytopathogens. AMB Express 12:54. doi: 10.1186/s13568-022-01393-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Zhou Y, Chen H, Jiang H, Yao Q, Zhu H. 2023. Characteristics of a lipase ArEstA with lytic activity against drug-resistant pathogen from a novel myxobacterium, Archangium lipolyticum sp. nov. Front Microbiol 14:1320827. doi: 10.3389/fmicb.2023.1320827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Wang C, Xiao Y, Wang Y, Liu Y, Yao Q, Zhu H. 2023. Comparative genomics and transcriptomics insight into myxobacterial metabolism potentials and multiple predatory strategies. Front Microbiol 14:1146523. doi: 10.3389/fmicb.2023.1146523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Kessler E, Safrin M, Olson JC, Ohman DE. 1993. Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J Biol Chem 268:7503–7508. doi: 10.1016/S0021-9258(18)53203-8 [DOI] [PubMed] [Google Scholar]
- 243. Gustin JK, Kessler E, Ohman DE. 1996. A substitution at His-120 in the LasA protease of Pseudomonas aeruginosa blocks enzymatic activity without affecting propeptide processing or extracellular secretion. J Bacteriol 178:6608–6617. doi: 10.1128/jb.178.22.6608-6617.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Kessler E, Safrin M, Abrams WR, Rosenbloom J, Ohman DE. 1997. Inhibitors and specificity of Pseudomonas aeruginosa LasA. J Biol Chem 272:9884–9889. doi: 10.1074/jbc.272.15.9884 [DOI] [PubMed] [Google Scholar]
- 245. Burke ME, Pattee PA. 1967. Purification and characterization of a staphylolytic enzyme from Pseudomonas aeruginosa. J Bacteriol 93:860–865. doi: 10.1128/jb.93.3.860-865.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Lache M, Hearn WR, Zyskind JW, Tipper DJ, Strominger JL. 1969. Specificity of a bacteriolytic enzyme from Pseudomonas aeruginosa. J Bacteriol 100:254–259. doi: 10.1128/jb.100.1.254-259.1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Brito N, Falcón MA, Carnicero A, Gutiérrez-Navarro AM, Mansito TB. 1989. Purification and peptidase activity of a bacteriolytic extracellular enzyme from Pseudomonas aeruginosa. Res Microbiol 140:125–137. doi: 10.1016/0923-2508(89)90046-6 [DOI] [PubMed] [Google Scholar]
- 248. Braun P, de Groot A, Bitter W, Tommassen J. 1998. Secretion of elastinolytic enzymes and their propeptides by Pseudomonas aeruginosa. J Bacteriol 180:3467–3469. doi: 10.1128/JB.180.13.3467-3469.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Bleves S, Viarre V, Salacha R, Michel GPF, Filloux A, Voulhoux R. 2010. Protein secretion systems in Pseudomonas aeruginosa: a wealth of pathogenic weapons. Int J Med Microbiol 300:534–543. doi: 10.1016/j.ijmm.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 250. Depluverez S, Devos S, Devreese B. 2016. The role of bacterial secretion systems in the virulence of Gram-negative airway pathogens associated with cystic fibrosis. Front Microbiol 7:1336. doi: 10.3389/fmicb.2016.01336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Spencer J, Murphy LM, Conners R, Sessions RB, Gamblin SJ. 2010. Crystal structure of the LasA virulence factor from Pseudomonas aeruginosa: substrate specificity and mechanism of M23 metallopeptidases. J Mol Biol 396:908–923. doi: 10.1016/j.jmb.2009.12.021 [DOI] [PubMed] [Google Scholar]
- 252. Razew A, Schwarz JN, Mitkowski P, Sabala I, Kaus-Drobek M. 2022. One fold, many functions-M23 family of peptidoglycan hydrolases. Front Microbiol 13:1036964. doi: 10.3389/fmicb.2022.1036964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Park S, Galloway DR. 1995. Purification and characterization of LasD: a second staphylolytic proteinase produced by Pseudomonas aeruginosa. Mol Microbiol 16:263–270. doi: 10.1111/j.1365-2958.1995.tb02298.x [DOI] [PubMed] [Google Scholar]
