Abstract
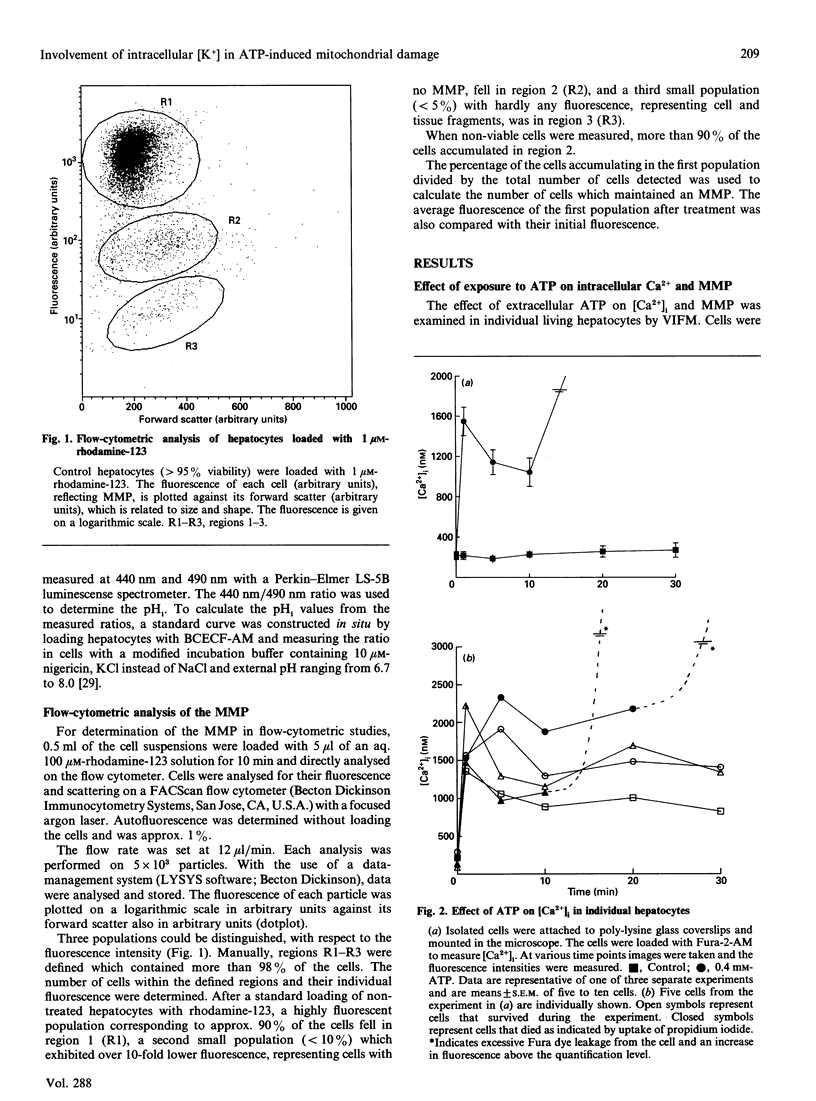
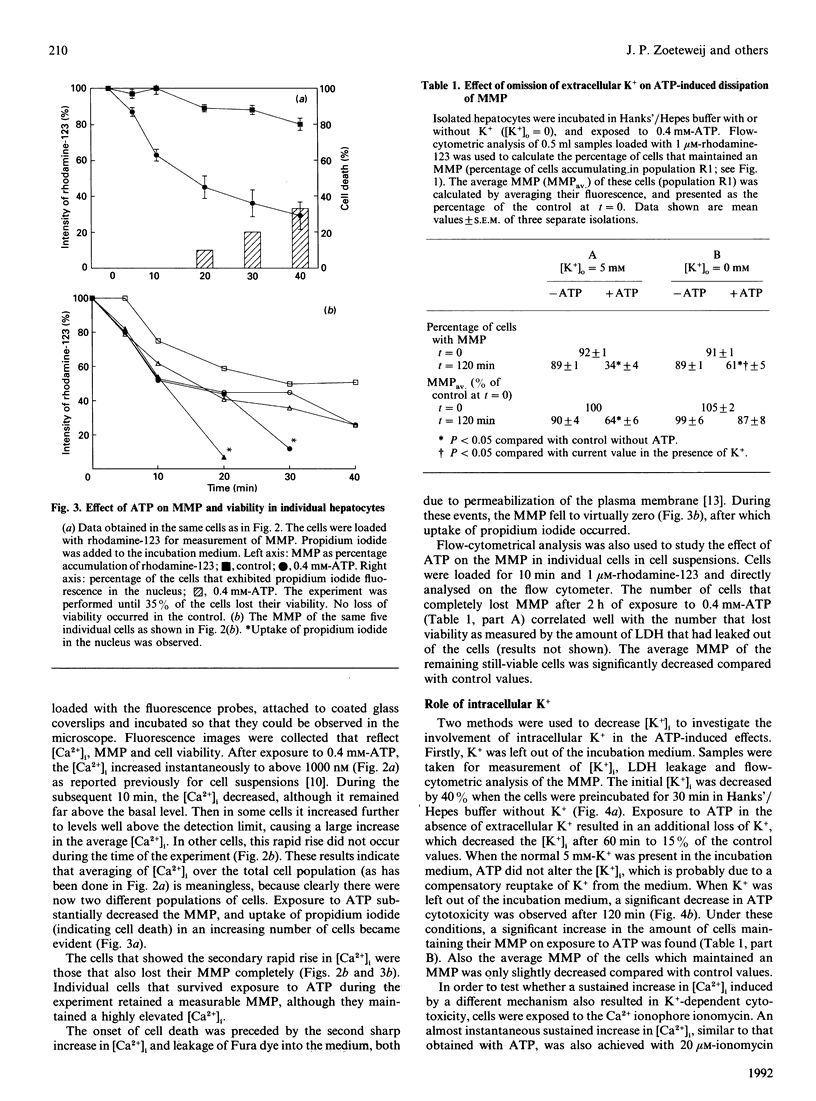
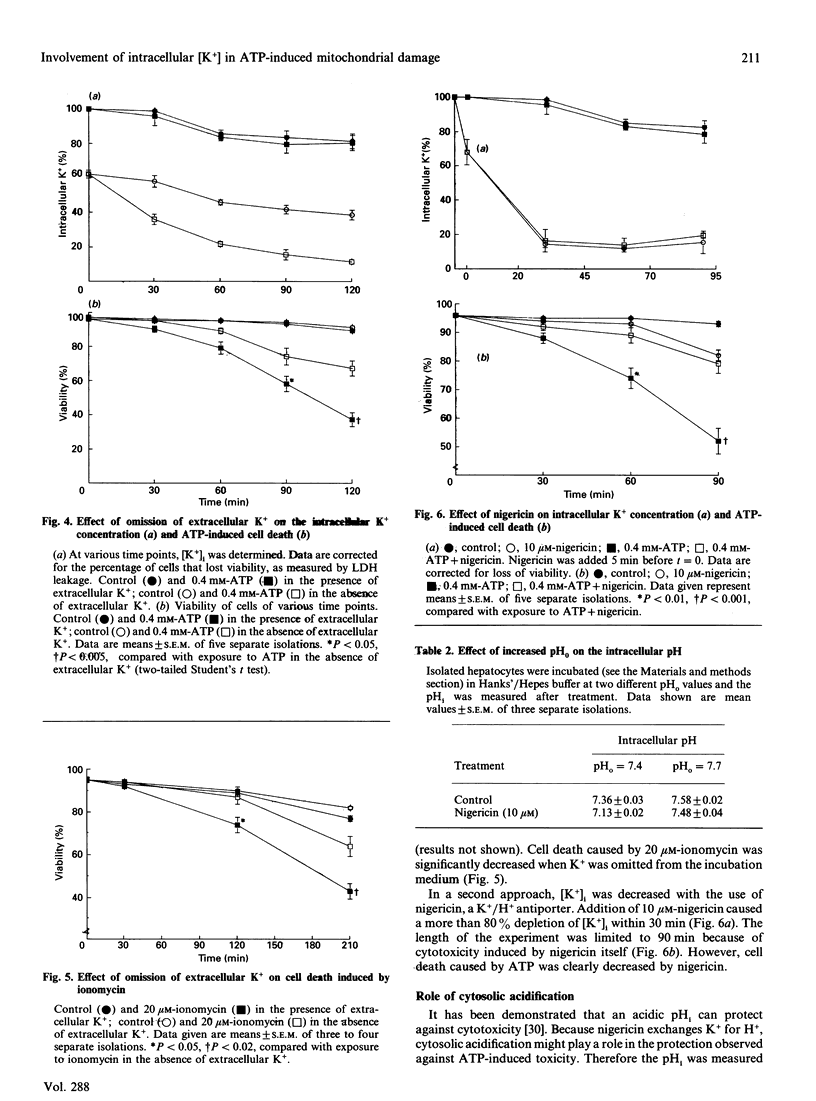
Isolated rat hepatocytes were incubated with extracellular ATP to induce a prolonged increase in intracellular Ca2+ ([Ca2+]i) and a loss of viability within 2 h. By using video-intensified fluorescence microscopy, the effects of exposure to extracellular ATP on [Ca2+]i, mitochondrial membrane potential (MMP) and cell viability were determined simultaneously in individual living hepatocytes. The increase in [Ca2+]i on exposure to ATP was followed by a decreasing MMP; there were big differences between individual cells. Complete loss of the MMP occurred before cell death was observed. Omission of K+ from the incubation medium decreased the cytotoxicity of ATP; under these conditions, intracellular K+ was decreased by more than 80%. Treatment with nigericin also depleted intracellular K+ and decreased ATP-induced toxicity. Protection against loss of viability by means of a decrease in intracellular [K+] was reflected by maintenance of the MMP. These observations suggest that ATP-induced cell death may be caused by a mechanism that has been described for isolated mitochondria: after an increase in Ca2+ levels, a K+ influx into mitochondria is induced, which finally disrupts the MMP and leads to cell death.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bright G. R., Fisher G. W., Rogowska J., Taylor D. L. Fluorescence ratio imaging microscopy: temporal and spatial measurements of cytoplasmic pH. J Cell Biol. 1987 Apr;104(4):1019–1033. doi: 10.1083/jcb.104.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekemeier K. M., Schmid P. C., Schmid H. H., Pfeiffer D. R. Effects of phospholipase A2 inhibitors on ruthenium red-induced Ca2+ release from mitochondria. J Biol Chem. 1985 Jan 10;260(1):105–113. [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Fabiato A., Leslie B. A., Putney J. W., Jr Calcium pools in saponin-permeabilized guinea pig hepatocytes. J Biol Chem. 1983 Dec 25;258(24):15336–15345. [PubMed] [Google Scholar]
