Abstract
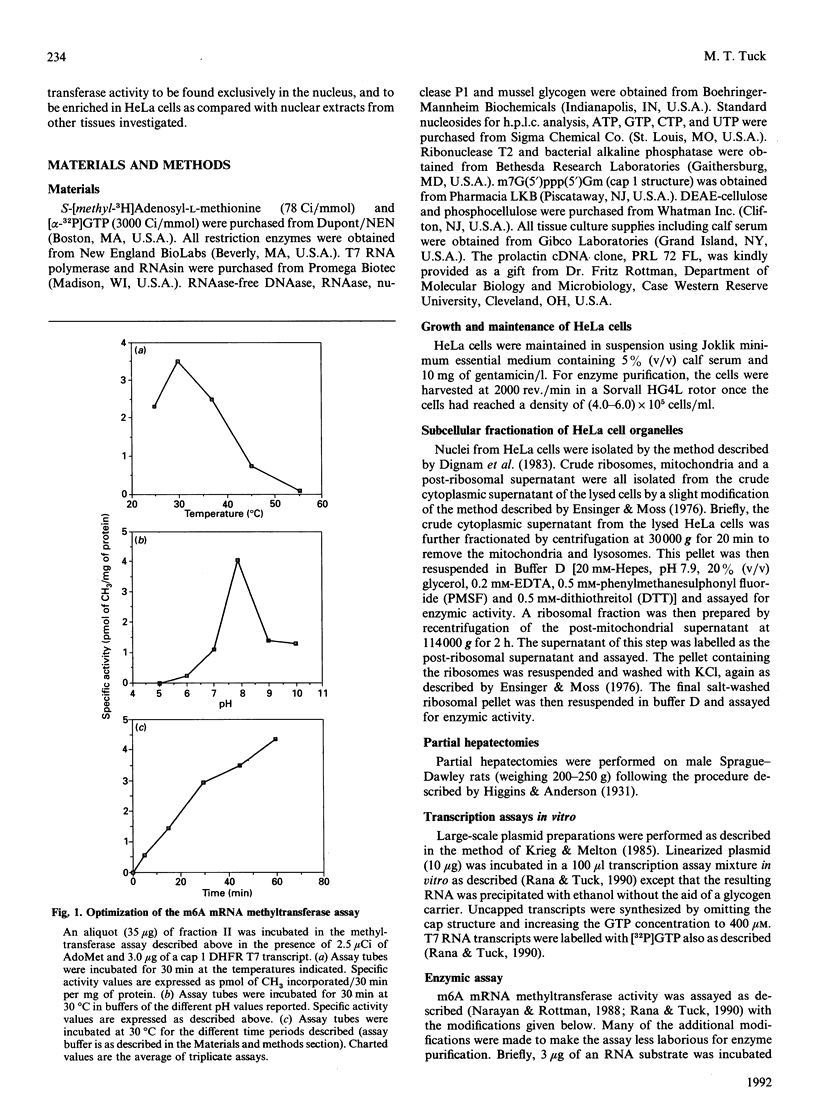
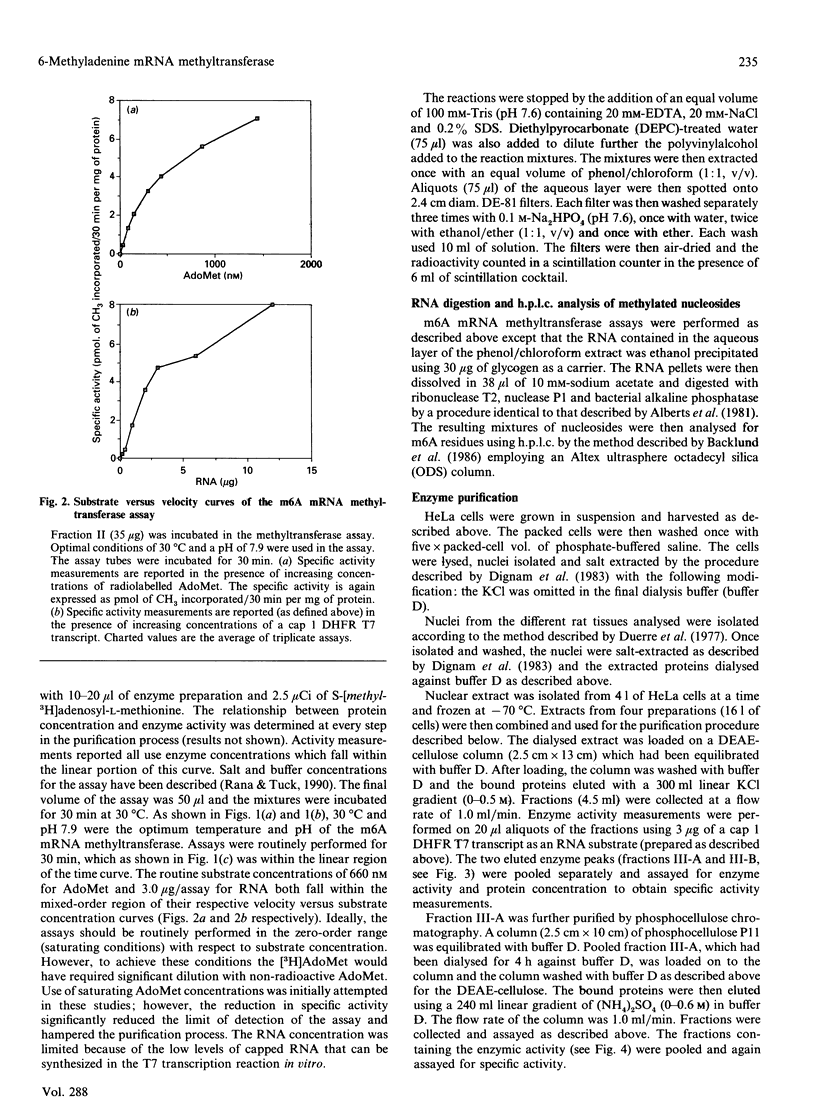
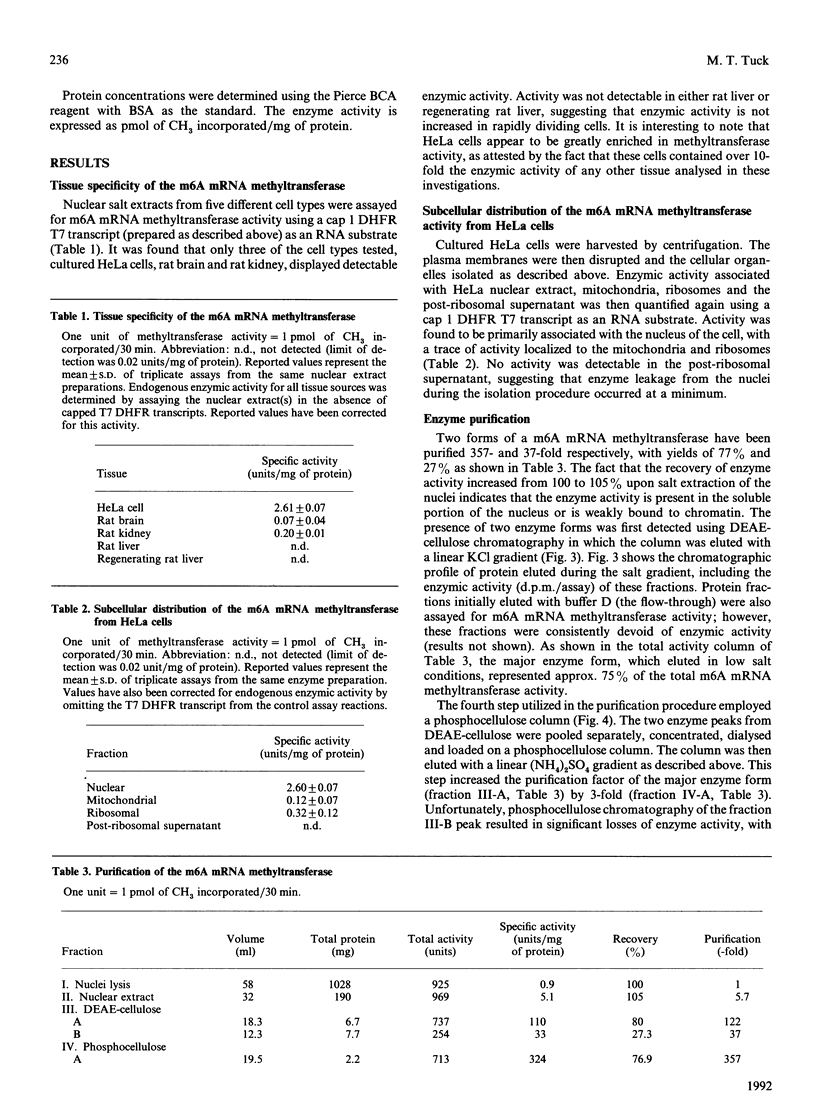
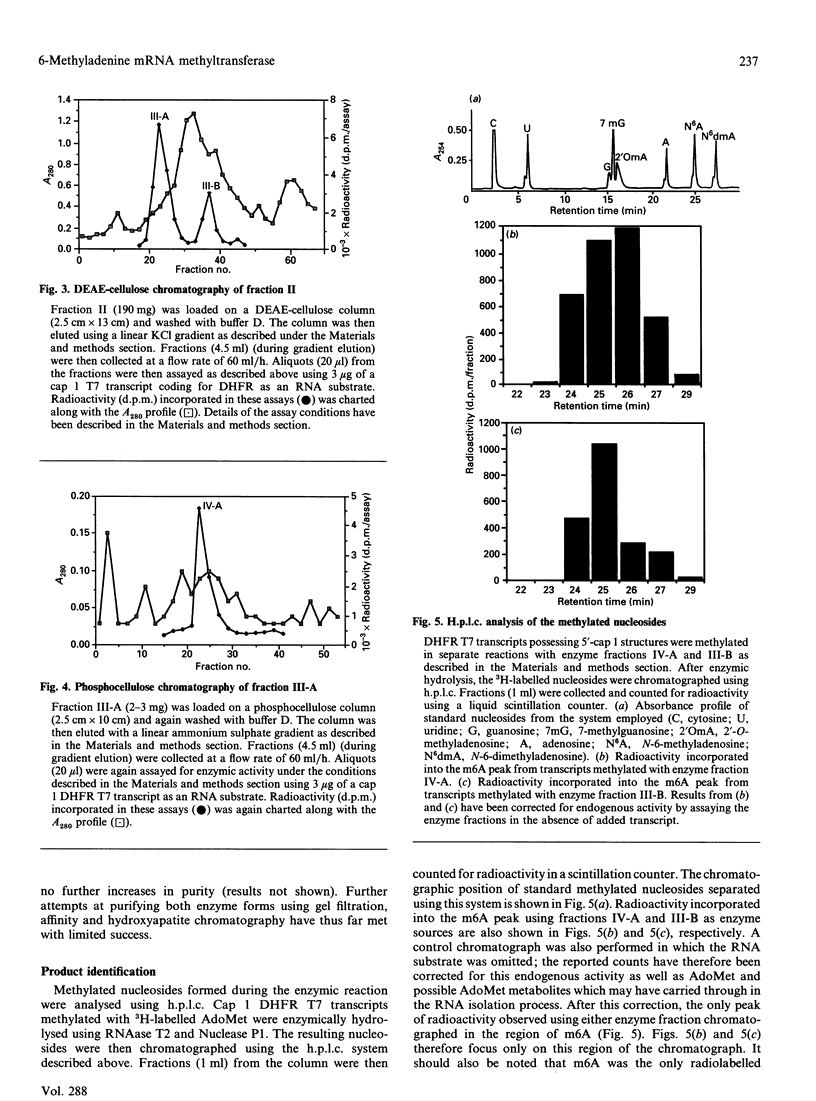
Two forms of a 6-methyladenine mRNA methyltransferase have been partially purified using a T7 transcript coding for mouse dihydrofolate reductase as an RNA substrate. Both enzyme forms modify internal adenine residues within the RNA substrate. The enzymes were purified 357- and 37-fold respectively from nuclear salt extracts prepared from HeLa cells using DEAE-cellulose and phosphocellulose chromatography. The activity of the first form of the enzyme eluted from DEAE-cellulose (major form) was at least 3-fold greater than that of the second (minor form). H.p.l.c. analysis of the hydrolysed, methylated mRNA substrates demonstrated that both forms of the enzyme produced only 6-methyladenine. The two forms of the enzyme differed in their RNA substrate specificity as well as in the dependence for a 5' cap structure. The 6-methyladenine mRNA methyltransferase activity was found to be elevated in HeLa nuclei as compared with nuclear extracts from rat kidney and brain. Enzymic activity could not be detected in nuclei from either normal rat liver or regenerating rat liver. In the case of the HeLa cell, activity could only be detected in nuclear extracts, with a small amount in the ribosomal fraction. Other HeLa subcellular fractions were void of activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Cory S. Modified nucleosides and bizarre 5'-termini in mouse myeloma mRNA. Nature. 1975 May 1;255(5503):28–33. doi: 10.1038/255028a0. [DOI] [PubMed] [Google Scholar]
