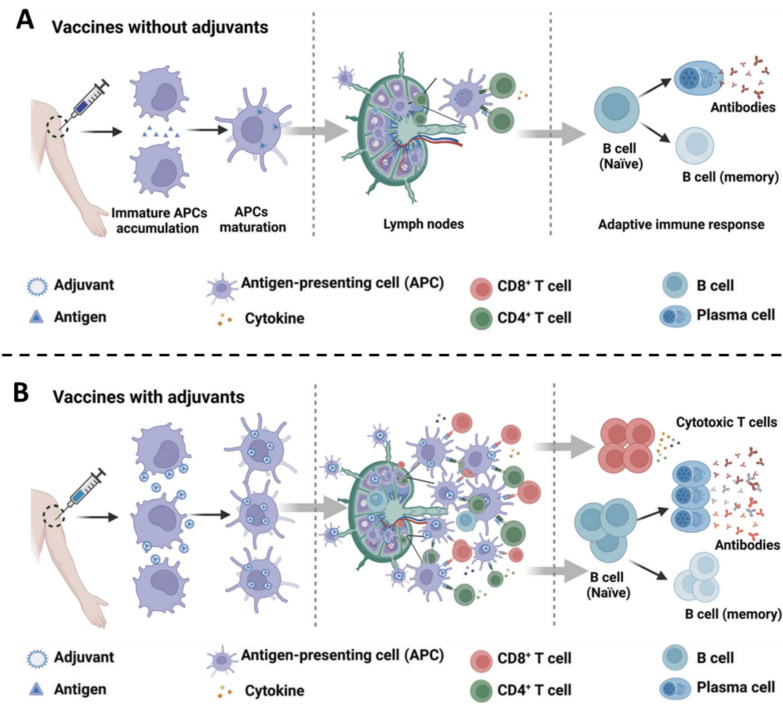
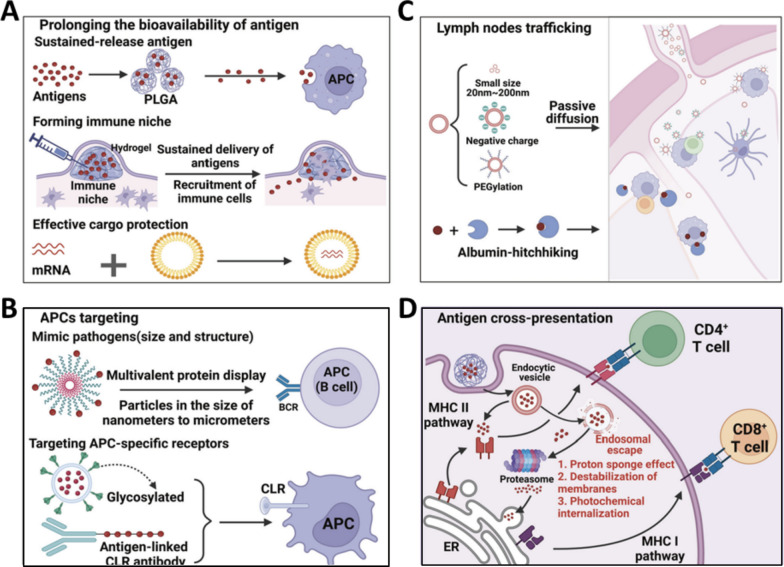
Abstract
Biomaterials are substances that can be injected, implanted, or applied to the surface of tissues in biomedical applications and have the ability to interact with biological systems to initiate therapeutic responses. Biomaterial-based vaccine delivery systems possess robust packaging capabilities, enabling sustained and localized drug release at the target site. Throughout the vaccine delivery process, they can contribute to protecting, stabilizing, and guiding the immunogen while also serving as adjuvants to enhance vaccine efficacy. In this article, we provide a comprehensive review of the contributions of biomaterials to the advancement of vaccine development. We begin by categorizing biomaterial types and properties, detailing their reprocessing strategies, and exploring several common delivery systems, such as polymeric nanoparticles, lipid nanoparticles, hydrogels, and microneedles. Additionally, we investigated how the physicochemical properties and delivery routes of biomaterials influence immune responses. Notably, we delve into the design considerations of biomaterials as vaccine adjuvants, showcasing their application in vaccine development for cancer, acquired immunodeficiency syndrome, influenza, corona virus disease 2019 (COVID-19), tuberculosis, malaria, and hepatitis B. Throughout this review, we highlight successful instances where biomaterials have enhanced vaccine efficacy and discuss the limitations and future directions of biomaterials in vaccine delivery and immunotherapy. This review aims to offer researchers a comprehensive understanding of the application of biomaterials in vaccine development and stimulate further progress in related fields.
Graphical Abstract
Keywords: Biomaterials, Vaccine delivery, Drug delivery, Vaccine adjuvant
Introduction
Vaccines are an important means of preventing and controlling infectious diseases, and effective vaccine systems are essential for vaccine protection. However, the application of conventional vaccines in the medical field is limited by numerous long-standing problems, such as susceptibility to breakdown by the body, poor immunogenicity, weak targeting, and difficulties in storage and delivery. Innovative approaches for vaccine development are urgently required to meet the growing demand for high-quality vaccines. To address this issue, a new class of biomimetic materials has been extensively explored for use in vaccines. These biomaterials offer targeted and sustained release of multiple immunotherapeutics while protecting them from enzymatic degradation and harsh pH conditions [1], thereby improving vaccine efficacy. This biomaterial-based vaccine delivery system has attracted considerable research attention because it focuses on enhancing the immune response of the body by delivering antigens and adjuvants together. In recent years, biomaterials have been extensively studied and utilized in various fields, such as drug delivery, vaccine development, gene therapy, stem cell therapy, and tissue regeneration, owing to their excellent biocompatibility and biodegradability [2–7]. Biomaterials are currently widely utilized as carriers for vaccine delivery, leading to improved vaccine efficacy for the treatment of diverse diseases. For instance, biomaterials have the ability to transport drugs through physical barriers, such as mucous membranes, and shield antigens from premature degradation, while also targeting lymphoid tissues and effectively presenting antigens and adjuvants to the appropriate immune cells, eliciting a robust immune response. Furthermore, certain biomaterials with immunomodulatory properties and controlled antigen release capabilities demonstrate a sufficient capacity for sustained drug release at the desired location [8, 9].
Certain biomaterials possess inherent immunogenicity and can serve as effective immunoadjuvants. Vaccine adjuvants play a pivotal role in augmenting the immune response to antigens, thereby bolstering the capacity to prevent infections. Consequently, they are indispensable for the development of safe and efficacious vaccines against a broad spectrum of infectious diseases. Recently, several vaccine adjuvants have been developed and approved for human use, including MF59, AS03, AF03, and other emulsion adjuvants [10]. Among these, MF59 nanoemulsion has been approved as an influenza vaccine adjuvant in the United States, with an excellent vaccination record [11, 12].
The exceptional potential of biomaterials positions them with extensive developmental prospects in the realm of biomedical engineering. However, the current challenges pertaining to vaccine transportation and storage conditions, delivery efficiency, cell targeting, and material safety remain significant hurdles that biomaterials must surmount in the field of vaccine delivery. Consequently, a systematic review of recent advancements in biomaterials for vaccine development to guide and inspire further innovation is imperative. This review summarizes the types of biomaterials and strategies used for their reprocessing, including polymer-based nanoparticles (PNPs), liposome-based nanoparticles (LNPs), hydrogels, and microneedles (MNs). Second, we discuss the physicochemical properties of biomaterials that affect immune reactions. These properties include morphology and structure, particle size, and surface properties. Third, we describe the routes of administration of the biomaterials used for vaccine delivery, such as injectable vaccination, transdermal immunization, intranasal administration, and oral administration. Subsequently, we summarize the design considerations for biomaterials as vaccine adjuvants and introduce the application of biomaterials in vaccine development. Finally, we provide an overview of some biomaterial deficiencies in vaccine development and immunotherapy, while highlighting avenues for future research. Ultimately, this review summarizes the advancements in biomaterial research for vaccines, aiming to enhance the understanding among researchers in related fields and facilitate the rational design and application of diverse biomaterials in vaccine development.
Types of biomaterials
Biomaterials can be injected, implanted, or applied to the surface of tissues in biomedical applications to interact with biological systems and trigger therapeutic responses [13]. The selection of biomaterials is critical in vaccine preparation because different materials can perform different functions. Biomaterials are typically categorized based on their origin as organic, inorganic, or biologically derived materials [2].
Organic nanomaterials
Nanomaterials are a class of materials with dimensions on the nanometer scale that are easily degradable, noncytotoxic, highly controllable, and versatile. They can serve as vaccine delivery systems to protect vaccine antigens from degradation by encapsulating them, improving their stability and biological activity, and enabling targeted delivery to specific cells or tissues through surface modification. Common organic nanomaterials include poly (lactic-co-glycolic acid) (PLGA), polyethyleneimine (PEI), chitosan (CS), self-assembled peptides (SAPs), liposomes, and immunostimulatory complexes (ISCOM). Their application in vaccine delivery can enhance the stability of immunogens, improve antigen presentation, and enhance the immune response.
Poly (lactic-co-glycolic acid)
The biodegradable polymer PLGA has been approved by the US Food and Drug Administration (FDA) for vaccine delivery [14]. It has been extensively studied for its potential application in vaccine delivery because of its exceptional biocompatibility, biodegradability, and safety in the human body [15]. PLGA nanoparticles offer two distinct advantages for vaccine applications. First, PLGA polymers demonstrate a suitable drug encapsulation effect because they are usually relatively robust, can form large pores, and can be used for bulk-loading drugs and proteins [16]. Second, they demonstrate a slow-release effect, such that drug encapsulation within PLGA nanoparticles can improve their loading capacity and facilitate controlled release [17]. The internalization of PLGA nanoparticles in cells typically occurs through lattice protein-mediated endocytosis; however, vaccine delivery is hindered as hydrophobic particles are cleared from the bloodstream or taken up by the reticuloendothelial system (RES) in the liver [18, 19]. Several surface modifications have been developed to address these limitations and protect nanoparticles from RES, such as coating the nanoparticle surface with polyethylene glycol (PEG) to conceal the hydrophobicity of PLGA owing to its hydrophilic properties [20, 21].
Notably, PLGA polymers have recently garnered increasing attention in the field of cancer therapy [22]. PLGA polymers undergo hydrolysis in vivo to produce metabolizable lactic acid and glycolic acid. Since both by-products are endogenous and can be converted within the tricarboxylic acid cycle in the body, the potential toxicity resulting from their use in drug delivery can be considered negligible [23]. From another perspective, current research indicates a close relationship between the metabolic environment and the formation of an immunosuppressive microenvironment within tumors [24]. In acidic conditions with lactic acid present, regulatory T cell populations may proliferate and differentiate, thereby playing a multifaceted role in maintaining the immunosuppressive microenvironment within the tumor. Moreover, a significant proportion of commercially available PLGA-based formulations currently approved for clinical use are employed in treating malignant tumors [25]. These findings may offer valuable insights for inspiring future cancer vaccine development and accelerating its pace. However, the accumulation of lactic acid and glycolic acid produced by the hydrolysis of PLGA can also lead to inflammation, thereby raising safety concerns [26].
Polyethyleneimine
PEI is a hydrophilic cationic polymer that aggregates negatively charged DNA and antigens in vivo. The amorphous mesh structure of PEI acts as a “proton sponge” in the lysosome, which is unique and remarkable, giving it a high transfection efficiency [27, 28]. PEIs are also rich in reactive amines, such as secondary and tertiary amines, which provide various structural modification possibilities, expanding their applications in targeting reagents and mitigating toxicity [29] and enabling them to bind strongly to nucleic acids. Therefore, they have been widely used for nucleic acid delivery for several decades. PEI-based nanoparticles can be used as adjuvants to improve the efficacy of conventional infectious and tumor vaccines [30–34].
Nanoparticles (NPs) offer a promising platform for vaccine delivery; however, NP-based vaccines often entail intricate synthetic and post-modification procedures, which present technical and manufacturing challenges in vaccine production. Facile modification of PEI can effectively address this issue. Nam et al. [35] combined the straightforward coupling chemistry of PEI with neoantigens, followed by electrostatic assembly with a cytosine-phosphate-guanine (CpG) adjuvant, thereby achieving self-assembly of PEI NPs. The engineered PEI NPs exhibited non-toxicity, possessed a particle size below 50 nm, and demonstrated a remarkable capability in promoting antigen-presenting cell activation and antigen cross-presentation. This method shows significant potential for streamlining the manufacturing of individualized cancer vaccines. However, it is crucial to emphasize that stringent control measures should be implemented to minimize its residue in pharmaceutical preparations owing to the limited biodegradability of PEI within the body.
