Abstract
Background
Illicit opioid overdose continues to rise in North America and is a leading cause of death. Mathematical modeling is a valuable tool to investigate the epidemiology of this public health issue, as it can characterize key features of population outcomes and quantify the broader effect of structural and interventional changes on overdose mortality. The aim of this study is to quantify and predict the impact of key harm reduction strategies at differing levels of scale-up on fatal and nonfatal overdose among a population of people engaging in unregulated opioid use in Toronto.
Methods
An individual-based model for opioid overdose was built featuring demographic and behavioural variation among members of the population. Key individual attributes known to scale the risk of fatal and nonfatal overdose were identified and incorporated into a dynamic modeling framework, wherein every member of the simulated population encompasses a set of distinct characteristics that govern demographics, intervention usage, and overdose incidence. The model was parametrized to fatal and nonfatal overdose events reported in Toronto in 2019. The interventions considered were opioid agonist therapy (OAT), supervised consumption sites (SCS), take-home naloxone (THN), drug-checking, and reducing fentanyl in the drug supply. Harm reduction scenarios were explored relative to a baseline model to examine the impact of each intervention being scaled from 0% use to 100% use on overdose events.
Results
Model simulations resulted in 3690.6 nonfatal and 295.4 fatal overdoses, coinciding with 2019 data from Toronto. From this baseline, at full scale-up, 290 deaths were averted by THN, 248 from eliminating fentanyl from the drug supply, 124 from SCS use, 173 from OAT, and 100 by drug-checking services. Drug-checking and reducing fentanyl in the drug supply were the only harm reduction strategies that reduced the number of nonfatal overdoses.
Conclusions
Within a multi-faceted harm reduction approach, scaling up take-home naloxone, and reducing fentanyl in the drug supply led to the largest reduction in opioid overdose fatality in Toronto. Detailed model simulation studies provide an additional tool to assess and inform public health policy on harm reduction.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12954-024-01069-9.
Keywords: Drug checking, Harm reduction, Interventions, Mathematical model, Opioids, Overdose risk, PWID, Opioid use disorder, Take-home naloxone, Individual-based model, Microsimulation model
Background
Overdose death due to unregulated opioid use has become a public health crisis. Globally, two-thirds of deaths attributed to drugs are opioid-related [1]. Use is most prevalent in North America, where opioid overdose deaths have been on the rise for two decades. In Canada, approximately 20 deaths per day occurred due to opioid overdose in 2022, nearly double the rate in 2019, prior to the COVID-19 pandemic [2]. The increasing use of opioids over the last few decades was driven initially by medical overuse and has since resulted in increased overdose deaths fuelled by the introduction of fentanyl and fentanyl analogs into the unregulated drug supply [3].
For people with opioid use disorder (OUD), injection has been the predominant administration route, to hasten the onset of action or due to increased tolerance [4]. However, in recent years, inhalation has become more common [5]. In Ontario in 2019, of those deaths where evidence of injection or pipe/foil equipment (or both) was found at the scene, 58.6% included injection equipment, while 67.6% included pipe/foil equipment [6]. Although equipment on scene does not necessarily imply its use as the mode of consumption, opioid overdose risk occurs across multiple modes of administration.
Prescription opioid misuse can lead to a switch to cheaper alternatives such as heroin [7]; in one study, 80% of heroin users first had misused prescription opioids [8]. More recently, synthetic opioids have become widespread due to their further reduction in cost and increase in potency, fuelling a recent acceleration in overdose mortality [9]. The most prevalent synthetic opioid, fentanyl, was implicated in 77% of overdose deaths in Canada in 2020 and has overtaken heroin as the opioid most commonly found in opioid-related deaths in Toronto [2, 10].
Although access varies, several interventions exist to combat the mortality risks associated with unregulated drug consumption. Examples include medication-assisted treatment (MAT) such as opioid agonist therapy (OAT) [11], supervised consumption sites (SCS) [12], and take-home naloxone (THN) kits [13], among others [14]. More recently, a pilot program to investigate the efficacy of a rapid drug-checking program was implemented in Toronto, Canada. These measures aim to reduce the associated harm, by decreasing an individual’s reliance on an unregulated and potentially toxic drug supply, by reducing mortality risk during consumption, or by disseminating information about the composition of unknown drug samples to increase risk competency.
The risk of overdose and overdose fatality for an individual with OUD is dependent on several factors. For instance, these risks can be mitigated by the uptake of harm reduction interventions, but the risks can increase if the individual has a history of previous overdose or was recently released from incarceration. Accordingly, these risks are dynamic features of individuals and emerge from the combined effects of use history, drug composition, harm reduction uptake, and various other factors [15–18].