- Albers R. J., Coffin B., Rottman F. M. Analysis of mRNA 5'-terminal cap structures and internal N6-methyladenosine by reversed-phase high-performance liquid chromatography. Anal Biochem. 1981 May 1;113(1):118–123. doi: 10.1016/0003-2697(81)90053-1. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Dhar R., Khoury G. Methylation of nuclear simian virus 40 RNAs. J Virol. 1979 Oct;32(1):52–60. doi: 10.1128/jvi.32.1.52-60.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund P. S., Jr, Carotti D., Cantoni G. L. Effects of the S-adenosylhomocysteine hydrolase inhibitors 3-deazaadenosine and 3-deazaaristeromycin on RNA methylation and synthesis. Eur J Biochem. 1986 Oct 15;160(2):245–251. doi: 10.1111/j.1432-1033.1986.tb09963.x. [DOI] [PubMed] [Google Scholar]
- Beemon K., Keith J. Localization of N6-methyladenosine in the Rous sarcoma virus genome. J Mol Biol. 1977 Jun 15;113(1):165–179. doi: 10.1016/0022-2836(77)90047-x. [DOI] [PubMed] [Google Scholar]
- Camper S. A., Albers R. J., Coward J. K., Rottman F. M. Effect of undermethylation on mRNA cytoplasmic appearance and half-life. Mol Cell Biol. 1984 Mar;4(3):538–543. doi: 10.1128/mcb.4.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani D., Kahana C., Lavi S., Groner Y. Identification and mapping of N6-methyladenosine containing sequences in simian virus 40 RNA. Nucleic Acids Res. 1979 Jun 25;6(8):2879–2899. doi: 10.1093/nar/6.8.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. M., Narayan P., Rottman F. M. N6-methyladenosine residues in an intron-specific region of prolactin pre-mRNA. Mol Cell Biol. 1990 Sep;10(9):4456–4465. doi: 10.1128/mcb.10.9.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Kiang S., Nevins J. R., Darnell J. E., Jr N-6-methyl-adenosine in adenovirus type 2 nuclear RNA is conserved in the formation of messenger RNA. J Mol Biol. 1979 Dec 15;135(3):733–752. doi: 10.1016/0022-2836(79)90174-8. [DOI] [PubMed] [Google Scholar]