Chitosan
CS is a naturally occurring polymer derived from the alkaline deacetylation of chitin and is commonly found in the exoskeletons of crustaceans and insects. It is a linear polysaccharide with a cationic charge, composed of D-glucosamine and N-acetyl-D-glucosamine [36]. These molecules contain reactive hydroxyl and amino groups. When dissolved in acidic media, the amino group of CS becomes highly protonated, allowing electrostatic interactions between CS and mucins, resulting in strong adhesion properties for CS. The adhesive properties of chitosan and its capacity to enhance the transport of macromolecules across mucosal surfaces are key factors contributing to its immunomodulatory properties [37, 38]. In addition, the presence of hydroxyl and amino groups in the CS molecule enables the incorporation of various functional groups through molecular or chemical modifications. This addresses the issue of reduced solubility in neutral and alkaline solutions, which allows for stability within a specific pH range. CS derivatives obtained after modification are mainly improved in terms of permeation enhancement and adhesion properties, and various CS derivatives, such as glycosylated or trimethyl CS, have been widely explored for vaccine delivery [39–42]. Recent studies have primarily focused on the application of CS-based nanovaccines in nasal mucosal delivery systems, capitalizing on the ability of CS to traverse tight junctions and its adhesive properties. These properties enhance mucosal adhesion and antigen uptake, while demonstrating intrinsic immunostimulatory capabilities [43].
For a considerable duration, the challenges of ensuring the safety and efficacy of live virus vaccines have posed significant obstacles to vaccine development. In light of this, Hao et al. [44] ingeniously encapsulated wild-type Zika virus within a chitosan gel matrix, aptly named Vax. This composite gel effectively confines the virus and orchestrates the recruitment of immune cells for viral eradication and antibody production throughout the organism. Remarkably, mice immunized with Vax in the animal trials were entirely shielded from the lethal effect of Zika virus. This groundbreaking discovery offers immense convenience for the expeditious preparation of safe and efficacious vaccines, as well as fortification against large-scale epidemics in future scenarios. Unfortunately, the intricate manufacturing and extraction processes associated with CS pose complexities that impede its purity enhancement, thus limiting its effectiveness in certain applications.
Self-assembling peptides
Peptide materials have unique properties and are made from simple amino acids under physiological conditions. They can self-assemble into nanostructures at various levels through non-covalent interactions to form functional materials with outstanding properties. The self-assembled morphology of peptide supramolecular structures and their diverse applications have been extensively investigated, showing significant potential for vaccine delivery and vaccine design owing to their excellent biocompatibility, facile preparation, and tunable functionality [45]. Zhu et al. [46] developed a peptide hydrogel nanoparticle containing functional peptide chains at the core and a framework of SAP, which exhibits significant biological functions, such as effective inhibition of local inflammation and elimination of non-resistant cells. It can independently induce immune activation without adjuvants and has a longer duration than simple peptides.
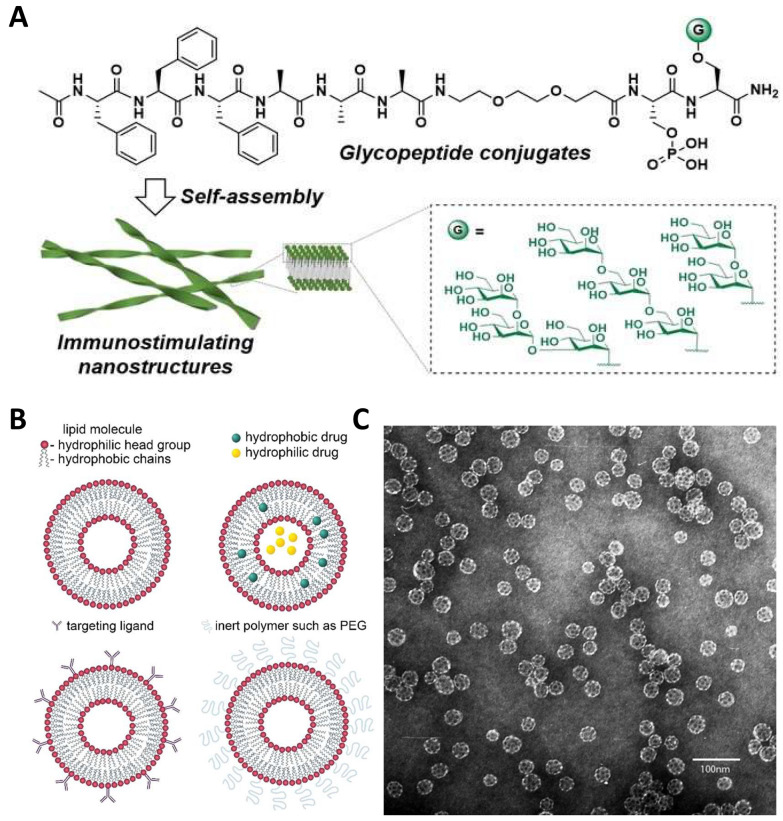
Wang et al. [47] developed a glycopeptide conjugate (GPC) (Fig. 1A), designed to effectively carry various mannan-oligosaccharides and self-assemble into stable nanoribbons, taking advantage of the immunostimulatory ability of high-mannose-type N-glycans. These GPCs exhibited varying affinities for C-type lectin receptor (CLR) proteins in in vitro binding assays, with some mannosylated peptides demonstrating strong immunostimulatory effects in vivo, highlighting their potential as vaccine adjuvants.
Fig. 1.
Structure of some biological materials. A Self-assembling GPCs systems. Reproduced with permission from Ref. [47], Copyright 2024, Angewandte Chemie International Edition. B Schematic representation of liposome, liposome encapsulating hydrophobic and hydrophilic drugs, immunoliposome functionalized with targeting ligands, and sterically stabilized (“stealth”) liposome functionalized with inert polymers such as PEG. Reproduced with permission from Ref. [49], Copyright 2021, American Chemical Society Nano. C The immunostimulatory complex ISCOMATRIX was observed by electron microscopy after negative staining (Bar = 100 nm.) Reproduced with permission from Ref. [50], Copyright 1995, In Vaccine Design: The Subunit and Adjuvant Approach
In conclusion, SAPs offer numerous advantages for drug delivery; however, there have been no documented instances of vaccines based on self-assembling peptides reaching the market. The intricate composition, challenges in large-scale production, and unpredictable modulation of the immune system present ongoing obstacles to the clinical application of self-assembling peptides [48]. It is anticipated that advancements in medical informatics and continual innovation in vaccine delivery systems will effectively address these issues.
Liposomes
Liposomes have been widely studied as nanodrug carriers for vaccine delivery systems. They are closed vesicles composed of lipid bilayers of phospholipids and cholesterol, with a biofilm-like structure that encapsulates water-soluble and lipid-soluble drugs (Fig. 1B) [49]. Liposomes have been extensively researched for their potential as vaccine delivery systems because of their biocompatibility, biodegradability, and non-toxic nature. They are commonly utilized as drug carriers and offer numerous advantages, such as high targeting specificity, effective protection of the encapsulated drug, controlled and gradual release of the drug, improved therapeutic index, and reduced side effects [51]. A key advantage of liposomes is their capacity to simultaneously deliver antigens and adjuvants to the same antigen-presenting cells (APCs), which is crucial for eliciting an effective immune response. Additionally, liposomes prevent antigen degradation, enhance APC uptake, facilitate cytoplasmic release, and possess immunostimulatory properties [52].
Liposomes can be classified as neutral, cationic, or anionic based on their composition [53, 54]. Cationic liposomes, which are composed of a stable complex of synthetic cationic lipids and anionic nucleic acids, are the most commonly utilized non-viral delivery systems for nucleic acid drugs [55]. Maria et al. recently reported a cationic liposome vaccine designed to co-deliver antigens and adjuvants capable of inducing a robust antigen-specific adaptive immune response [56]. Cationic liposomes consisting of dimethyl dioctadecylammonium bromide (DDAB), cholesterol (CHAL), and oleic acid (OA) with an imiquimod (IMQ) adjuvant were validated using mouse immunization as an effective delivery platform for protein antigens capable of targeting and inducing maturation via dendritic cells (DCs). Furthermore, Farrhana et al. [57] utilized standard peptide synthesis to couple the human papillomavirus (HPV)-16 E7 protein peptide (8Qm) to polyleucine, and then, self-assembled nanoparticles or incorporated the peptide into liposomes for cancer research. Following a single immunization, liposomal delivery of the 8Qm-coupling significantly enhanced survival, reduced tumor growth in mice, and eradicated mature tumors growing for seven days in mice. This experimental breakthrough illustrates that fully defined natural hydrophobic amino acid-based polymers coupled with peptide antigens can function as effective vaccine delivery systems. Given the demand for vaccine therapies targeting intracellular infectious diseases and malignant tumors, it is anticipated that the vaccine delivery systems presented herein will be utilized in future applications. However, liposomal formulations of anticancer drugs lack cancer-cell targeting abilities when administered intravenously, leading to numerous adverse reactions. Therefore, appropriate delivery modalities are required to maximize their effectiveness.
Immunostimulatory complexes
ISCOMs are cage-structured nanoparticles, approximately 40 nm in size, initially reported in 1984 as antigen delivery systems that possess strong immunostimulatory properties [58]. They can be formulated by mixing antigens, saponins, phospholipids, and cholesterol, using lecithin-assisted hydrophobic modulation. Therefore, they have the potential to serve as reliable vaccine delivery systems and enhancers. For example, when antigen 85 composite is incorporated into ISCOMs, it can boost both humoral and cellular immune responses to Mycobacterium tuberculosis following pulmonary immunization [59]. However, a major drawback of traditional ISCOM synthesis is the need to add antigenic proteins, which limits the variety of antigens that can be used, making the process complex and difficult to control. To address these challenges, the ISCOMATRIX® solution was developed, which is a prefabricated particle resembling a cage (Fig. 1C) [50] with a diameter typically ranging from 40 to 50 nm. It consists of purified saponins (ISCOPREP® saponin), phospholipids, and cholesterol [60]. This potent immunomodulatory agent can affect both innate and adaptive immune responses. ISCOMATRIX vaccines have demonstrated a broad spectrum of antibody- and antigen-specific T-cell responses in cancer immunotherapy and infectious disease treatment [61]. The utilization of ISCOM in vaccine preparation has been extensively researched for numerous years and has significantly contributed to the advancement of veterinary and human vaccine development.
Inorganic nanomaterials
Inorganic nanomaterials are usually structurally stable but not biodegradable, and their physicochemical properties must be modified to enhance their biocompatibility for better use in nanovaccines. However, inorganic nanomaterials are inexpensive to produce and safe for various applications, and their controlled synthesis and rigid structure are two of their primary advantages. A variety of metals and their oxides, inorganic salts, and non-metallic oxides have potential as nanomaterials for vaccines, such as gold, carbon, and silicon dioxide, which are already widely used [62–64]. Nanomaterials are composed of nanoparticles whose synthesis can result in a range of shapes, sizes, and surface modifications.
Metals and their oxide nanoparticles
Metallic nanoparticles, including gold nanoparticles (GNPs) and silver nanoparticles (AgNPs), are widely used in vaccine delivery systems owing to their distinct optical and electrical characteristics. These nanoparticles are typically smaller than 1 µm, which allows them to interfere with viral replication in the host cells. This provides an advantage over other non-nanomaterials [65]. Surface-modified or functionalized metallic nanoparticles can enhance the stability and delivery efficiency of vaccine antigens as well as boost the immune response by activating immune cells or altering the immune environment. GNPs have low toxicity and good biocompatibility and are more colloidally stable than AgNPs; thus, they have been extensively studied [66]. GNPs have demonstrated remarkable immunomodulatory and adjuvant properties. Moreover, intradermal injection of GNPs containing glucagon peptidogens C3-A1 leads to the recruitment and retention of immune cells in the skin of patients with type 1 diabetes [67].
In the last two years, GNPs have been increasingly developed for various viral assays and diagnostics [68, 69]. Notably, GNPs can cause cytotoxicity and activate inflammatory responses at high doses (> 8 mg/kg) [70]. Additionally, several types of metal-based nanoparticles are potent antiviral drugs with significant antiviral effects when used bare or after surface modification, including nanoparticles composed of metals such as silver or metal oxides such as zinc oxide, titanium dioxide, and iron dioxide [71, 72].
Carbon nanoparticles
Carbon nanoparticles (CNPs) with sizable mesopores and macropores demonstrate exceptional capabilities as antigen carriers. When utilized as adjuvants for oral vaccines, they can elicit robust immune responses mediated by immunoglobulin G (IgG), immunoglobulin A (IgA), Th1, or Th2 [63]. Furthermore, CNPs can synthesize nanotubes that can simultaneously load tumor testicular antigens and adjuvants to build effective tumor vaccines [73]. Carbon nanotubes show promise as a method for delivering vaccines; however, their potential toxicity in living organisms is still uncertain owing to their irregular shape and strong pro-inflammatory effects [2].