Mathematical modeling has been used to study opioid overdose from a number of perspectives (see [19] for a comprehensive review). Broadly, approaches can be taken at the population level, such as compartmental models (e.g., [20]) which dynamically track the sizes of defined subpopulations, or individual level, where each member of the population is resolved. For complex systems where interactions between individuals are important, agent-based models are suitable, and allow for dynamic network effects to arise [21]. Here, an individual-based micro-simulation model approach [22, 23] is used, where each individual is represented by a set of dynamic characteristics, suitable for a heterogeneous population where an individual’s own current and past state determines the future state. We quantify and predict the impact of key interventions at differing levels of scale-up on overdose incidence among a population of people with OUD in Toronto, Ontario, to determine how individual overdose risk factors, including access to relevant interventions, translate collectively to population-level outcomes. The resulting model offers an additional tool to inform harm reduction policy.
Methods
Study setting
This study focuses on the urban population of people with OUD in Toronto, Canada’s largest city. Opioid overdose is the leading cause of death among 20- to 39-year-olds in Ontario, with Toronto at the epicentre [2, 10]. Toronto is chosen as the study setting due to the established network of harm-reduction services, comprehensive data reporting on opioid overdose, and recent high-quality survey data [24–26].
Like elsewhere in North America, opioid-related deaths have increased dramatically in Toronto: between 2015 and 2017, such deaths increased by 125% before stabilizing into 2019, when 295 deaths due to opioid toxicity were confirmed. However, 2020 saw another dramatic rise of nearly 80%, to 545 deaths, rising further to 585 deaths in 2021, an increase exacerbated by the effects of the SARS-CoV-2 pandemic [27, 28]. Our model calibration is based on 2019 data to decouple results from pandemic-related effects.
Toronto employs a well-developed harm reduction strategy, including a network of SCS, naloxone distribution and training services, and OAT options [29]. Recent federal and provincial funding announcements aimed at combatting the opioid overdose crisis provide an opportunity to improve harm reduction options in Toronto. In 2019, a five-year pilot program was launched to implement a network of multi-site drug-checking services (DCS) in the city, unique among DCS across Canada [10].
Two survey studies (OiSIS-Toronto, and the I-Track Phase 2 study) have focused on the population of people who inject drugs (PWID) in Toronto [24–26]. Size estimates of this population vary (9,000–17,700) [30–32]. Although restricted to one form of administration (injection), and so not fully capturing recent trends toward inhalation, these survey data provide the best available description of demographic features and use behaviour in our study population. Together with this survey data, the availability of opioid overdose data (e.g., the Toronto Overdose Information System) and up-to-date data on the illicit drug supply in Toronto allow for accurate calibration of many model parameters [24, 26, 29, 33]. Toronto is, therefore, a natural setting to model opioid overdose dynamics and evaluate the effect of various harm reduction scenarios.
Model framework
We constructed a dynamic, stochastic, individual-based model for injection and overdose in a population of people with OUD. Each person in the population is modeled directly instead of tracking the sizes of defined subpopulations (i.e., compartmental modeling). An individual in the model is defined to be a person aged 14 and above engaging in unregulated opioid consumption, and is described by a set of ten dynamic attributes composing the relevant demography, use history, intervention usage, and drug composition. The model framework we describe here results from an expansive review of the literature for significant quantitative observations of factors modifying opioid overdose morbidity and mortality risk. Distilled from this review, the individual attributes we consider are age at initiation of use, current age to obtain length of drug use (equal to the difference of age and age of initiation), frequency, whether (and how many) previous nonfatal overdoses have occurred, whether the user’s drug contains fentanyl, and incarceration (whether or not an individual is in prison, or has been released from prison within the last three weeks).1 Additionally, individuals can access four harm reduction services: the status of whether an individual is currently accessing these services (described below) comprises an additional four attributes.
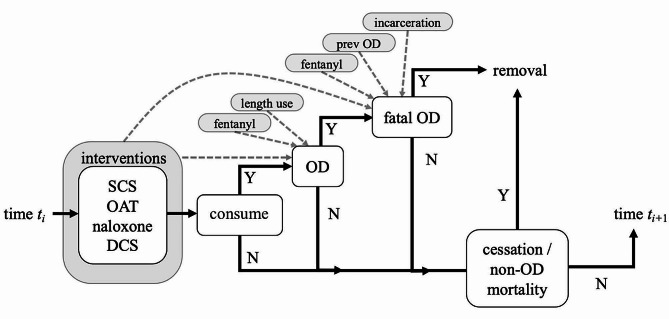
The model is time-dependent, and at each timestep, a person flows through a set of states as outlined in Fig. 1. Four different harm reduction strategies are available prior to consumption: SCS use, OAT, having THN present and available to be administered, and use of DCS to examine drug makeup and potency. First, the dichotomous use status of each intervention for a person is updated. Then the person may consume at the current timestep, with a probability determined by their use frequency. If consumption takes place, the person may overdose, with a probability based on the interventions used, whether fentanyl is present, and the length of use for that person. Should the person overdose, fatality can occur with a probability determined by a base risk, scaled by intervention usage, number of previous overdoses, presence of fentanyl, and time since release from incarceration (if applicable). If the overdose is fatal, the individual is removed from the population. Other removal (other-cause mortality, cessation of injection drug use) can also occur. Last, the set of attributes for each person is then updated either deterministically (age, length of use, previous overdose history) or probabilistically (incarceration state, fentanyl presence, use of the four harm-reduction strategies). Time then advances, and the process repeats.