- Csepany T., Lin A., Baldick C. J., Jr, Beemon K. Sequence specificity of mRNA N6-adenosine methyltransferase. J Biol Chem. 1990 Nov 25;265(33):20117–20122. [PubMed] [Google Scholar]
- Desrosiers R. C., Friderici K. H., Rottman F. M. Characterization of Novikoff hepatoma mRNA methylation and heterogeneity in the methylated 5' terminus. Biochemistry. 1975 Oct 7;14(20):4367–4374. doi: 10.1021/bi00691a004. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimock K., Stoltzfus C. M. Sequence specificity of internal methylation in B77 avian sarcoma virus RNA subunits. Biochemistry. 1977 Feb 8;16(3):471–478. doi: 10.1021/bi00622a021. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., Taylor R. H. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res. 1975 Oct;2(10):1653–1668. doi: 10.1093/nar/2.10.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J., Moss B. Modification of the 5' terminus of mRNA by an RNA (guanine-7-)-methyltransferase from HeLa cells. J Biol Chem. 1976 Sep 10;251(17):5283–5291. [PubMed] [Google Scholar]
- Finkel D., Groner Y. Methylations of adenosine residues (m6A) in pre-mRNA are important for formation of late simian virus 40 mRNAs. Virology. 1983 Dec;131(2):409–425. doi: 10.1016/0042-6822(83)90508-1. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Shatkin A. J., Jelinek W., Salditt-Georgieff M., Darnell J. E. Methylated, blocked 5 termini in HeLa cell mRNA. Proc Natl Acad Sci U S A. 1975 May;72(5):1904–1908. doi: 10.1073/pnas.72.