Non-metallic oxide nanoparticles
Silicon dioxide nanoparticles (SiNPs) are prominent non-metallic oxide nanoparticles. They are excellent vaccine carriers with small particle sizes, stable physicochemical properties, good biocompatibility, and high drug-carrying capacity. They have been widely used as feed additives, veterinary drugs, and new animal vaccines because they can encapsulate drugs, providing protective and controlled release. They can also cross the blood–brain barrier and biofilms and can target other transport pathways [74]. SiNPs are also candidates for transdermal vaccines and gene therapy through controlled and sustained drug release into the skin and enhanced skin penetration by encapsulating drug components [75]. SiNPs can induce Th1- and Th2-mediated immune reactions, making them powerful vaccine adjuvants [76, 77].
Mesoporous silica has a unique molecular sieve structure and strong adsorption properties, and injecting mesoporous silica nanoparticles (MSNs) into mice effectively enhances the immune response [78]. Furthermore, mesoporous silica microrods can adsorb PEI and tumor antigens through electrostatic action and can promote the secretion of inflammatory cytokines when synergistic with PEI, showing broad prospects in anti-tumor therapy [79]. It is widely acknowledged that vaccines typically rely on cryopreservation and cold chain transport, rendering them highly susceptible to inactivation at non-optimal temperatures. In light of this, scientists have successfully preserved the vaccine's efficacy for up to three years at room temperature by encapsulating the protein with a layer of silica coating, thereby safeguarding its structural integrity while preserving immunogenicity [80, 81]. As an advanced material, SiNPs have immense potential for various applications; however, appropriate processes and additives must be employed during preparation to maintain their stable dispersion state and prevent any adverse impact on the performance or application effectiveness owing to their tendency to agglomerate and precipitate in water-based dispersion systems.
Biologically derived materials
Unlike the organic and inorganic nanomaterials mentioned earlier, biologically derived materials are primarily sourced from cells, bacteria, and viruses. This category includes virus-like particles (VLPs) and outer membrane vesicles (OMVs) [82].
Virus-like particles
VLPs are self-assembled multiprotein nanoparticles. They consist of biocompatible envelope proteins without a viral genome [83]. They resemble viruses in appearance and structure, and their lack of viral nucleic acids prevents their replication in vitro. They are biodegradable nanoparticles of approximately 20–100 nm in size, which are optimal for DCs and other APC-recognizing particles. They can rapidly pass through tissue barriers to reach draining lymph nodes and effectively activate an immune response [84]. Innate and adaptive immune responses can be activated in the absence of viral replication through the presentation of pathogenic structural proteins. Furthermore, VLPs have a highly repetitive and rigid structure, allowing them to display multivalent antigenic epitopes on their surface and form extensive cross-links with B cell receptors, thereby stimulating B cells and eliciting a strong and long-lasting antibody response [85].
VLPs have been extensively utilized in vaccine development because of their favorable safety profiles and strong immunogenicity. Recently, several VLP-based vaccines have been introduced, including those targeting HPV (Cervarix®, Gardasil®, and Gardasil9®) and hepatitis B (Engerix®, Sci-B-Vac™, RTS®, and S VLP®) [86]. VLPs have the potential to transport both native viral antigens and modified foreign antigens attached to VLPs through chemical bonding or gene fusion. For example, a VLP vaccine designed for breast cancer incorporates human epidermal growth factor receptor 2 (HER-2) epitopes linked to cowpea mosaic virus (CPMV), demonstrating efficacy in stimulating specific immune responses and offering protection against tumors in a mouse model [87]. Several VLP vaccines are currently undergoing clinical trials, including those targeting chikungunya virus, neocoronavirus, influenza, cancer, and encephalitis [88]. However, the application of VLPs is somewhat limited owing to the challenges associated with their low expression yield and complex preparation.
Outer membrane vesicles
OMVs are naturally derived from gram-negative bacteria and range in size from 20 to 250 nm. They possess a bilayer lipemic membrane nanostructure containing lipopolysaccharides, membrane proteins, phospholipids, toxins, enzymes, periplasmic proteins, RNA, DNA, and peptidoglycans [89]. OMVs are generally stable under various treatments and temperatures, making them highly versatile for engineering the expression of a wide range of antigens [90, 91]. Furthermore, OMVs are appealing as adjuvants for delivering vaccines because they contain immunogenic components from the host bacterium that can stimulate both humoral and cell-mediated immune responses to antigens [92]. They offer many advantages in terms of drug loading and delivery. Hong et al. [93] investigated a senescent cancer cell-derived nanovesicle (SCCNV), which contains interferon-γ and tumor necrosis factor-α expressed during cell senescence. The presence of these endogenous factors confers immunogenicity to the vaccine without the need for exogenous adjuvants. Furthermore, the delivery system based on red blood cell membrane (RBCm) offers the advantage of prolonging anti-tumor therapy and overcoming accelerated blood clearance; however, its limited application is attributed to low drug-loading capacity and lack of tumor-targeting ability. To tackle this challenge, Zhang et al. [94] engineered a versatile delivery system by employing hyaluronic acid (HA)–hybridized RBCm (HA&RBCm) as a coating to surmount the endothelial barrier of the reticuloendothelial system. Meanwhile, the honeycomb architecture within the system expanded the encapsulation capacity, thereby establishing a secure and efficacious anti-tumor therapeutic carrier. At present, Several OMV-based vaccines have shown promising results in clinical trials, including the OMV Meningitis B (MenB) vaccine [95].
Application of exosomes
In recent years, exosomes and cell-derived nanovesicles (CDNs) have received much attention due to their potential therapeutic applications in a variety of diseases. Exosomes are nanoscale vesicles secreted by cells which are endogenous natural drug carriers that are stable, biosecure, and less immunogenic than exogenous carriers [2]. They are able to effectively penetrate biofilms and remain stable in the blood, thereby enhancing vaccine targeting and preventing premature antigen leakage. In the field of targeted drug delivery, bioinspired simulated exosome nanovesicles have been developed with higher yields compared to natural exosomes to deliver chemotherapy drugs to tumor tissues [96]. Recently, exosomal noncoding RNAs (ncRNAs) have shown potential for treating bacterial infections, such as tuberculosis (TB. Exosomal ncRNAs play a pivotal role in modulating the immune response to M. tuberculosis infection by activating specific signaling pathways that regulate the infection process [97]. Progress in sequencing technologies has increasingly revealed the role of exosomal ncRNAs in regulating the transcriptional and post-transcriptional expression of host genes, thereby influencing M. tuberculosis to the host environment and its pathogenicity [98]. Additionally, their role in delivering vaccine components across different infectious diseases offers promising prospects to develop more effective vaccine strategies against M. tuberculosis in the future [99].
Synergies between exosomes and cell-derived nanovesicles
In addition, exosomes can be used in the treatment of autoimmune diseases in synergy with CDNs. A nanotherapeutic drug delivery strategy has been developed using fused nanovesicles derived from plant and animal cells with high clinical potential. Up to now, the existing therapeutic methods for autoimmune diseases still have problems such as low efficacy, serious side effects and difficulty in reaching target tissues [100]. A multifunctional fusion nanovesicle that can target lesions is designed for the treatment of autoimmune skin diseases. By utilizing centrifugal force and micropores, CDNs can be generated on a large scale, which provides a cost-effective method for producing these vesicles for a variety of applications [101]. In addition, in the context of regenerative medicine, stem cell-derived exosomes and cell-engineered nanovesicles have shown promise in promoting cell proliferation, migration, and anti-aging, and can be used to treat wound injuries and skin aging [102]. These extracellular vesicles contain biomolecules that promote intercellular communication and tissue regeneration, making them attractive candidates for therapeutic interventions. Overall, the literature on exosomes and CDNs demonstrates their different roles in immune response, targeted drug delivery, cancer progression, and regenerative medicine. Further studies are needed to fully understand the mechanisms of its therapeutic effects and to optimize its use in clinical Settings [103].
This section provides a detailed introduction to the classification and properties of biological materials, along with a summary of their advantages and disadvantages in vaccine applications (Table 1).
Table 1.
Classification, properties, and advantages and disadvantages of biological materials
| Types | Classification | Properties | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Organic nanomaterials | Poly (lactic-co-glycolic acid) | Biocompatible, biodegradable, safe, and particle size controllability | Bioactive molecules can be encapsulated, with accurately targeted delivery, and long-term slow release | Phagocytosis may accelerate degradation. It is hydrophobic and poorly encapsulated for hydrophilic drugs | [104, 105] |
| Polyethyleneimine | Hydrophilic, easily modified structurally, and low toxicity | Has a “proton sponge” effect, a high transfection efficiency, and strongly binds nucleic acids | It is not biodegradable and may contain drug residues | [27, 28] | |
| Chitosan | Adhesive, biocompatible, biodegradable, low toxicity, and easy to obtain and modify | Enhances the adhesion and absorption of antigen by mucosa and prolongs the time of drug action | Relatively high cost, poor stability, purity issues | [37, 38] | |
| Self-assembling peptide | Biocompatible and controllable | Can self-assemble into various layered nanostructures; preparation is simple, and function can be controlled | The composition is complex, not easy to mass produce, and the stability is poor at low pH | [45] | |
| Liposomes | Biocompatible, biodegradable, and non-toxic | Can encapsulate water-soluble and fat-soluble drugs and offers targeted delivery and slow drug release | High production costs, oxidation and fusion problems, structural instability, RES clearance | [49] | |
| Immunostimulatory complexes | Safe | It serves the dual purpose of presenting antigens and acting as an immune adjuvant, leading to a comprehensive, long-lasting, and robust immune response | Preparation is complex and difficult to control | [58, 106] | |
| Inorganic nanomaterials | Metals and their oxide nanoparticles | Biocompatible, has low toxicity, and has optical and electrical properties | Interferes with viral replication in host cells, is easy to synthesize various shapes, and is easy to surface modify or functionalize | Surface ligands and charges may cause toxicity | [65, 107] |
| Carbon nanoparticles | Biocompatibility, stability, controllability, easy modification | The specific surface area is extensive, with diverse sizes and shapes, allowing for selective adsorption and potential functionalization | Impurities or surface ligands may cause toxicity | [63, 107] | |
| Non-metallic oxide nanoparticles | Biocompatibility, particle size control, large specific surface area, easy modification, thermal stability | Strong drug loading capacity, easy synthesis and functionalization, and is conducive to long-term preservation of vaccines as coatings | Agglomeration and precipitation are easy to occur, and there is a certain toxicity | [80, 81, 108] | |
| Biologically derived materials | Virus-like particles | Biodegradable, small particle size, safety, easy to design | Uses the advantages of its own viral structure to enhance the immune response while avoiding viral replication | The expression yield is low, the preparation is complex, and there are stability problems | [84, 85, 109] |
| Outer membrane vesicles | Biocompatible and has low immunogenicity | The immunogenic components derived from its parent bacteria elicit both humoral and cell-mediated antigenic immune responses | The lipopolysaccharide component of the surface may cause an inflammatory response | [110–112] |
Reprocessing strategies for biomaterials in delivery systems
Biomaterials that allow the spatial and temporal control of loaded reagents have evolved into powerful tools for vaccine delivery and localization [113, 114]. Current biomaterial platforms encompass a variety of dynamic systems including PNPs, LNPs, hydrogels, and MN arrays. Each strategy has been used in various synergistic therapies. The characteristics and design considerations of the delivery system are listed in Table 2.
Table 2.