- Carafoli E. Calcium pump of the plasma membrane. Physiol Rev. 1991 Jan;71(1):129–153. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- Carbonera D., Azzone G. F. Permeability of inner mitochondrial membrane and oxidative stress. Biochim Biophys Acta. 1988 Aug 18;943(2):245–255. doi: 10.1016/0005-2736(88)90556-1. [DOI] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Erdahl W. L., Krebsbach R. J., Pfeiffer D. R. A comparison of phospholipid degradation by oxidation and hydrolysis during the mitochondrial permeability transition. Arch Biochem Biophys. 1991 Mar;285(2):252–260. doi: 10.1016/0003-9861(91)90357-o. [DOI] [PubMed] [Google Scholar]
- Gores G. J., Nieminen A. L., Wray B. E., Herman B., Lemasters J. J. Intracellular pH during "chemical hypoxia" in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J Clin Invest. 1989 Feb;83(2):386–396. doi: 10.1172/JCI113896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths E. J., Halestrap A. P. Further evidence that cyclosporin A protects mitochondria from calcium overload by inhibiting a matrix peptidyl-prolyl cis-trans isomerase. Implications for the immunosuppressive and toxic effects of cyclosporin. Biochem J. 1991 Mar 1;274(Pt 2):611–614. doi: 10.1042/bj2740611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Halestrap A. P. The regulation of the matrix volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism. Biochim Biophys Acta. 1989 Mar 23;973(3):355–382. doi: 10.1016/s0005-2728(89)80378-0. [DOI] [PubMed] [Google Scholar]
- Hughes B. P., Milton S. E., Barritt G. J. Effects of vasopressin and La3+ on plasma-membrane Ca2+ inflow and Ca2+ disposition in isolated hepatocytes. Evidence that vasopressin inhibits Ca2+ disposition. Biochem J. 1986 Sep 15;238(3):793–800. doi: 10.1042/bj2380793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass G. E., Duddy S. K., Moore G. A., Orrenius S. 2,5-Di-(tert-butyl)-1,4-benzohydroquinone rapidly elevates cytosolic Ca2+ concentration by mobilizing the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool. J Biol Chem. 1989 Sep 15;264(26):15192–15198. [PubMed] [Google Scholar]