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. E., Miceli S. M., Roberts R. J., Manley J. L. Sequence specificity of the human mRNA N6-adenosine methylase in vitro. Nucleic Acids Res. 1990 Oct 11;18(19):5735–5741. doi: 10.1093/nar/18.19.5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland R. A., Cline M. G. Post-transcriptional modifications of oat coleoptile ribonucleic acids. 5'-Terminal capping and methylation of internal nucleosides in poly(A)-rich RNA. Eur J Biochem. 1980 Feb;104(1):271–277. doi: 10.1111/j.1432-1033.1980.tb04425.x. [DOI] [PubMed] [Google Scholar]
- Horowitz S., Horowitz A., Nilsen T. W., Munns T. W., Rottman F. M. Mapping of N6-methyladenosine residues in bovine prolactin mRNA. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5667–5671. doi: 10.1073/pnas.81.18.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. E., Beemon K. Inhibition of methylation at two internal N6-methyladenosine sites caused by GAC to GAU mutations. J Biol Chem. 1987 Mar 5;262(7):3422–3427. [PubMed] [Google Scholar]
- Kennedy T. D., Lane B. G. Wheat embryo ribonucleates. XIII. Methyl-substituted nucleoside constituents and 5'-terminal dinucleotide sequences in bulk poly(AR)-rich RNA from imbibing wheat embryos. Can J Biochem. 1979 Jun;57(6):927–931. doi: 10.1139/o79-112. [DOI] [PubMed] [Google Scholar]
- Lavi S., Shatkin A. J. Methylated simian virus 40-specific RNA from nuclei and cytoplasm of infected BSC-1 cells. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2012–2016. doi: 10.1073/pnas.72.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi U., Fernandez-Muñoz R., Darnell J. E., Jr Content of N-6 methyl adenylic acid in heterogeneous nuclear and messenger RNA of HeLa cells. Nucleic Acids Res. 1977 Jan;4(1):63–69. doi: 10.1093/nar/4.