Characteristics and design considerations of several delivery systems
| Delivery systems | Formation | Functional characteristics | Design considerations | References |
|---|---|---|---|---|
| Polymer-based nanoparticles | Composed of natural/synthetic high polymer | The loaded active compounds can be embedded in the polymer core or adsorbed onto the polymer core surface | Controlled delivery of vaccines is achieved by controlling size, shape, and surface characteristics | [115, 116] |
| Liposome-based nanoparticles | The composition includes lipids that can be ionized, cholesterol, phospholipids, and lipids modified with polyethylene glycol | Can encapsulate water-soluble and fat-soluble drugs, has a high degree of targeting, can effectively protect the encapsulated drugs, and can control and slowly release drugs | Its formulation composition, size, and surface charge affect its efficacy and pharmacokinetics, and these factors can be controlled to optimize vaccine delivery | [117, 118] |
| Hydrogels | High hydrophilic polymers form three-dimensional network structures by cross-linking in water | High doses of non-specific drug distribution can be accurately delivered to specific sites while achieving continuous controlled release of drug molecules | Generally, drug-containing hydrogels are mainly diffused and released by controlling the degradation, swelling, and mechanical deformation of the hydrogel mesh, ultimately leading to controlled drug release | [119–121] |
| Microneedles | Consists of a plurality of micrometer small needle tips connected in an array on the base | Can be directed through the stratum corneum, avoiding blood vessels and nerves to deliver the drug precisely and effectively below the stratum corneum; allows the active ingredient to be released in a continuous or controlled manner | The length, size, and shape of the microneedles determine their mechanical properties, insertion capacity, drug-loading capacity, and drug-delivery efficiency and can be individually designed according to therapeutic needs | [122, 123] |
Vaccine delivery systems
Polymer-based nanoparticles
PNPs are a class of nanomaterials composed of synthetic polymers capable of loading active compounds and encapsulating them within the polymer core or adsorbing them onto the surface, typically in the form of nanocapsules and nanospheres (Fig. 2A) [115]. Nanospheres have a matrix with a physical and uniform dispersion of the active ingredient, whereas nano-capsules have a vesicular structure, with the active ingredient enclosed within a cavity surrounded by a polymer membrane. Polymeric micelles are another type of PNP. Amphiphilic polymer molecules self-assemble in aqueous solutions to form colloids with a hydrophobic core and hydrophilic shell. The distinctive architecture of polymeric micelles offers significant advantages in vaccine delivery. The hydrophobic core can encapsulate a large amount of the hydrophobic drug, which markedly improves its solubility in water. Additionally, the structure allows for interactions with proteins, cells, and other biological components, influencing pharmacokinetic behavior and drug distribution [116].
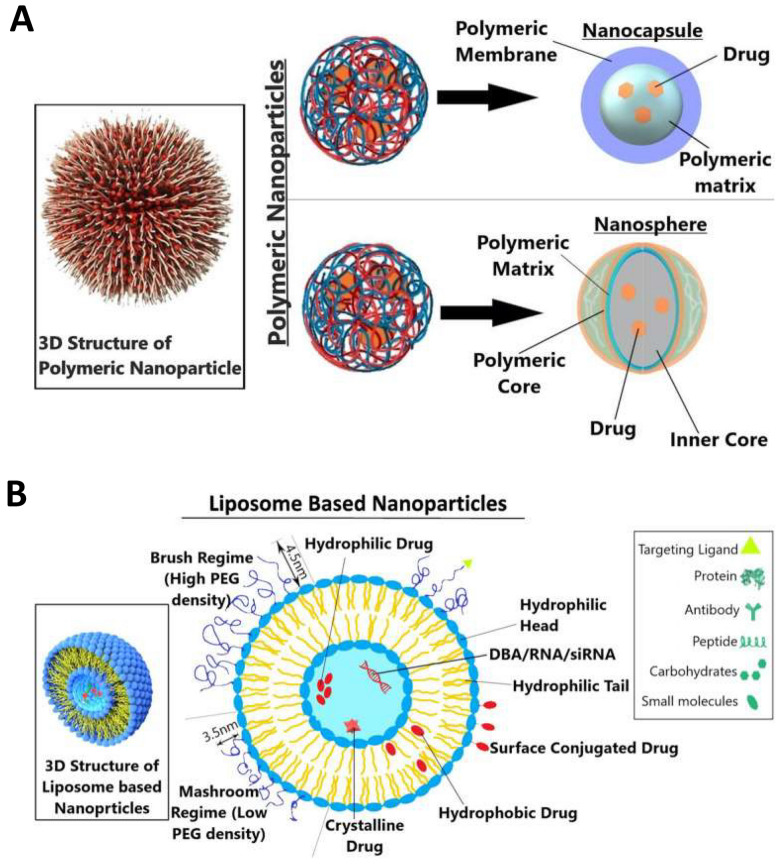
Fig. 2.
Vaccine delivery system. A Types of polymeric nanocarriers—nanosphere and nanocapsule—are depicted in the diagram. A polymeric shell that regulates the drug’s release profile from the core of the nanocapsule surrounds an oily core, typically where the medication dissolves. Using a continuous polymeric network as its foundation, nanospheres allow the medicine to either be maintained inside or adsorbed onto its surface. Reproduced with permission from Ref. [115], Copyright 2023, Technology in Cancer Research & Treatment. B Vesicle structure of nanoparticles based on liposomes. The polymer of the mashroom regime (low PEG density) consists of independent random coils of number Flory radius RF3 on the surface of the member. The polymer chain of the brush regime more mutually interacts and is densely packed. Reproduced with permission from Ref. [115], Copyright 2023, Technology in Cancer Research & Treatment
Over the past decade, PNPs have achieved significant advancements in vaccine development because of their substantial potential for precise drug and vaccine delivery [124–127]. Owing to their exceptional customizability, biocompatibility, and biodegradability, lipid nanoparticles have been extensively utilized in the development of particulate vaccines for emerging infectious diseases [128–130]. They can be formed from a range of materials, including natural polymers commonly occurring in nature, and synthetic polymers manufactured artificially [131, 132]. Their rich chemistry and multifunctional combinatorial structures provide a new platform for the development of vaccines with high efficiency and efficacy and can modulate vaccine delivery and immune activation effects by changing the chemical structure and physical properties of polymers. Additionally, they have controllable size, morphology, and surface properties that enable controlled delivery of vaccines and increase immunogen stability and immune responses by controlling the delivery rate.
Liposome-based nanoparticles
LNPs are lipid vesicle delivery systems that are non-viral and multifunctional, comprising ionizable lipids as the primary components, cholesterol, phospholipids for structural integrity, and PEG-modified lipids to aid in maintaining stability (Fig. 2B) [117]. Following extensive research, LNPs have been utilized for the administration of various vaccines and have significantly enhanced both humoral and cellular immune responses. LNPs can stimulate robust follicular Th cells, durable plasma cells, B cells in germinal centers, and memory B cells, resulting in enhanced humoral immune reactions after vaccination [133]. Recently, LNPs have been extensively studied as potential carriers for mRNA vaccines in clinical trials of COVID-19, tumor vaccines, and influenza vaccines. For example, emergency marketing authorizations were granted in 2020 for Pfizer’s BNT162b2 vaccine (Comirnaty) and Moderna’s mRNA-1273 vaccine (Spikevax), both of which utilize LNPs for mRNA antigen delivery [134, 135]. The successful use of these two mRNA vaccines has yielded exciting results and made significant contributions to the fight against COVID-19. LNPs have accelerated the utilization of mRNAs in humans, and their promising prospects in vaccine development are highly anticipated.
Solid lipid nanoparticles (SLNs) are a type of lipid-based nanocarrier comprising active pharmaceutical ingredients and solid lipids, along with surfactants and/or co-surfactants. They serve as vaccine delivery systems that entrap drugs within natural or synthetic solid lipids, such as lecithin, acting as carriers [136]. Their internal oil phase was replaced with a solid lipid to reduce the limitations associated with conventional lipid formulations, avoid the use of organic solvents during preparation, provide better stability, reduce drug degradation, and maintain relative stability during storage. SLNs play a pivotal role in various applications in nanotechnology, such as vaccine delivery and cancer treatment, owing to their capacity to encapsulate both hydrophilic and hydrophobic compounds, biocompatibility, and ease with which their surface can be modified [118].
Over the past few years, SLNs have been widely studied as a means of delivering anticancer drugs for breast cancer treatment [137, 138]. For example, ultrasound technology has been used to prepare radiolabeled trastuzumab (TRZ)-loaded SLNs [139]. In vitro studies have shown that TRZ-SLNs are biocompatible and effective for inducing apoptosis in human MCF-7 adenocarcinoma cells. Moreover, parenteral injection of TRZ-SLNs into rats showed promising results in terms of SLNs serving as cancer diagnostic agents. Additionally, SLNs possess the dual properties of liposomes and nanoparticles, offering the advantages of low toxicity, good biocompatibility, and biodegradability. However, issues such as low drug loading and poor stability can arise owing to the complex internal structure of SLNs, which consists of multiple phases. Problems such as excessive drug loading leading to gelation, particle size growth, and drug degradation during storage limit their widespread application.
Hydrogels
Introduction to hydrogels
Hydrogels are formed by polymers, creating a three-dimensional network in water through cross-linking and possessing the ability to rapidly absorb a significant amount of water while maintaining their volume without dissolution [140]. The capacity of the hydrogel to absorb water is attributed to the hydrophilic functional groups on its polymer backbone. Additionally, its insolubility in water is a result of cross-linking of the network chains. Hydrogels have garnered significant attention in the fields of immunomodulation and vaccine development owing to their unique physicochemical properties. These properties include high water content, drug-carrying capacity, biodegradability, biocompatibility, and ease of construction and manipulation (Fig. 3A) [119]. The physical properties of hydrogels are conducive to vaccine delivery, which allows the precise delivery of high doses of non-specifically distributed drugs to specific sites while achieving the sustained and controlled release of drug molecules [120, 121].
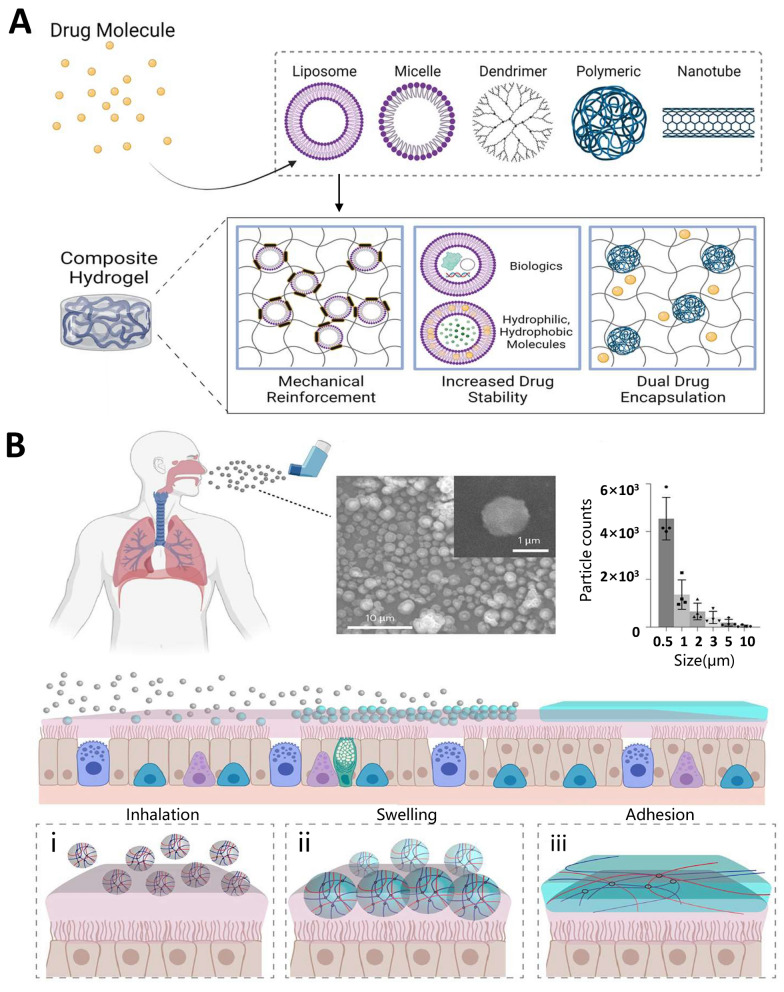
Fig. 3.
Hydrogel. A Nanocarrier-hydrogel composites for vaccine delivery. Reproduced with permission from Ref. [119], Copyright 2019, Applied Materials Today. B Changes in the contact process of the inhalable bioadhesive hydrogel with the mucus surface. Dry SHIELD particles (grey spheres) are inhaled and they become swollen (blue spheres) once they are in contact with the mucus layer (pink layer). Finally, it forms a layer of hydrogel (blue layer) and adheres to the mucus layer. The process includes inhalation (i), swelling (ii) and adhesion (iii). The representative SEM image showing the morphology of SHIELD particles before swelling. The inset shows a zoomed-in view of one particle. Aerodynamic diameter of SHIELD particles. Data are mean ± s.d.; n = 4 independent experiments. Reproduced with permission from Ref. [143], Copyright 2023, Nature Materials
Types and applications of hydrogels
Reformulated hydrogels, which are composed of a blend of natural and synthetic materials, have demonstrated efficacy as carriers for vaccine delivery. Recent advancements in artificial modifications have resulted in improved hydrogel compositions and extended-release cycles for vaccines. Furthermore, the antiviral ability of hydrogels can be enhanced by combining antiviral components. Additionally, hydrogels can be designed as gel microspheres, injectable hydrogels, and gel films to cater to various applications [141, 142]. For example, mucous membranes are the first line of defense of the immune system and often rely on the mucus barrier to provide protection when viruses invade the body through the respiratory tract. To enhance the mucus barrier, an inhalable bioadhesive hydrogel against SARS-CoV-2 infection was prepared, which was made of food-grade materials and could be conveniently delivered through a dry powder inhaler. This resulted in a dense hydrogel network that covered the respiratory tract and interacted with mucus, which ultimately enhanced its diffusion barrier properties and reduced viral penetration, which in turn enhanced lung protection (Fig. 3B) [143]. Some protein nanoparticles degrade very easily in the body and require multiple doses to ensure long-lasting immunity. The slow-release properties of hydrogels can solve this problem. Injectable hydrogels were prepared from CS and glycerophosphate, which were compounded with CPMV nanoparticles for slow release and prolonged immune stimulation [144]. Receptor binding domain (RBD) retention can be enhanced by utilizing injectable hydrogel scaffolds; the hydrogel acts as a persistent RBD reservoir and a localized inflammatory ecological niche for innate immune cell activation [145]. Until now, most vaccines have relied on cold chains for distribution and storage. To address this issue, a reversible PEG-based hydrogel platform was designed for the encapsulation, thermal stabilization, and on-demand release of biologics by borate cross-linking using novel hydrogel encapsulation technology, which can stabilize multiple types of biologics up to a high temperature of 65 °C to maintain their vaccine activity [146]. This advancement signifies a significant milestone in the efforts to mitigate the financial burden and potential risks associated with cold chains.