- Kehrer J. P., Jones D. P., Lemasters J. J., Farber J. L., Jaeschke H. Mechanisms of hypoxic cell injury. Summary of the symposium presented at the 1990 annual meeting of the Society of Toxicology. Toxicol Appl Pharmacol. 1990 Nov;106(2):165–178. doi: 10.1016/0041-008x(90)90238-p. [DOI] [PubMed] [Google Scholar]
- Kraus-Friedmann N. Calcium sequestration in the liver. Cell Calcium. 1990 Nov-Dec;11(10):625–640. doi: 10.1016/0143-4160(90)90017-o. [DOI] [PubMed] [Google Scholar]
- Moldéus P., Högberg J., Orrenius S. Isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- Nagelkerke J. F., Barto K. P., van Berkel T. J. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983 Oct 25;258(20):12221–12227. [PubMed] [Google Scholar]
- Nagelkerke J. F., Dogterom P., De Bont H. J., Mulder G. J. Prolonged high intracellular free calcium concentrations induced by ATP are not immediately cytotoxic in isolated rat hepatocytes. Changes in biochemical parameters implicated in cell toxicity. Biochem J. 1989 Oct 15;263(2):347–353. doi: 10.1042/bj2630347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Bellomo G., Orrenius S. The role of Ca2+ in cell killing. Chem Res Toxicol. 1990 Nov-Dec;3(6):484–494. doi: 10.1021/tx00018a001. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Hartzell P., Baldi C., Svensson S. A., Bellomo G., Orrenius S. Cystamine induces toxicity in hepatocytes through the elevation of cytosolic Ca2+ and the stimulation of a nonlysosomal proteolytic system. J Biol Chem. 1986 Nov 5;261(31):14628–14635. [PubMed] [Google Scholar]
- Nieminen A. L., Dawson T. L., Gores G. J., Kawanishi T., Herman B., Lemasters J. J. Protection by acidotic pH and fructose against lethal injury to rat hepatocytes from mitochondrial inhibitors, ionophores and oxidant chemicals. Biochem Biophys Res Commun. 1990 Mar 16;167(2):600–606. doi: 10.1016/0006-291x(90)92067-a. [DOI] [PubMed] [Google Scholar]