- 254. Folders J, Tommassen J, van Loon LC, Bitter W. 2000. Identification of a chitin-binding protein secreted by Pseudomonas aeruginosa. J Bacteriol 182:1257–1263. doi: 10.1128/JB.182.5.1257-1263.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Cahan R, Axelrad I, Safrin M, Ohman DE, Kessler E. 2001. A secreted aminopeptidase of Pseudomonas aeruginosa. Identification, primary structure, and relationship to other aminopeptidases. J Biol Chem 276:43645–43652. doi: 10.1074/jbc.M106950200 [DOI] [PubMed] [Google Scholar]
- 256. Zhao HL, Chen XL, Xie BB, Zhou MY, Gao X, Zhang XY, Zhou BC, Weiss AS, Zhang YZ. 2012. Elastolytic mechanism of a novel M23 metalloprotease pseudoalterin from deep-sea Pseudoalteromonas sp. CF6-2: cleaving not only glycyl bonds in the hydrophobic regions but also peptide bonds in the hydrophilic regions involved in cross-linking. J Biol Chem 287:39710–39720. doi: 10.1074/jbc.M112.405076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Lisboa J, Pereira C, Rifflet A, Ayala J, Terceti MS, Barca AV, Rodrigues I, Pereira PJB, Osorio CR, García-Del Portillo F, Gomperts Boneca I, do Vale A, Dos Santos NMS. 2021. A secreted NlpC/P60 endopeptidase from Photobacterium damselae subsp. piscicida cleaves the peptidoglycan of potentially competing bacteria. mSphere 6:e00736-20. doi: 10.1128/mSphere.00736-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Griffin ME, Klupt S, Espinosa J, Hang HC. 2023. Peptidoglycan NlpC/P60 peptidases in bacterial physiology and host interactions. Cell Chem Biol 30:436–456. doi: 10.1016/j.chembiol.2022.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Barnett MJ, Pinheiro J, Keown JR, Biboy J, Gray J, Lucinescu I-W, Vollmer W, Hirt RP, Simoes-Barbosa A, Goldstone DC. 2023. NlpC/P60 peptidoglycan hydrolases of Trichomonas vaginalis have complementary activities that empower the protozoan to control host-protective lactobacilli. PLoS Pathog 19:e1011563. doi: 10.1371/journal.ppat.1011563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Szczesny R, Jordan M, Schramm C, Schulz S, Cogez V, Bonas U, Büttner D. 2010. Functional characterization of the Xcs and Xps type II secretion systems from the plant pathogenic bacterium Xanthomonas campestris pv vesicatoria. New Phytol 187:983–1002. doi: 10.1111/j.1469-8137.2010.03312.x [DOI] [PubMed] [Google Scholar]
- 261. Barequet IS, Habot-Wilner Z, Mann O, Safrin M, Ohman DE, Kessler E, Rosner M. 2009. Evaluation of Pseudomonas aeruginosa staphylolysin (LasA protease) in the treatment of methicillin-resistant Staphylococcus aureus endophthalmitis in a rat model. Graefes Arch Clin Exp Ophthalmol 247:913–917. doi: 10.1007/s00417-009-1061-2 [DOI] [PubMed] [Google Scholar]
- 262. Barequet IS, Bourla N, Pessach YN, Safrin M, Yankovich D, Ohman DE, Rosner M, Kessler E. 2012. Staphylolysin is an effective therapeutic agent for Staphylococcus aureus experimental keratitis. Graefes Arch Clin Exp Ophthalmol 250:223–229. doi: 10.1007/s00417-011-1822-6 [DOI] [PubMed] [Google Scholar]
- 263. Radlinski L, Rowe SE, Kartchner LB, Maile R, Cairns BA, Vitko NP, Gode CJ, Lachiewicz AM, Wolfgang MC, Conlon BP. 2017. Pseudomonas aeruginosa exoproducts determine antibiotic efficacy against Staphylococcus aureus. PLoS Biol 15:e2003981. doi: 10.1371/journal.pbio.2003981 [DOI] [PMC free article] [PubMed] [Google Scholar]