Hydrogel systems hold significant promise for vaccine delivery in light of their favorable biocompatibility, efficient loading of nucleic acid drugs, and capacity to target and regulate the release of antigens at a local level. The recent development of various stimulus-responsive hydrogels has enabled precise spatial and temporal control over vaccine delivery to disease sites or disease-related tissues, rendering it a prominent area of research [147]. Furthermore, hydrogels can be integrated with other delivery technologies such as nanoparticles and microneedles for diverse clinical applications. It is anticipated that the integration of new technologies and platforms will lead to the development of novel hydrogel drug delivery systems in the near future.
Microneedles
Introduction to microneedles
MNs consist of small tips connected to a base and typically have a body measuring 10–2000 µm in height and 10–50 µm in width. Their length, size, and shape can be customized based on the specific treatment requirements. MNs allow for puncturing of the skin and can be guided through the outermost layer to create tiny channels for vaccine delivery, enabling drugs to reach the underlying skin layers in a minimally invasive manner without causing damage to blood vessels and nerves in the dermis [148, 149]. When MNs are used for vaccine delivery, the drug is loaded into an array of MNs. These MNs induce a driving force by establishing a concentration gradient between the medication and subcutaneous tissue fluid. Consequently, the drug can be released in a sustained or controlled manner [122]. MNs offer stabilized transdermal absorption rates, convenient delivery, and the potential for more precise and rapid drug administration at stable dosages. They have considerable advantages in vaccine delivery applications owing to their minimally invasive, convenient, precise, and effective modes of action. In recent years, they have been widely used for pharmaceutical delivery and immunization [150].
Types and characteristics of microneedles
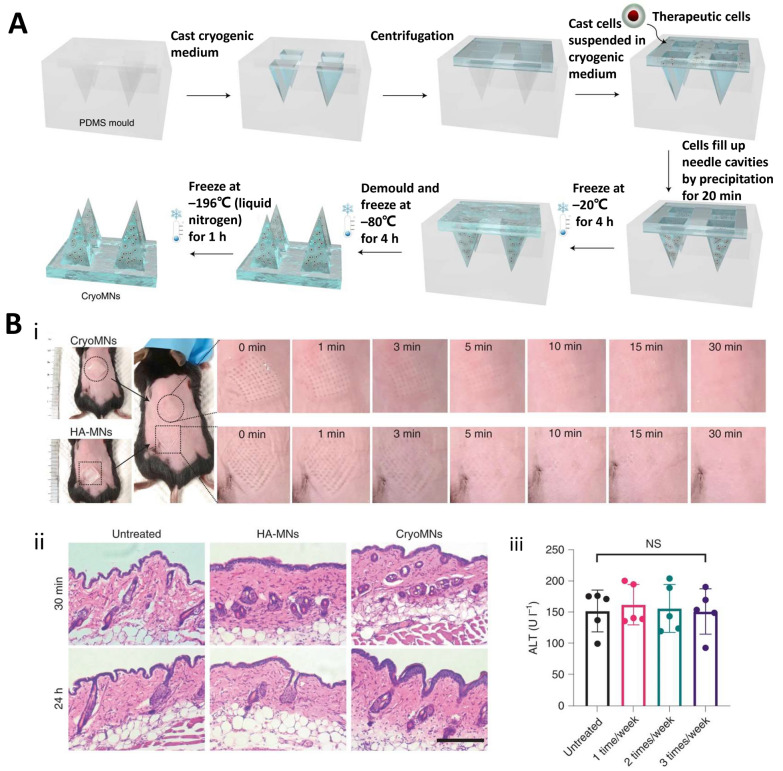
According to their transdermal mode of action, MNs can be categorized into many types, such as solid, encapsulated, dissolved, hollow, hydrogel, and frozen MNs [122, 123]. Solid MNs have high mechanical strength; after penetrating the skin stratum corneum to form microscopic pores, drugs are coated on the skin surface, which is conducive to promoting the local or systemic administration of drugs. In encapsulated MNs, drugs are encapsulated on the surface, and the drug enters the skin when the microneedles penetrate the stratum corneum. Dissolved MNs are made by microprocessing biodegradable polymers, which can be dissolved in the skin tissue after penetrating the skin, releasing the encapsulated drugs. Hollow MNs have hollow channels that act on the skin to provide dermal channels for vaccine delivery and tissue fluid extraction. Hydrogel MNs are composed of drug-loaded hydrogels; the hydrogel enters the skin and mixes with the tissue fluid to dissolve and release the loaded drug, with MN removal following drug administration. Frozen MNs (also known as cryomicroneedles [cryoMNs]) are a new concept of microneedling technology developed in the last two years. They were made using pre-designed MN molds optimized for cryogenic media with pre-suspended cells and further processed using a stepwise cryogenic micromolding method (Fig. 4A) [151]. CryoMNs have a high mechanical strength and can easily penetrate the skin and promote the proliferation of loaded live cells within the skin. CryoMN-delivered cells in mice maintained their viability and proliferative capacity, indicating their biocompatibility and safety (Fig. 4B).
Fig. 4.
Frozen microneedles. A Preparation of frozen microneedles. Reproduced with permission from Ref. [151], Copyright 2021, Nature Biomedical Engineering. B Evaluation of in vivo compatibility and safety of cryoMNs. (i) Assessment of skin recovery following application of frozen MN; (ii) Displaying skin H&E staining images at 30 min and 24 h post MN application, Scale bar, 200 μm; (iii) Showing images of skin stained with TUNEL (green) and Hoechst (blue) after MN application. Reproduced with permission from Ref. [151], Copyright 2021, Nature Biomedical Engineering
These MNs offer several advantages but also have some limitations [122]. For instance, while solid and encapsulated MNs demonstrate superior performance, the former are susceptible to needle breakage owing to their high mechanical strength, posing potential safety hazards. Additionally, the latter may not be suitable for drugs that require large dosages. Dissolved MNs and hydrogel MNs are simple to use and enable precise control over the release kinetics of drugs or biologics. However, the manufacturing materials of these two MNs have high requirements, including good biocompatibility. In addition, while hollow MNs can release drugs in a controlled manner and facilitate the delivery of large doses, their production cost is high, and the manufacturing process is challenging. Conversely, cryoMNs can be stored for several months under conventional storage conditions, making them easily transportable and usable, highlighting their clinical potential. The greatest clinical potential is the intradermal delivery of DC vaccines. However, many issues regarding the materials, production conditions, and drug-loading capacity need to be addressed.
Physicochemical properties of biomaterials affecting the immune response
Biomaterial-based vaccine delivery systems, particularly NPs, have been widely employed in vaccine research and development. The physicochemical properties of nanoparticles (including morphology and structure, particle size, surface charge, and antigen loading pattern) significantly influence vaccine adjuvancy and immune response [152].
Morphology and structure
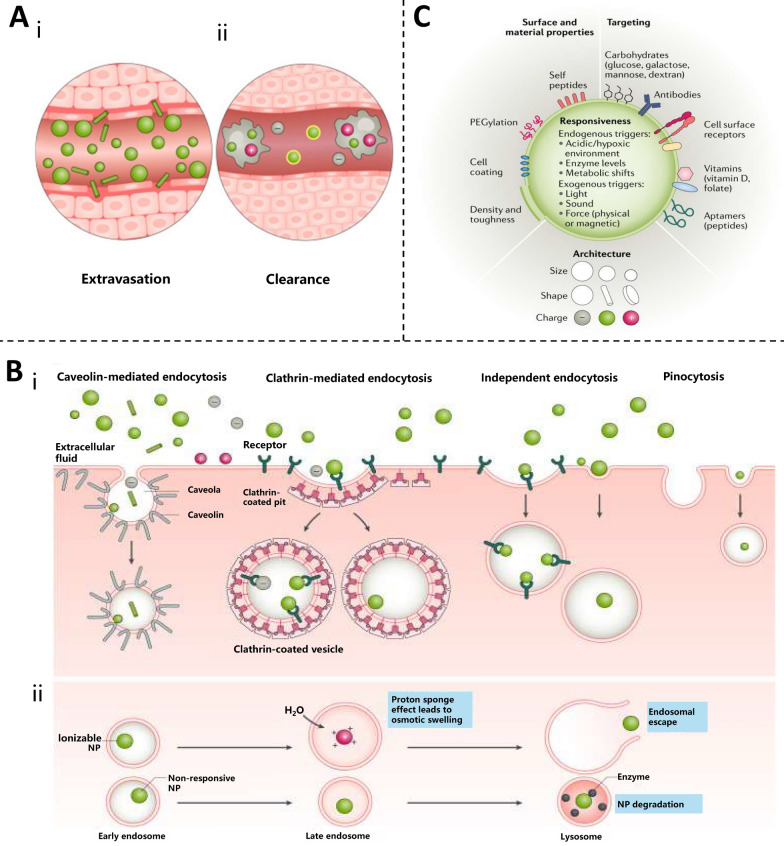
The shape of a biomaterial affects its interaction with APCs or other immune cells, which in turn affects its adjuvant activity and immunization effects. Ellipsoidal particles can induce cytotoxic T lymphocytes (CTLs) more efficiently with greater antigen specificity and stronger in vivo stimulation of immune cells than spherical particles under the same synthesis conditions [153, 154]. Nanoparticles with artificial APCs have been developed for cancer immunotherapy. Non-spherical particles have a larger surface area and flatter T cell-engaging surfaces than spherical particles [155]. This property may enhance interactions with T cells and stimulate antigen-specific T cells more effectively, leading to antitumor efficacy in a murine melanoma model, which could not be achieved with spherical particles. However, such interactions between nanoparticles and immune cells cannot be generalized and may vary depending on the cell type, as demonstrated by studies showing that macrophages prefer the uptake of rigid and spherical nanoparticles over soft and cylindrical nanoparticles [156]. Additionally, spherical and larger particles are relatively easy to marginalize, whereas rod particles are more prone to extravasation in blood circulation (Fig. 5A) [157].
Fig. 5.
Physicochemical properties of biomaterials affecting immune response. A The influence distribution of NP characteristics. (i) Spherical and larger NPs marginate more easily during circulation, whereas rod-shaped NPs extravasate more readily; (ii) Uncoated or positively charged NPs are cleared more quickly by macrophages. Reproduced with permission from Ref. [158], Copyright 2021, Nature Reviews Drug Discovery. B Pathway and fate of NP uptake. (i) endocytosis of NP: upon interaction with the cell surface, NPs—depending on their surface, size, shape and charge—are taken up by various types of endocytosis or pinocytosis via non-specific interactions, such as membrane wrapping, or specific interactions, such as with cell surface receptors; (ii) Ionizable NPs contribute to endosome escape: responsive NPs—such as ionizable NPs that become charged in low-pH environments-aid in endosomal escape and allow for intracellular delivery whereas unresponsive NPs often remain trapped and are destroyed by lysosome acidity and proteolytic enzymes [158]. C NP surface characteristic design for enhanced delivery. Reproduced with permission from Ref. [158], Copyright 2021, Nature Reviews Drug Discovery
Particle size
The particle size of biomaterials varies from nanometers to micrometers or larger particles [1]. Although nanoparticles can reach hundreds of nanometers in size, biomaterial nanoparticles with particle sizes ≤ 25 nm are more likely to target antigen delivery to the lymph nodes, whereas larger particles are trapped at the injection site and internalized before being delivered to the lymph nodes by these APCs [159, 160]. The efficiency and rate of uptake of 50 nm polystyrene (PS) by macrophages and dendritic cells is much higher than that of 500 nm PS [161]. At the same time, APCs ingesting 50 nm PS particles expressed more co-stimulatory markers than those ingesting 500 nm particles. In additional reports employing antigen-coated PS rods and spheres of various sizes, smaller spherical particles promoted adjuvant Th1 immune responses [162]. In contrast, longer rod-shaped particles tended to promote Th2 immune responses rather than thicker particles. This may be because longer particles present denser antigens and a larger surface area, which are conducive to attachment or internalization by cells. These results indicate that particle size is a key factor in the immune system, such as transport, uptake, and activation of APCs, and affects the clinical application of drugs.