Fig. 1.
A graphical representation of the model flow at one timestep. Black lines indicate flow pathways between events. ‘Y’ indicates yes for an event that occurs, whereas ‘N’ indicates no for an event that does not occur. Dotted arrows indicate probability dependencies from the individual attributes (grey) to a given event. Removal refers to an individual who is removed from the population
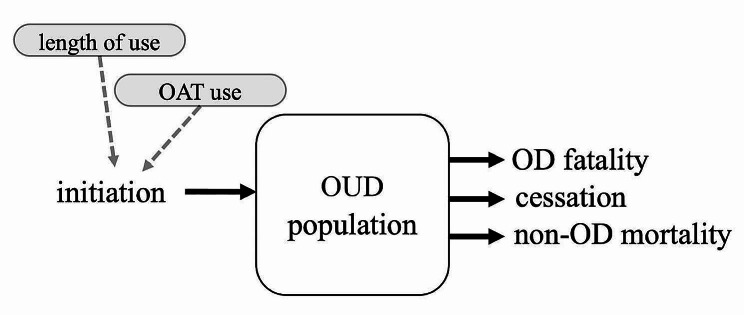
Because the model resolves each person, the population size at a given time is determined simply by summing the number of individuals at that time. Population-level dynamics are determined by the inflow and outflow of individual persons (see Fig. 2). At each timestep, a person may initiate another into opioid use and thus into the population, with a probability dependent on the length of use for the initiating person and whether the initiating person is currently using OAT [34, 35]. The summation of initiation over all individuals determines the inflow into the population. Outflow is determined by the summation of removal of individuals as described above and in Fig. 1- through fatal OD, other mortality, or through cessation of use.
Fig. 2.
Population-level dynamics determined by the summation of individuals entering and leaving the model population. Solid arrows (black) indicate flows into and out of the population stock. The dotted arrow indicates model dependency on attribute (grey). At each timestep, population inflow is the sum of initiation of new persons by all individuals, and outflow is the sum of all OD fatality, cessation, and non-OD all-cause mortality by all individuals
The detailed resolution of the individual mandates many parameters. These parameters determine the initialization of the population, the probabilities underpinning overdose and overdose fatality (and how each attribute modifies the risk of each), cessation, other-cause mortality, and initiation. Owing to the large parameter set, the model is computational in nature, where we look to numerically simulate the population dynamics over a fixed time frame (here, one year) for chosen parameter values versus obtaining analytical results (i.e., solutions to model equations or relationships between model parameters). The model is implemented in the scientific computing environment Matlab R2022b [36].
To quantify how individual attributes affect overdose and overdose fatality risk, base risk probabilities pOD and pODf (overdose and overdose fatality probability, respectively) were formulated and then scaled according to an individual’s set of specific factors to form an actual risk of overdose and fatality for that individual. Whereas the population-level actual risk can be deduced from annual public health reporting on overdose and overdose fatality, pOD and pODf are unknown, in that it is not possible to study a population of people with OUD who do not exhibit any of the risk-modifying factors we consider in this model. Our approach, therefore, is to set the scaling parameters governing how factors influence overdose and fatality risk, and then fit pOD and pODf such that model output matches reported overdose and fatality incidence in the chosen population, described below.
Model scenarios and outputs
Model simulations are run relative to a baseline parametrization, using all parameters calibrated to the Toronto study setting using 2019 overdose data from the Toronto Overdose Information System [27]. This baseline scenario is run with 80 trials for model validation, particularly for the calibrated values of pOD and pODf. From the baseline scenario, different harm reduction scenarios are explored. By varying in a range around the baseline value, the parameters controlling: SCS usage, the proportion using OAT, the probability of THN administered at OD, and the probability of using DCS prior to use, each intervention is tested independently, in turn. Additionally, the proportion of injections containing fentanyl is varied to explore changes in the composition of the drug supply. All other parameters are kept at baseline values. For each parameter combination, the model is run 25 times. Response to each of the parameter variations is measured through the outputs of total incidence of fatal and nonfatal OD in the population over the 365-day duration of the model run (representing the calendar year of 2019), averaged over the set of simulations for the parameter combination.