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockard R. E., Lane C. Requirement for 7-methylguanosine in translation of globin mRNA in vivo. Nucleic Acids Res. 1978 Sep;5(9):3237–3247. doi: 10.1093/nar/5.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Gershowitz A., Stringer J. R., Holland L. E., Wagner E. K. 5'-Terminal and internal methylated nucleosides in herpes simplex virus type 1 mRNA. J Virol. 1977 Aug;23(2):234–239. doi: 10.1128/jvi.23.2.234-239.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P., Rottman F. M. An in vitro system for accurate methylation of internal adenosine residues in messenger RNA. Science. 1988 Nov 25;242(4882):1159–1162. doi: 10.1126/science.3187541. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Friderici K., Rottman F. The methylated constituents of L cell messenger RNA: evidence for an unusual cluster at the 5' terminus. Cell. 1975 Apr;4(4):387–394. doi: 10.1016/0092-8674(75)90159-2. [DOI] [PubMed] [Google Scholar]
- Rana A. P., Tuck M. T. Analysis and in vitro localization of internal methylated adenine residues in dihydrofolate reductase mRNA. Nucleic Acids Res. 1990 Aug 25;18(16):4803–4808. doi: 10.1093/nar/18.16.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Dane R. W. Accumulation of spliced avian retrovirus mRNA is inhibited in S-adenosylmethionine-depleted chicken embryo fibroblasts. J Virol. 1982 Jun;42(3):918–931. doi: 10.1128/jvi.42.3.918-931.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C. M., Gershowitz A., Moss B. 5'-Terminal and internal methylated nucleotide sequences in HeLa cell mRNA. Biochemistry. 1976 Jan 27;15(2):397–401. doi: 10.1021/bi00647a024. [DOI] [PubMed] [Google Scholar]