Surface property effect
The surface charge of nanoparticles is crucial for determining their interaction with and uptake by immune cells. Immune cells showed a greater tendency to internalize positively charged NPs. Additionally, they appear to have the ability to escape lysosomes after internalization and localize to the perinuclear region. Meanwhile, nanoparticles with negative and neutral charges tend to colocalize within lysosomes [163]. For example, PS particles with diameters of ≤ 0.5 μm were most readily absorbed by DCs using an in vitro model [164]; however, the uptake of larger particles (such as those with a diameter of 1 μm) by DCs was greatly enhanced by transforming the surface of the particles into a positively charged one. In the presence of macrophages, positively charged and uncoated particles tend to be eliminated the fastest [165]. In addition to charge, the antigen loading pattern also affects the immune response [166]. Three PLGA nanoparticles were synthesized, each with unique surface charges and antigen loading patterns, and were loaded with ovalbumin (OVA). Two of the nanoparticles encapsulated antigens using Angelica polysaccharides and PEI, whereas the remaining nanoparticles were designed to adsorb antigens onto the PEI-coated surface. Antigen-encapsulated nanoparticles induced a stronger and longer-lasting antigen-specific antibody response than nanoparticles with adsorbed antigens in vivo [156].
In summary, the physicochemical properties of NPs (including morphology, structure, particle size, and surface properties) have an impact on vaccine delivery and immunization effects, which may not be caused by a single factor alone; other factors need to be considered. Nanoparticles can be absorbed by cells in various ways, but their fate is not the same. During endocytosis, the stability of nanoparticles is extremely easily destroyed by the low pH environment of endosomes, and the design of ionizable materials can effectively prevent the degradation of NP by acidic conditions and facilitate the escape of endosomes (Fig. 5B) [158]. For example, negatively charged PLGA nanoparticles can be transformed into neutral or positively charged NPs by means of surface modifications such as polyethylene glycolization or CS coating to break through the RES biological barrier and facilitate vaccine delivery [15]. Therefore, the physicochemical properties of NPs can be altered to improve their suitability for vaccine development, and intelligent design is of great significance for enhancing delivery capabilities and immune effects (Fig. 5C).
Vaccine delivery routes related to biomaterials
Receiving a vaccine via different administration routes can significantly impact its effectiveness owing to variations in the local environment, antigen distribution and presentation, and the resulting immune response. Therefore, it is crucial to understand and plan vaccination routes, especially with the emergence of new diseases and the lack of effective vaccines against many untreatable pathogens, which urgently necessitates the development of innovative vaccination strategies.
Injections
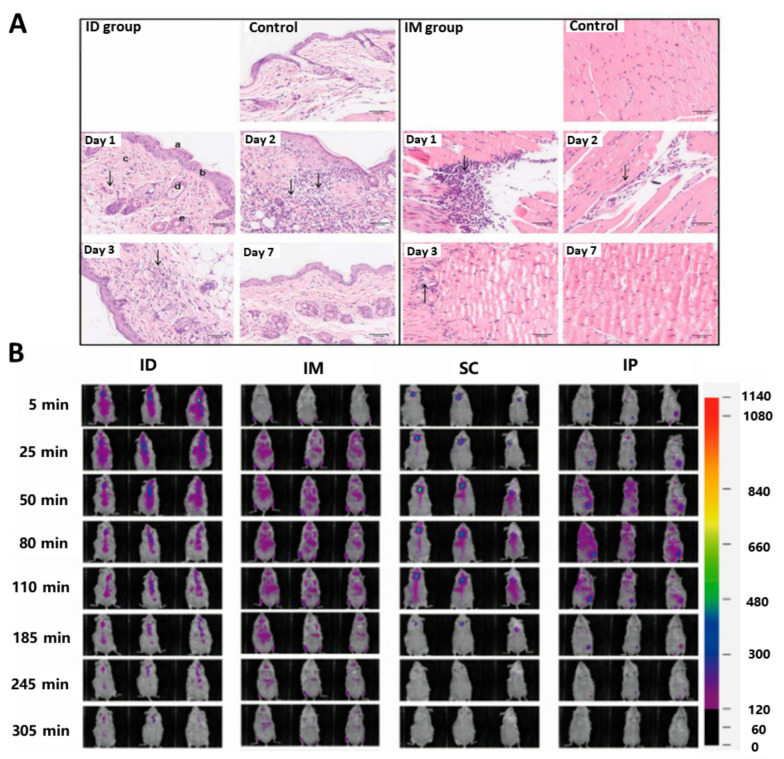
Injections are the most common form of vaccination and are generally categorized as intradermal, subcutaneous, intramuscular, intravenous, or intraperitoneal. Injections usually require puncturing of the skin surface to deposit the drug deep into the subcutaneous or muscle tissue; therefore, this injection method can be cumbersome and painful. Moreover, injections can easily cause local infections and adverse reactions; nevertheless, this type of vaccine delivery is accurate and direct and provides rapid systemic action and absorption [167]. As such, injections have been widely used in various drug therapies, including those used to deliver sedatives, antiemetics, hormones, analgesics, and immunizations [168]. Currently, intramuscular and subcutaneous injections are used clinically for vaccinations. It is important to note that intramuscular injection (IM) has been shown to be more effective than needle-free injection technology-based intradermal injection (ID) in stimulating antibody production (Fig. 6A) [169]. This phenomenon may be attributed to the more pronounced tissue damage caused by intramuscular injection, resulting in accelerated blood circulation within the muscle and recruitment of a greater number of inflammatory cells. The study also found that the longer the antigen is deposited at the site, the more favorably it enhances the uptake of antigens by APCs. Both intramuscular and intradermal injections have a great advantage in this regard (Fig. 6B) [169]. Recently, researchers have uncovered that intravenous injection nanocrystals (INCs) possess inherent potential in anti-tumor applications due to their high drug loading, low toxicity, and controllable size. This discovery holds promise for addressing the current challenges of dissolution, bioavailability, and toxicity in vaccine delivery [170].
Fig. 6.
Inoculation by injection. A In order to investigate the local inflammatory response that occurred in skin and muscle following ID and IM administration routes, hematoxylin and eosin staining was conducted on days 1, 2, 3 and 7 after inoculation. Analysis of histological changes in skin tissue and muscle tissue at different time points following administration. Reproduced with permission from Ref. [169], Copyright 2023, International Journal of Molecular Sciences. B Investigation into the local retention and diffusion dynamics of the model antigen Cy7-OVA after injection into ICR mice via different routes. Reproduced with permission from Ref. [169], Copyright 2023, International Journal of Molecular Sciences
Pain associated with vaccination has always been one of the reasons why many patients are afraid of receiving vaccinations. To alleviate the pain and fear of needles, the World Health Organization (WHO) has recommended that vaccination be promoted more effectively by introducing as many pain-modifying or pain-reducing regimens as possible [171]. As such, future research efforts should focus on evaluating the efficacy and feasibility of these regimens associated with injections to improve patient experience and vaccination rates.
Transdermal immunization
Transdermal vaccination involves the application of antigens to the skin to facilitate efficient transportation to immune cells in the skin for a rapid and effective immune response. However, it is challenging to penetrate the outermost layer of the skin known as the stratum corneum. Additionally, poor uptake of free antigens and chemokines by cells further limits the efficacy of transdermal immunization. Various adjuvants and delivery systems have been developed using different physical and chemical methods to enhance immune response without the need for needles. The previously mentioned MN patch is an increasingly popular technique for transdermal vaccine delivery within the medical field, as it effectively delivers antigens to APCs in the epidermal and mucocutaneous compartments [172].
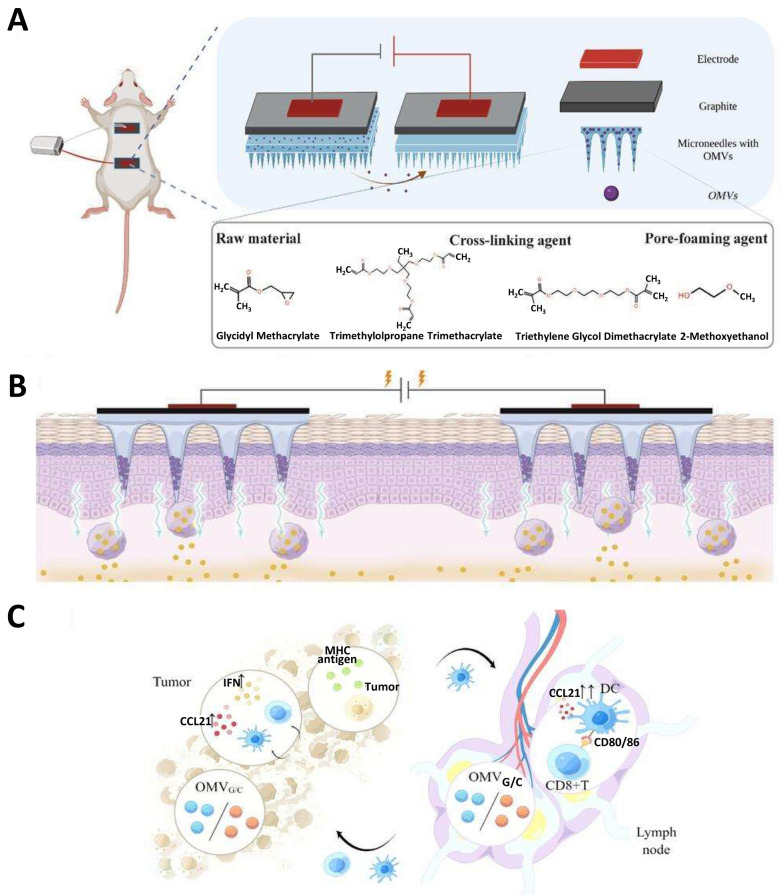
In recent years, iontophoresis therapy in combination with MNs has further increased transdermal drug penetration [173]. This was used to construct a triple platform of IPMN-GC electrons-MN-exosomes for the transdermal delivery of antigens and chemokine proteins for the painless inoculation of skin tumors and cancer vaccines [174] (Fig. 7A). A pair of MN patches were combined by iontophoresis using electrical currents to introduce ionic medicinal compounds into the body. A secure voltage can be applied to MN patches to facilitate the penetration of negatively charged vesicles into the dermis. This can be achieved by incorporating a graphite layer into the patches, rendering them electrically conductive (Fig. 7B). The MN patch vaccination elicited a robust immune response in mice, leading to the activation of numerous T cells, suggesting that IPMN-GC significantly augmented the T cell-mediated immune response for cancer suppression (Fig. 7C). Overall, transdermal immunization has a promising future as an excellent vaccination route. Compared with oral administration, transdermal immunization can bypass the metabolism of drugs in the digestive system and allow for sustained drug release. Compared with intramuscular injection, it reduces pain and improves adherence in elderly and infant patients.
Fig. 7.
Percutaneous immunization. A Composition and structure of MN. Reproduced with permission from Ref. [174], Copyright 2023, Small Science. B IPMN-G or IPMN-C facilitates transdermal delivery of gp100 and CCL21. Reproduced with permission from Ref. [174], Copyright 2023, Small Science. C Schematic of Anti-tumor mechanism of IPMN-GC in vivo. Reproduced with permission from Ref. [174], Copyright 2023, Small Science
Intranasal administration
Administering vaccines through nasal tissues is a highly effective method because it enables lower injection doses and prevents the drug from being exposed to extreme pH or digestive enzymes, offering distinct advantages. The mucosal layer is the human immune system's first barrier of defense, and intranasal antigens can block infection and transmission of respiratory pathogens at the point of entry by inducing the production of IgA antibodies and the initiation of T cells in nasal tissues, which in turn induces local mucosal immunity and systemic humoral responses [175, 176].