Model parametrization and calibration
The primary data source for model parametrization is the Ontario Integrated Supervised Injection Services cohort in Toronto (hereafter OiSIS), an open prospective cohort of 701 PWID aged 18 and over, sampled from 2018 to 2020 [26]. Additional parametrization was drawn from the I-Track Phase 2 survey (Toronto site) of PWID to monitor HIV, Hepatitis C, and associated risk behaviours (hereafter, I-Track), part of a national survey framework [24]. The Toronto site involved 255 PWID surveyed from 2006 to 2007. Additional parametrization was based on the latest available data and populations reasonably similar to our study setting. Full details of parametrization are included in the Appendix, including a summary list of all model parameters in Table A.1.
Results
Baseline model simulation results
At baseline, the study population of Toronto grew by 1.4% after one year, from 9000 to 9124 (95% CI: 9115–9133). There were 3690.6 (95% CI: 3679–3703) nonfatal overdoses and 295.4 (95% CI: 291.4-299.4) fatal overdoses. Of those experiencing a nonfatal overdose, 34.0% (95% CI: 33.8-34.2%) were receiving OAT, and 14.4% (95% CI: 14.3-14.5%) occurred at a SCS, whereas for fatal overdoses, 12.9% (95% CI: 12.4-13.3%) were receiving OAT, and none occurred at a SCS. Naloxone was present for 17.7% (95% CI: 17.6-17.8%) of nonfatal overdoses, but fatal overdoses with naloxone present were very rare (0.253% (95% CI: 0.19-0.32%) of fatal overdoses). At baseline, it is assumed no drug-checking services were being used.
Fentanyl was present in 81.1% (95% CI: 80.8-81.1%) of nonfatal overdoses but in 92.0% (95% CI: 91.7-92.4%) of fatal overdoses. The high-frequency injection class (daily use) composed 91.7% (95% CI: 91.6-91.8%) of all overdoses. The average age of individuals was similar across those experiencing a nonfatal overdose (42.32; 95% CI: 42.27–42.36) and fatal overdose (42.42; 95% CI: 42.30-42.54%).
27.6% (95% CI: 27.4-27.8%) of nonfatal overdoses occurred in individuals released from prison. Of those, 7.2% (95% CI: 7.0-7.4%) had been released within the two weeks before the overdose. In contrast, 40.8% (95% CI: 40.2-41.4%) of fatal overdoses occurred in those released from incarceration, and of those, 36.9% (95% CI: 35.9-37.8%) had been released within two weeks of the fatal overdose.
Of those experiencing a nonfatal overdose, 62% (95% CI: 61.8-62.1%) had no previous overdoses, 31.8% (95% CI: 31.7-32.0%) had one, 5.4% (95% CI: 5.3-5.5%) had two, and 0.8% (95% CI: 0.76-0.82%) had three or more. For those experiencing a fatal overdose, 46.1% (95% CI: 45.5-46.7%) had no previous overdose, 46.0% (95% CI: 45.4-46.7%) had one, 7.0% (95% CI: 6.7-7.3%) had two, and 0.85% (95% CI: 0.73-0.96%) had three or more.
Harm reduction interventions
From the baseline set of parameters, the impact of scaling up or down the four different harm reduction strategies were explored.
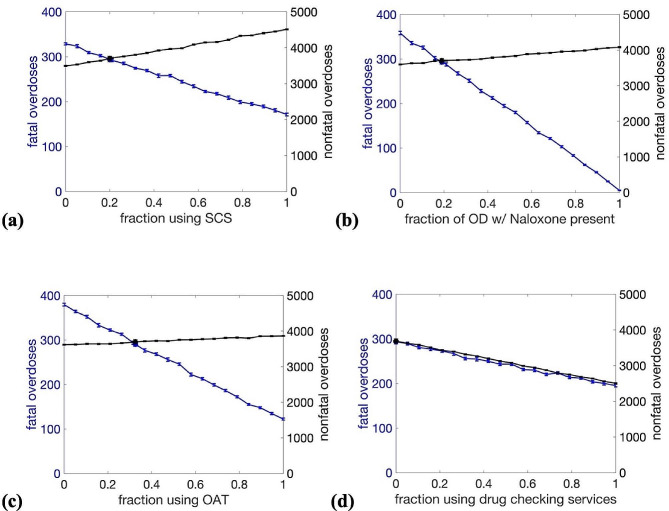
In Fig. 3.a., the fraction of the study population using any SCS services are scaled from 0 to 1 (i.e., fraction using SCS for none of their consumption scaling from 1 to 0), with a baseline value of 0.2. The relative proportion of use across classes of most, some, and few is kept constant. Scaling up SCS uptake from baseline leads to a moderate increase in nonfatal overdose due to the observed increased overdose risk at SCS [25]. At full scale-up of the entire population using SCS for a few, some, or most of their opioid use, an additional 820 overdoses were observed. Conversely, fatal overdoses decreased linearly, with 124 fatalities reduced from baseline at full use.
Fig. 3.