- Nieminen A. L., Gores G. J., Wray B. E., Tanaka Y., Herman B., Lemasters J. J. Calcium dependence of bleb formation and cell death in hepatocytes. Cell Calcium. 1988 Dec;9(5-6):237–246. doi: 10.1016/0143-4160(88)90004-8. [DOI] [PubMed] [Google Scholar]
- Olafsdottir K., Pascoe G. A., Reed D. J. Mitochondrial glutathione status during Ca2+ ionophore-induced injury to isolated hepatocytes. Arch Biochem Biophys. 1988 May 15;263(1):226–235. doi: 10.1016/0003-9861(88)90631-5. [DOI] [PubMed] [Google Scholar]
- Petit P. X., O'Connor J. E., Grunwald D., Brown S. C. Analysis of the membrane potential of rat- and mouse-liver mitochondria by flow cytometry and possible applications. Eur J Biochem. 1990 Dec 12;194(2):389–397. doi: 10.1111/j.1432-1033.1990.tb15632.x. [DOI] [PubMed] [Google Scholar]
- Pounds J. G., Rosen J. F. Cellular Ca2+ homeostasis and Ca2+-mediated cell processes as critical targets for toxicant action: conceptual and methodological pitfalls. Toxicol Appl Pharmacol. 1988 Jul;94(3):331–341. doi: 10.1016/0041-008x(88)90275-x. [DOI] [PubMed] [Google Scholar]
- Richelmi P., Mirabelli F., Salis A., Finardi G., Berte F., Bellomo G. On the role of mitochondria in cell injury caused by vanadate-induced Ca2+ overload. Toxicology. 1989 Jul 3;57(1):29–44. doi: 10.1016/0300-483x(89)90032-2. [DOI] [PubMed] [Google Scholar]
- Richter C., Kass G. E. Oxidative stress in mitochondria: its relationship to cellular Ca2+ homeostasis, cell death, proliferation, and differentiation. Chem Biol Interact. 1991;77(1):1–23. doi: 10.1016/0009-2797(91)90002-o. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Spät A., Bradford P. G., McKinney J. S., Rubin R. P., Putney J. W., Jr A saturable receptor for 32P-inositol-1,4,5-triphosphate in hepatocytes and neutrophils. Nature. 1986 Feb 6;319(6053):514–516. doi: 10.1038/319514a0. [DOI] [PubMed] [Google Scholar]
- Thomas C. E., Reed D. J. Effect of extracellular Ca++ omission on isolated hepatocytes. II. Loss of mitochondrial membrane potential and protection by inhibitors of uniport Ca++ transduction. J Pharmacol Exp Ther. 1988 May;245(2):501–507. [PubMed] [Google Scholar]