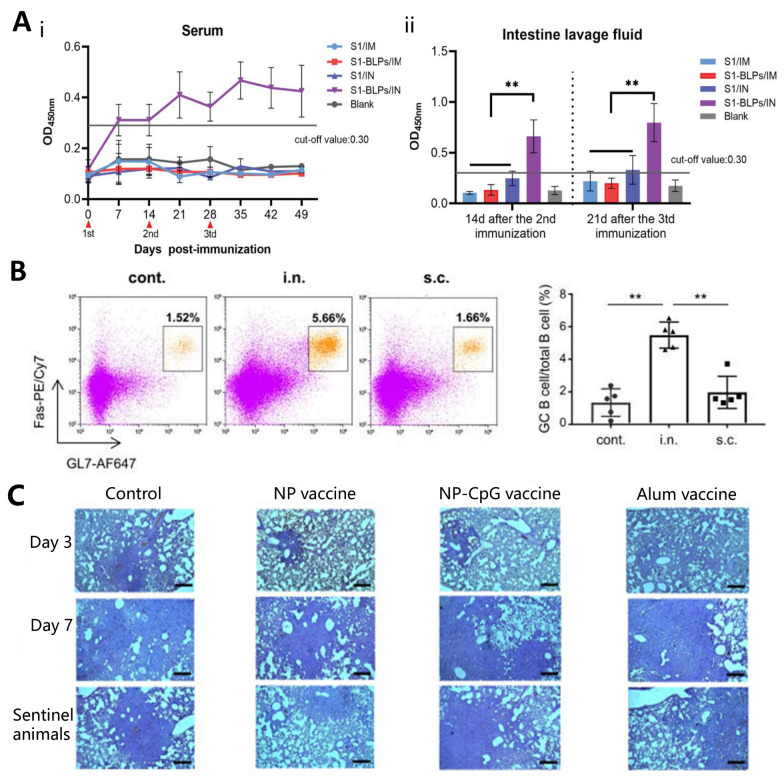
Recently, specific IgA antibodies were detected in the serum and lavage fluid of mice that were inoculated by intranasal administration of bacterial-like particles (S1-BLPs) but were not injected intramuscularly to develop a mucosal vaccine against porcine epidemic diarrhea virus [177]. This demonstrates that intranasal administration of S1-BLPs is effective in stimulating a specific secretory IgA response in the intestinal mucosa (Fig. 8A). Nagisa et al. [178] immunized mice subcutaneously or intranasally with SARS-CoV-2 inactivated whole-virion (WV) as an antigen and assessed the percentage of germinal center B cells in the nasal passages out of the total B cells by flow cytometry (Fig. 8B). Intranasal administration of inactivated WV triggers strong production of S protein-specific IgA in the nasal mucosa.
Fig. 8.
Intranasal administration. A IgA levels in serum and the quantity of IgA secreted in lavage solution were evaluated using ELISA. (i) The temporal changes of specific IgA in serum; (ii) Specific secretory IgA in small intestinal lavage fluid were analyzed. Reproduced with permission from Ref. [177], Copyright 2023, Frontiers in Immunology. B The proportion of germinal center (GC) B cells within nasal B cells was assessed using flow cytometry. Reproduced with permission from Ref. [178], Copyright 2023, Frontiers in Immunology. C Histological examination showed pathological changes in the lung tissue of the animals on days 3 and 7 post-attack. Reproduced with permission from Ref. [181], Copyright 2023, Scientific Reports
In conclusion, intranasal administration represents a needle-free method for vaccine delivery that circumvents the blood–brain barrier and mitigates systemic adverse reactions. For instance, Ruan et al. [179] developed a nasal-to-brain delivery system capable of swiftly detaching microneedles from their base, thereby reducing administration time and enhancing drug transport to the brain. Intranasal administration also offers practical advantages in terms of cost and ease of management; however, notably, its efficacy may vary depending on the duration of drug retention. This issue can be mitigated by employing chitosan, which offers advantages in adhesion to mucous membranes, or by utilizing lectins [180]. Mice challenged with wild-type SARS-CoV-2 were effectively protected by an intranasal vaccine containing SARS-CoV-2 spiny receptor-binding domain protein encapsulated in mannose-conjugated CS nanoparticles, as demonstrated by significant reductions in body weight loss, lung viral load, and lung pathology (Fig. 8C) [181]. Enhanced binding of CS to the nasal epithelial surface extends its retention, resulting in increased drug availability for absorption.
Oral administration
Oral administration is a standard needleless technique specifically used to treat influenza, polio, rotavirus, typhoid, cholera, and other diseases [182]. Compared with injections, oral administration is milder, cheaper, and less likely to cause damage to the skin or mucous membranes. Furthermore, oral administration can provide local or systemic delivery of a broad range of drug molecules (from small molecules to biomolecules) and is frequently considered the preferred delivery route.
Despite the obvious advantages of the oral route of administration, only a few vaccines are currently available in oral formulations because oral administration remains challenging owing to the complex environment of the human gastrointestinal tract. These challenges include poor drug stability, poor solubility, and low drug permeability across the mucosal barrier. For example, antigens should propagate intact into the intestinal lumen without degradation by the acidic stomach environment [183, 184]. Damage to the digestive system can reduce the immune response generated by vaccines and their ability to protect themselves against pathogens. Therefore, it is crucial to develop new vaccine carriers that can withstand the acidic conditions of the stomach, remain in the small intestine, and be released effectively. Simultaneously, the adjuvant capacity of the delivery vector should be further optimized [185]. To address these concerns, a safe genetically modified rice vaccine has been developed that can withstand the acidic environment of the stomach [186]. In addition, there have been advancements in the development of oral rice-based vaccines for allergies, cholera, and diarrhea [187–189]. In a separate study, Oliveira et al. [190] utilized CS nanoparticles to deliver a plasmid DNA vaccine encoding the Rho1 GTP protein of Schistosoma mansoni. In vivo experiments demonstrated that oral administration could potentially alleviate liver pathology by upregulating the expression of regulatory IL-10. Yagnik et al. [191] engineered a recombinant form of Lactococcus lactis to express Shigella dysenteriae type 1 protein A in the outer membrane. The immune response elicited by oral administration was significantly stronger than that induced by intranasal administration. Overall, these findings suggest that oral vaccines have a promising potential for the prevention of various diseases.
This section mainly discusses the different vaccination methods, and their application, advantages and disadvantages are summarized in Table 3.
Table 3.
Application, advantages and disadvantages of different vaccination routes
| Vaccination routes | Application | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Injections | Sedatives, antiemetics, hormone therapy, analgesics and vaccinations | Administration is accurate and direct, providing rapid systemic action and absorption | It is more troublesome and makes patients feel stronger pain, and it is easy to cause local infection and adverse reactions | [167, 168] |
| Transdermal immunization | Cosmetic skin, vaccination, disease chemotherapy, biological therapy, immunotherapy, and gene therapy | Avoid first pass effect, eliminate adverse reactions, improve vaccine stability, increase patient compliance | The stratum corneum of the skin is difficult to penetrate | [172, 173] |
| Intranasal administration | Central nervous system diseases, pain management, hormone replacement therapy, vaccinations, and treatment of special diseases | Reduce injection dose, activate mucosal immunity and bypass blood–brain barrier | The effect is often different due to the length of residence of the drug | [175, 176] |
| Oral administration | Vaccines for influenza, polio, rotavirus, typhoid, cholera and other diseases | Convenient administration, stronger immune response, high compliance | The stability and solubility of the drug were poor, and the permeability of the drug through the mucosal barrier was low | [182–184] |
Design considerations for biomaterials used in vaccine adjuvants
Autoimmune diseases, inflammatory disorders, and organ and tissue graft rejection are underpinned by adverse immune responses at the cellular, tissue, and systemic levels, and biomaterials offer powerful opportunities to control interactions at these levels to promote immune tolerance for selective control of inflammation [192]. A highly important aspect of immune system regulation is ensuring that proper signals reach the appropriate immune cells at the right time, dosage, and location. The varied utilization of biomaterials can effectively stimulate the immune system and prevent uncontrolled systemic immune reactions, thereby addressing these challenges [13]. Biomaterials are currently extensively utilized as adjuvants in vaccines to improve the efficacy, quality, and longevity of vaccine responses. The better the design of these biomaterials, the greater their potential to extend and improve the functionality of the vaccine.
Prerequisites for biomaterials as adjuvants
Reinforcing adjuvants have the potential to boost the adaptive immune response of vaccines by promoting the activation of cells in the innate immune system [193]. Some prerequisites must be met for DCs to effectively activate the innate immunity. First, the adjuvant must effectively deliver antigenic targeting to DCs, and second, DCs must be activated to release antigens for further antigen processing and presentation [194]. The particulate biomaterial itself is readily phagocytosed by DCs; however, it is also readily phagocytosed by surrounding macrophages, a situation that would result in ineffective activation of naïve T cells. Therefore, we can expect improved efficacy by preferentially targeting biomaterial adjuvants to DCs in vivo. For instance, nanoscale particles can migrate and concentrate in DC-rich lymph nodes [195]. Additionally, they can release chemokines or cytokines to recruit DCs to the injection site and stimulate a specific immune response [196, 197]. Furthermore, the use of liposome-encapsulated antigens and immunomodulatory factors to target antigens and maturation signals directly to DCs in vivo improves DC uptake and generates immunity [198, 199].
The use of biomaterials as adjuvants has two advantages. First, they activate the innate immune system. Second, combining an innate immunity activator with a biomaterial adjuvant is relatively simple. Once the biomaterial particles deliver their payload to the DC, the DC must be properly activated. Therefore, if the biomaterial adjuvant efficiently activated the innate immune system, the vaccine would be more effective. Achieving this can be complex as the interactions between toll-like receptors (TLRs) that affect the maturation of the phagosome and the presentation of antigens are intricate and can potentially induce autoimmunity or tolerance [200–202]. Therefore, a more profound understanding of the operational mechanisms of adjuvants will be beneficial for improving their design and refinement.
Adjuvants enhance vaccine immunogenicity. The vaccine itself, without adjuvants, induced the production of moderate amounts of helper T-cell factors, activated T cells, and antibodies (Fig. 9A). In contrast, adjuvanted vaccines promote the expansion of additional APCs, enhance the interaction between APCs and T cells, and increase the production of helper T cell factors, versatile T cells, and antibodies. Moreover, it offers the advantage of requiring lower dosage and antigen usage. Consequently, it generated a broad and long-lasting immune response (Fig. 9B). Adjuvants can function as immunostimulants or delivery systems, depending on their functional mechanisms. The following section describes the specific mechanisms of adjuvant action of both immunostimulants and delivery systems. This will serve as a valuable tool for the development and progression of adjuvants.
Fig. 9.
Adjuvant enhances vaccine immunogenicity. A Vaccines without adjuvants induce modest production of T helper-polarizing cytokines, antibodies, and activated T cells. Reproduced with permission from Ref. [203], Copyright 2023, Signal Transduction and Targeted Therapy. B In contrast, vaccines with adjuvants promote the maturation of more APCs, increase the interaction between APCs and T cells, promote the production of greater numbers and more types of T helper-polarizing cytokines, multifunctional T cells, and antibodies, leading to broad and durable immunity, as well as dose and antigen savings. Reproduced with permission from Ref. [203], Copyright 2023, Signal Transduction and Targeted Therapy
Mechanisms of immunostimulants
Immunostimulants are a class of molecules that activate immune responses by engaging with TLRs and other pattern-recognition receptors (PRRs), thereby signaling danger and enhancing antigenic and costimulatory signaling. This ultimately leads to the maturation and activation of APCs and enhances adaptive immune responses. Different types of immunostimulatory signaling through distinct PRRs result in the secretion of various cytokines [204, 205]. The specific mechanisms by which these molecules exert adjuvant potency are complex, including targeting the TLR, cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon genes (cGAS-STING), CLR, and other PRRs. A more detailed description of these specific mechanisms can be found in [203] as we did not extensively delve into this topic in this study.
Several researchers have incorporated known innate immune activators into biomaterial adjuvant systems to regulate innate immune activation. Although the range of innate activators is vast, TLR ligands play a direct role in adaptive immunity [206]. Controlled release of TLR ligands from the particle and attachment to the surface of the particle are viable delivery methods [207]. The delivery method should be selected based on the mechanism of action of the ligand. For example, TLR-1, -2, -4, and -6 are expressed on the cell surface, similar to NOD-like receptors (NLRs), whereas TLR-3, -7, -8, and -9, as well as NLRs, are expressed intracellularly [208, 209]. Further studies on innate immunity may reveal the effects of TLR ligand mixtures and their optimal timings. Therefore, it is feasible to develop combinatorial and sequential release strategies to guide and augment the adaptive immune responses.
Mechanisms of delivery systems
The delivery system serves as a carrier material to enhance antigen presentation. This is achieved by increasing the biological availability of the loaded antigen and by targeting it to the lymph nodes or APCs. The process of antigen presentation involves recognition, uptake, and internalization of the antigen by the APC, followed by the positioning and expression of major histocompatibility complex (MHC) molecules on the surface of the APC [210]. This delivery system can enhance antigenic signals on the APC surface via one or more routes.