Parameter exploration of the four harm reduction strategies considered here: (a) supervised consumption site (SCS) use, (b) rate of presence of naloxone at overdose, (c) fraction of PWID population using opioid agonist treatment (OAT), and (d) fraction of population who use drug checking services prior to injection. Fatal overdoses (blue) and nonfatal overdoses (black) are plotted with standard error bars, and values corresponding to baseline parameters are indicated by blue and black circles, respectively
In Fig. 3.b., the probability of THN being present (and able to be administered) at an overdose is scaled from 0 to 1, with the baseline value at 0.19. Fatal overdose decreased most dramatically out of the four interventions considered. Current THN usage (at the baseline value) prevents 63 fatalities annually. At full scale-up, only five overdose fatalities occurred, an additional reduction of 290 fatalities. Nonfatal overdose increased slightly as THN presence increased due to the reduction in overall fatality, leading to a larger population and, thus, more use events. An additional 388 nonfatal overdoses were observed when THN was present for all injections.
In Fig. 3.c., the fraction of the population using OAT is scaled from 0 to 1, with a baseline value of 0.325. A significant reduction in overdose fatality is seen as OAT prevalence increases. At baseline usage, 85 fatalities are prevented versus having no OAT available, and additional scale-up beyond the baseline value further decreases fatalities, with 173 additional fatalities prevented at full usage. As in Fig. 3.b., there is a slight increase in nonfatal overdose (171 more overdoses at full usage) due to the resultant increase in population caused by the reduction in overdose fatality.
In Fig. 3.d., the probability that an individual will use drug-checking services before use is scaled from 0 to 1, with baseline at 0. Both nonfatal and fatal overdoses decrease linearly as DCS usage increases, with a reduction of 100 fatalities and 1185 nonfatal overdoses at full scale-up. Among the interventions tested, drug checking is the only one to reduce nonfatal overdose.
Fentanyl prevalence
A substantial increase in both fatal and nonfatal overdose is observed as the presence of fentanyl in the drug supply increases, with baseline prevalence estimated to be 0.5, based on drug checking service data in Toronto [33]. With no fentanyl in the drug supply, model simulations indicate that the population would experience an 84% reduction in fatal overdoses to just 47 overdoses (Fig. 4). Conversely, as the prevalence fraction increases to 1, fatal overdoses rise to 545. Similar trends are seen in nonfatal overdose, with such overdoses rising from 1434 with no fentanyl to 5851 with full fentanyl presence, a 58% increase from baseline nonfatal overdose rates.
Fig. 4.
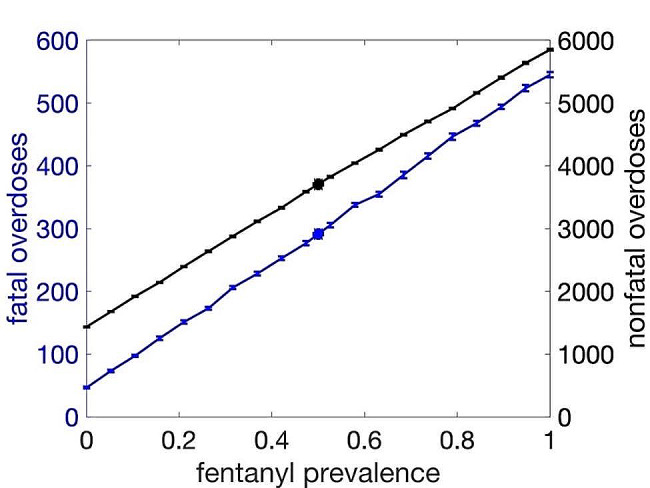
Effect of the prevalence of fentanyl in the illicit drug supply on fatal overdoses (blue) and nonfatal overdoses (black), plotted with standard error bars. Results of fatal and nonfatal overdose based on current baseline prevalence (0.50) is indicated by blue and black circles, respectively
Discussion
Computational models are a crucial tool for aiding the development of informed policy to address the opioid crisis. The model developed here uses an individual-based approach to combine multiple risk factors for overdose and overdose fatality to more fully understand how changes to demography, drug composition, and harm reduction interventions affect outcomes for a population of people with OUD.
Although this study focused on urban Toronto, the model is adaptable to other locations in a straightforward manner, provided sufficient data exists to parametrize the model specific to that population. Continuing and expanding high-quality survey studies of people with OUD are essential to further develop and update appropriate models. Results reported here center mainly on harm reduction, but the model is also useful to guide policy around incarceration, changes to the illicit drug supply, initiation, and effects of shifting demographics.
Results from this study suggest that expanding THN programs is the most effective harm-reduction strategy to reduce overdose fatality, with 35 lives saved per 10% increase in availability at overdose. THN is also one of the most cost-effective approaches to harm reduction. Improving access to THN at overdose depends on expanding kit distribution, increasing training on administering naloxone at overdose, and encouraging consumption in the presence of others who have access to a kit [37]. In our model framework, we implement THN intervention via a probability of administration at overdose. To more fully resolve this process as being administered from one individual to another, a framework with network dynamics would be required.
Expanding SCS services also significantly reduces overdose fatalities (approximately 16 fewer fatalities annually per 10% increase in SCS use). These services not only acutely reduce overdose fatality risk with available medical intervention but also connect users to education, recovery programs, and other harm reduction programs. The increase in non-fatal overdose observed with scale-up of SCS services reflects the increased risk factor as found in [25], but is likely not causative in nature, and more likely reflects a tendency of SCS to attract people with higher-risk use profiles [24, 25]. In practice, this association would likely reduce at higher levels of scale-up as differences in risk profiles of those using and not using SCS services would be less pronounced. OAT, such as methadone maintenance programs, began in the 1960s and remains an effective treatment for opioid use disorder [38]. Model results show a reduction in approximately 26 overdose fatalities annually per 10% increase in OAT use. The COVID-19 pandemic precipitated several changes to OAT services aimed at improving access, including federal exemptions to facilitate OAT prescription re-fills, deliveries, take-home (non-observed) doses, and reduced drug screening for patients [39]. Both SCS and OAT services also offer benefits not captured in this study, such as reducing blood-borne virus transmission in PWID by reducing injections or improving access to clean syringes [40].
As part of the multi-site drug-checking services initiative launched in Toronto in 2019, the advanced technique of using mass spectrometry is currently offered through three SCS sites. However, there are opportunities for more standardized drug testing (i.e., immunoassay test strips) independent of SCS at designated locations and events where drugs are expected to be present. Drug checking can reduce overdose risk beyond the individual checking their sample, as results are often shared with the broader population [41]. It is also an important outreach approach to attract users who may not access drug-related services otherwise [10].Where drug checking is offered through SCS, there is potential to improve the reach of other harm reduction services. DCS vary in cost, technology, and whether services are mobile or permanent. This approach gives the flexibility to provide support given various budget constraints, demand, and detectability standards.
The model was parametrized based on pre-pandemic surveys and statistical data. Using pre-pandemic data was a deliberate choice to generate model predictions independent of the effects of the pandemic. During the pandemic, harm reduction efforts experienced service closures, treatment disruptions, reduced operating hours, and reduced client capacity at SCS [42, 43]. Furthermore, directives on social distancing conflicted with the increased risks associated with injecting alone. By reflecting these changes in model parameter values, this study framework could predict the specific, quantified effects of each disruption, tested against the observed increases in overdose and overdose fatality since 2020.
Recent trends in route of administration of opioids presents a limitation of this work. Available survey data used here describe people who inject drugs, but those at risk of opioid overdose have increasingly also used inhalation as the route. Determining how demography, use behaviour, and overdose risk change in response to these shifting trends in usage will enable a more accurate description of the population at risk of opioid overdose.
Although this model tested the effect of each harm reduction strategy separately, efficacy and reach are optimized by scaling up these strategies collectively. Where resource limitations dictate the prioritization of investment into harm reduction strategies, model studies are useful to guide specific allocations toward combating the opioid crisis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to Dr. Daniel Werb for many helpful discussions and guidance.
Author contributions
KG and RL contributed to research design, creation of software, analysis of data, and drafting and revision of the manuscript.
Funding
This research was supported by a Nova Scotia Health Research Foundation Scotia Scholar Award and a Natural Sciences and Engineering Research Council Discovery Grant.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Research conducted by Groot et al. (2016) suggests the increase in overdose risk observed post-incarceration returns to baseline after three weeks.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Nations. World Drug Report 2023. [https://www.unodc.org/unodc/en/data-and-analysis/world-drug-report-2023.html]
- 2.Government of Canada. Opioid- and Stimulant-related Harms in Canada. 2023.
- 3.Belzak L, Halverson J. The opioid crisis in Canada: a national perspective. Health Promot Chronic Dis Prev Can. 2018;38(6):224–33. 10.24095/hpcdp.38.6.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strang JS, McDonald R. Preventing opioid overdose deaths with take-home naloxone. Publications Office; 2016.
- 5.Alambyan V, Pace J, Miller B, et al. The emerging role of inhaled heroin in the opioid epidemic: a review. JAMA Neurol. 2018;75(11):1423–34. 10.1001/jamaneurol.2018.1693. 10.1001/jamaneurol.2018.1693 [DOI] [PubMed] [Google Scholar]
- 6.Gomes T, Murray R, Kolla G, Leece P, Bansal S, Besharah J, Cahill T, Campbell T, Fritz A, Munro C, Toner L, Watford J, on behalf of the Ontario Drug Policy Research Network. Office of the Chief Coroner for Ontario and Ontario Agency for Health Protection and Promotion (Public Health Ontario). Changing circumstances surrounding opioid-related deaths in Ontario during the COVID-19 pandemic. Toronto, ON: Ontario Drug Policy Research Network; 2021. [Google Scholar]
- 7.Drug Enforcement Administration. National Drug Threat Assessment Summary 2013.
- 8.National Institute on Drug Abuse. Drug Overdose Death Rates 2023. https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates
- 9.Frank RG, Pollack HA. Addressing the fentanyl threat to public health. N Engl J Med. 2017;376(7):605–7. 10.1056/NEJMp1615145 [DOI] [PubMed] [Google Scholar]
- 10.Maghsoudi N, McDonald K, Stefan C, Beriault D, Mason K, Barnaby L, et al. Evaluating networked drug checking services in Toronto, Ontario: study protocol and rationale. Harm Reduct J. 2020;17:1–10. 10.1186/s12954-019-0336-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tenore PL. Psychotherapeutic benefits of opioid agonist therapy. J Addict Dis. 2008;27(3):49–65. 10.1080/10550880802122646 [DOI] [PubMed] [Google Scholar]
- 12.Pardo B, Kilmer B, Caulkins JP. Assessing the evidence on supervised drug consumption sites. Volume 5. RAND; 2018 Dec.
- 13.Moustaqim-Barrette A, Dhillon D, Ng J, Sundvick K, Ali F, Elton-Marshall T, Leece P, Rittenbach K, Ferguson M, Buxton JA. Take-home naloxone programs for suspected opioid overdose in community settings: a scoping umbrella review. BMC Public Health. 2021;21:1–6. 10.1186/s12889-021-10497-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wakeman SE. Harm reduction approaches for opioid use disorder. Treat Opioid Addict. 2019:169–80.
- 15.Keen C, Kinner SA, Young JT, Snow K, Zhao B, Gan W, et al. Periods of altered risk for non-fatal drug overdose: a self-controlled case series. Lancet Public Health. 2021;6(4):e249–59. 10.1016/S2468-2667(21)00007-4 [DOI] [PubMed] [Google Scholar]
- 16.Stoové MA, Dietze PM, Jolley D. Overdose deaths following previous non-fatal heroin overdose: record linkage of ambulance attendance and death registry data. Drug Alcohol Rev. 2009;28(4):347–52. 10.1111/j.1465-3362.2009.00057.x [DOI] [PubMed] [Google Scholar]
- 17.Binswanger IA, Blatchford PJ, Mueller SR, Stern MF. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592–600. 10.7326/0003-4819-159-9-201311050-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall BD, Krieger MS, Yedinak JL, Ogera P, Banerjee P, Alexander-Scott NE, et al. Epidemiology of fentanyl-involved drug overdose deaths: a geospatial retrospective study in Rhode Island, USA. Int J Drug Policy. 2017;46:130–5. 10.1016/j.drugpo.2017.05.029 [DOI] [PubMed] [Google Scholar]
- 19.Cerdá M, Jalali MS, Hamilton AD, DiGennaro C, Hyder A, Santaella-Tenorio J, Kaur N, Wang C, Keyes KM. A systematic review of simulation models to track and address the opioid crisis. Epidemiologic reviews. 2021;43(1):147-65. 10.1093/epirev/mxab013 [DOI] [PMC free article] [PubMed]
- 20.Battista NA, Pearcy LB, Strickland WC. Modeling the prescription opioid epidemic. Bull Math Biol. 2019;81:2258–89. 10.1007/s11538-019-00605-0 [DOI] [PubMed] [Google Scholar]
- 21.Brandon DL, Marshall S, Galea. Formalizing the role of Agent-based modeling in causal inference and epidemiology. Am J Epidemiol. 2015;181(2):92–9. 10.1093/aje/kwu274 [DOI] [PMC free article] [PubMed]
- 22.Ritter A, Shukla N, Shanahan M, Van Hoang P, Lam Cao V, Perez P, M. Farrell. Building a microsimulation model of heroin use careers in Australia. Int J Microsimulation. 2016;9(3):140–76. 10.34196/IJM.00146. 10.34196/IJM.00146 [DOI] [Google Scholar]
- 23.Shukla N, Van Hoang M, Shanahan M, Ritter A, Lam Cao V, Perez P. (2014). A lifetime individual sampling model (ISM) for heroin use and treatment evaluation in Australia. [10.13140/2.1.1833.4404]
- 24.Public Health Agency of Canada. I-Track - Enhanced Surveillance of HIV, Hepatitis C and associated risk behaviours among people who inject drugs in Canada: Phase 2 Report 2014. https://www.canada.ca/en/public-health/services/hiv-aids/publications/i-track-enhanced-surveillance-hiv-hepatitis-associated-risk-behaviours-people-who-inject-drugs-canada-phase-2.html
- 25.Scheim AI, Bouck Z, Tookey P, Hopkins S, Sniderman R, McLean E, et al. Supervised consumption service use and recent non-fatal overdose among people who inject drugs in Toronto, Canada. Int J Drug Policy. 2021;87:102993. 10.1016/j.drugpo.2020.102993 [DOI] [PubMed] [Google Scholar]
- 26.Scheim AI, Sniderman R, Wang R, Bouck Z, McLean E, Mason K, et al. The Ontario Integrated Supervised Injection Services Cohort Study of People Who Inject Drugs in Toronto, Canada (OiSIS-Toronto): Cohort Profile. J Urban Health. 2021;98(4):538–50. 10.1007/s11524-021-00547-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.City of Toronto. Toronto Overdose Information System 2023. [https://www.toronto.ca/community-people/health-wellness-care/health-inspections-monitoring/toronto-overdose-information-system/].
- 28.Gomes T, Kitchen SA, Murray R. Measuring the Burden of opioid-related mortality in Ontario, Canada, during the COVID-19 pandemic. JAMA Netw Open. 2021;4(5). [DOI] [PMC free article] [PubMed]
- 29.City of Toronto. Toronto Overdose Action Plan: Status Report 2020.
- 30.Archibald CP, Jayaraman GC, Major C, Patrick DM, Houston SM, Sutherland D. Estimating the size of hard-to-reach populations: a novel method using HIV testing data compared to other methods. Aids. 2001;15:S41–8. 10.1097/00002030-200104003-00006 [DOI] [PubMed] [Google Scholar]
- 31.Remis RS, Strathdee SA, Millson M, Leclerc L, Degani N, Palmer RW et al. Consortium to characterize injection drug users in Montreal, Toronto and Vancouver, Canada. Contract for Health Canada. 1998.
- 32.Bayoumi AM, Bayoumi AM, Strike. C (co-principal investigators). In: Jairam J, Watson T, Enns E, Kolla G, Lee A, Shepherd S, Hopkins S, Millson M, Leonard L, Zaric G, Luce J, Degani N, Fischer B, Glazier R, O’Campo P, Smith C, Penn R, Brandeau M, editors. Report of the Toronto and Ottawa supervised Consumption Assessment Study, 2012. Toronto, Ontario: St. Michael’s Hospital and the Dalla Lana School of Public Health, University of Toronto; 2012. [Google Scholar]
- 33.McDonald K, Maghsoudi N, Thompson H, Werb D. What’s in Toronto’s drug supply? Results from samples checked by Toronto’s drug checking service: October 10, 2019-March 31, 2020. Centre on Drug Policy Evaluation. Toronto. 2020.
- 34.Marks C, Borquez A, Jain S, Sun X, Strathdee SA, Garfein RS, et al. Opioid agonist treatment scale-up and the initiation of injection drug use: a dynamic modeling analysis. PLoS Med. 2019;16(11):e1002973. 10.1371/journal.pmed.1002973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mittal ML, Jain S, Sun S, DeBeck K, Milloy MJ, Hayashi K, et al. Opioid agonist treatment and the process of injection drug use initiation. Drug Alcohol Depend. 2019;197:354–6. 10.1016/j.drugalcdep.2018.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MATLAB version. 9.13. 0 (R2022b), Natick, Massachusetts: The MathWorks Inc.; 2022.
- 37.Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158(1):1–9. 10.7326/0003-4819-158-1-201301010-00003 [DOI] [PubMed] [Google Scholar]
- 38.Murray JB. Effectiveness of methadone maintenance for heroin addiction. Psychol Rep. 1998;83(1):295–302. 10.2466/pr0.1998.83.1.295 [DOI] [PubMed] [Google Scholar]
- 39.Bouck Z, Scheim AI, Gomes T, Ling V, Caudarella A, Werb D. Evaluating interventions to facilitate opioid agonist treatment access among people who inject drugs in Toronto, Ontario during COVID-19 pandemic restrictions. Int J Drug Policy. 2022;104:103680. 10.1016/j.drugpo.2022.103680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Degenhardt L, Wolfe D, Hall W, Hickman M, Chang J, Bruneau J, et al. Strategies to reduce drug-related harm: responding to the evidence base. Lancet. 2019;394(10208):1490–3. 10.1016/S0140-6736(19)32232-9 [DOI] [PubMed] [Google Scholar]
- 41.Charlois T, editor. Safer nightlife in Europe. 5th Meeting EXASS Network; 2009.
- 42.Russell C, Ali F, Nafeh F, Rehm J, LeBlanc S, Elton-Marshall T. Identifying the impacts of the COVID-19 pandemic on service access for people who use drugs (PWUD): a national qualitative study. J Subst Abuse Treat. 2021;129:108374. 10.1016/j.jsat.2021.108374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The Global Fund. Results Report 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.