Prolonging the bioavailability of antigens
Prolongation of antigen bioavailability can be achieved by ensuring that sufficient antigen is available to the APC while simultaneously promoting MHC antigenic peptide signaling. The delivery system can extend antigen bioavailability in three ways: (1) slow release of the antigen, (2) creation of an immunological niche, and (3) protection of the antigen (Fig. 10A).
Fig. 10.
Action mechanism diagram of conveying system. A Extending the duration of antigen bioavailability. Reproduced with permission from Ref. [203], Copyright 2023, Signal Transduction and Targeted Therapy. B Targeting the APC antigen. Reproduced with permission from Ref. [203], Copyright 2023, Signal Transduction and Targeted Therapy. C Direct delivery of antigens to lymph nodes. Reproduced with permission from Ref. [203], Copyright 2023, Signal Transduction and Targeted Therapy. D Promoting cross-presentation of antigens. Reproduced with permission from Ref. [203], Copyright 2023, Signal Transduction and Targeted Therapy
First, delivery systems can prolong the presence of antigens through gradual release [211]. For example, injectable hydrogels allow the continuous presentation of antigens by APCs through slow release, facilitating the production of larger numbers and higher-affinity antibodies [212–214]. A hydrogel mesh also plays a major role in slow release, and the mesh size can usually be adjusted to control the sustained-release time [215]. PLGA polymer particles can encapsulate antigens and provide a sustained release [216, 217]. Additionally, self-assembled scaffolds with macroporous structures can slowly release antigens while releasing inflammatory signals through the loading of immune stimulants [218].
Second, the delivery system could also potentially boost immune activation by establishing an immune microenvironment at the injection site [219], thereby attracting more immune cells for infiltration at the injection site. Developers can incorporate certain cytokines into the delivery system for slow release to promote immune cell migration. Sun et al. recently developed a thermosensitive hydrogel containing granulocyte–macrophage colony-stimulating factor (GM-CSF) and ovalbumin that can be injected to improve the attraction of DCs to the injection site and enhance antigen absorption [220]. In a similar study, injecting mice with a mesoporous silica rod increased the infiltration of innate immune cells at the injection site and enhanced the immune response [221].
Furthermore, the administration of antigens can improve their bioavailability by shielding them from enzymatic degradation in the body and preserving their physiological activities. For example, encapsulating mRNA antigens in LNPs can protect them from enzymatic hydrolysis in extracellular serum [222, 223].
In conclusion, it is essential to consider the duration of antigen and adjuvant exposure, as well as their bioavailability when formulating adjuvants to enhance the strength, longevity, and effectiveness of adaptive immune responses.
Targeting APCs
The delivery system can target APC in two ways, as described below, enabling APC to capture antigens efficiently (Fig. 10B). Enhanced antigen uptake increases the number of MHC-antigenic peptides present on the APC surface and induces a more potent adaptive immune reaction [203].
On the one hand, the first delivery system mimics the size and structure of the disease agent to increase the probability of APCs taking up the antigens. For example, liposomal nanoparticle encapsulation of antigens enhances APC recognition and uptake, thereby facilitating antigen presentation [224]. Self-assembled protein nanoparticles and other polyvalent particles can mimic the spatial structure of the pathogen to enhance APC antigen uptake [225–228]. Additionally, antigen uptake and cellular immunity can be selectively enhanced in vitro and in vivo using delivery systems that target immunogens to the Fc receptors on APCs [229–232].
On the other hand, modifying or tailoring the size of nanoparticles can also enable delivery systems to actively target specific cell groups. For example, when the receptor Clec9A antibodies are attached to nanoparticles, they favorably target normal type 1 DC (cDC1) [233]. By engineering their size to be between 50 and 100 nm, nanoparticles can effectively target and remain within follicular dendritic cells (FDCs) [234, 235]. This targeted approach significantly enhances the generation of a highly affinitive and long-lasting antibody immune response [236].
Lymph node transport
Lymph nodes accumulate large numbers of internal immune cells and lymphocytes and are the site of the initial immune response [237]. Delivery systems often deliver antigens directly to the lymph nodes either by passive diffusion or binding to albumin (Fig. 10C).
It is crucial to carefully control the size of the delivery system to facilitate passive diffusion of the drug to the lymph nodes. The optimal size range should be 5 and 100 nm [238]. This is because delivery systems that are too small tend to easily enter the capillaries, whereas those that are too large may not be able to enter the lymphatic vessels. Furthermore, the hydrophilic nature of the delivery system influences the efficiency of vaccine transport [239]. When administered subcutaneously, the 50 nm PEG-modified polymers demonstrated enhanced hydrophilicity and increased retention in rat lymph nodes compared with unmodified particles [240].
The second method involves the presence of endogenous albumin in the lymphatic system through which the antigen can be directly transported to the lymph nodes by binding to endogenous albumin [241, 242]. Albumin is specifically designed to transport fatty acid molecules and can bind and incorporate a restricted quantity of lipid molecules [243]. Researchers have utilized this mechanism to create a tumor peptide vaccine containing a lipophilic tail that strongly binds to albumin. Consequently, the vaccine accumulates in large quantities in the lymph nodes, leading to a notable increase in its antitumor effectiveness when administered to mice [244]. Additionally, certain dyes such as Evans blue can bind endogenous albumin [245]. By employing this functionality, conjugation of the Evans blue derivative to the antigen enables the vaccine to effectively bind to albumin and transport it to the lymph nodes [246]. The vaccine has been shown to effectively inhibit tumor growth in mice based on the results of experiments conducted in living organisms.
Promoting antigen cross-presentation
Foreign antigens are presented in a process known as antigen cross-presentation to CD8 + T cells through MHC I molecules. In contrast, foreign antigens are typically present in CD4 + T cells via MHC II molecules without cross-presentation [247, 248]. The delivery system can achieve cross-presentation of the antigen by releasing it through the proton sponge effect, membrane destabilization, and photochemical internalization (Fig. 10D).
The first mechanism, known as the proton sponge effect, involves the internalization of cationic polymers and lipid delivery systems containing protonamine groups that absorb a large number of protons to buffer the acidic environment within the endosomes or lysosomes [249]. For instance, PEI can absorb protons through its protonamine group under acidic endosomal conditions, leading to endosomal swelling and rupture. This results in the release of antigens into the cytosol and facilitates cross-presentation of MHC I molecular antigens [250]. Additionally, the proton sponge effect enhances antigen cross-presentation in several other cationic polymers and liposomes [251–253]. Secondly, some delivery systems may enhance cross-presentation by destabilizing the endosomal/lysosomal membrane through fusion or bonding, leading to the release of the antigen into the cytosol. This allows the presentation to CD8 + T cells via MHC-I molecules [254].
The third method, photochemical internalization [255, 256], involves loading the photosensitizer into the delivery system along with the antigen. Upon exposure to a light source, the endosomal membrane is disrupted, causing the release of the antigen from the delivery system into the cytoplasm [257]. Ji et al. [258] developed a cationic nanoparticle that electrostatically binds to the photosensitizer AlPcS2a and delivers the antigen. This approach significantly enhances the release of antigens from endosomes/lysosomes into the cytosol when a 660 nm light source is applied, thereby promoting an immune response mediated by CD8 T cells.
Biomaterials have been extensively utilized to enhance vaccine development. It is essential to gain a more comprehensive understanding of their ability to stimulate the immune system and deliver vaccines to guide and improve adaptive immune responses. Enhancers play a major role in activating and enhancing adaptive immunity, whereas delivery systems can increase the number of antigens delivered to the surfaces of APCs by increasing antigen bioavailability, targeting these cells, delivering vaccines to lymph nodes, and promoting antigen cross-presentation. This further enhances the effectiveness, duration, and robustness of adaptive immune responses. It is important to note that the stability of the delivery system, the efficiency of antigen release, and other factors can collectively impact the targeting effect of lymph nodes, making these aspects crucial. Designers should take into account these factors during adjuvant development and incorporate them into the design process. Furthermore, as adjuvants play a pivotal role in vaccines, they must be tailored to effectively enhance immune responses while ensuring minimal systemic toxicity and optimal bioavailability. Thus, achieving a balance between efficacy and safety poses a significant challenge.
Applications of biomaterials in vaccine development
Numerous communicable diseases occur annually in developing nations, including acquired immunodeficiency syndrome (AIDS), malaria, tuberculosis, dengue fever, influenza, and various respiratory illnesses. These outbreaks continue to pose a significant threat to human health and well-being. It is crucial to implement strategies for disease prevention and the development of specific medications and protective vaccines.
Traditional vaccines play a crucial role in preventing and controlling infectious diseases. However, they are associated with limitations, such as poor safety, low immunogenicity, instability, and challenges in generating long-lasting immune responses. In recent years, significant research has been conducted on the use of biomaterials in vaccine development. Biomaterials have the potential to enhance the efficacy and tolerability of vaccines, leading to a more rational approach in antigen and adjuvant design as well as improving delivery methods. Currently, biomaterials are being tested for their use in a broad range of diseases. This paper focuses on the development and research progress of biomaterial delivery vehicles for cancer vaccines, as well as vaccines for infectious diseases such as AIDS, influenza, COVID-19, TB, malaria, and hepatitis B. Table 4 provides a summary of the biomaterials used in these vaccines, their forms of delivery, and their therapeutic effects.
Table 4.
Biomaterials, delivery methods and therapeutic effects in vaccine applications
| Applications | Biomaterials | Delivery methods | Therapeutic effects | References |
|---|---|---|---|---|
| Cancer vaccine | Hydrogels and nanoparticles | • Nanovaccine and ICB-hydrogel complex NvIH | • It enhances the accumulation of tumor antigens, stimulates the systemic anti-tumor immune response, and effectively suppresses the growth of large tumors with an abscess effect | [259] |
| AIDS vaccine | Liposomes and nanoparticles |
• Liposomes containing HIV envelope proteins • Nanoparticles containing immunomodulatory substances |
• Enhanced antigen capture and antigen cross-expression by APCs • Altered immunostimulatory function of drugs |
[260] |
| Influenza vaccine | Nanoparticles and microneedles | • HMNF nanoparticles loaded with and protecting inactivated viral antigens | • Stimulates a robust and durable immune response, generating antibodies and cellular immunity for broad protection against various subtypes of influenza A and B vaccines | [261] |
| COVID-19 vaccine | Liposomes | • LNPs wrap around and protect mRNA | • Promotes uptake of mRNA by cells in the body and induces endosomal disruption for delivery of nucleic acids to the cytoplasm of the host cell, triggering a powerful immune response | [262] |
| Tuberculosis vaccine | Nanoparticles and exosomes |
• Chitosan-coated with Phipps BCG and CFP-PLGA combination • Exosomes loaded with vaccine components |
• Induces a strong immune response and reduces infection rates and bacteria count • Modulates host resistance to Mycobacterium tuberculosis infection |
[263]、[97] |
| Malaria vaccine | Hydrogels, nano-capsules, and nano-emulsions |
• As an API vector to target vaccine delivery • R21 fuses with HBsAg and combines with Matrix-M adjuvant |
• Reduces parasite resistance and increases antimalarial activity of API • Enhances vaccine efficacy and duration of protection and improves CSP immune response |
[264, 265] |
| Hepatitis B vaccine | Self-assembling peptides and nanoparticles |
• Ferritin NP-preS1 nanovaccine • Chimeric HBV-HuNoV P particles |
• Induces a high-level and long-lasting anti-PRES1 response in the body • Induces a more robust humoral and cellular immune system response to infections |
[228, 266] |
ICB immune checkpoint blockade, NvIH (Nanovaccines + ICBs)-in-hydrogel, CFP culture filtrate protein, API active pharmaceutical ingredients, CSP cyclosporum protein, HuNov human norovirus
Cancer vaccines
In recent years, cancer immunotherapy has demonstrated significant potential for inhibiting tumor growth and metastasis by activating the immune system to recognize and eradicate specific tumor cells. This approach has become a fundamental strategy for clinical cancer treatment, with cancer vaccines emerging as a particularly promising form of immunotherapy. Typically, they deliver antigens combined with immune-activating adjuvants to the lymph node tissues in vivo via vectors. APCs utilize extracted vectors to enhance tumor-specific immune responses and promote immune memory, thereby initiating the cancer immune cycle [267]. However, the effectiveness of cancer vaccination is hindered by various limitations, including immunosuppressive tumor microenvironment (TME), limited immunogenicity, and poor targeting. To overcome these obstacles, many delivery systems such as nanocarrier systems and micelles have been used for cancer vaccines [268, 269]. However, these carriers can restrict the effectiveness of cancer vaccines owing to issues such as inefficient antigen encapsulation, poor targeting, complex preparation processes, and systemic toxicity [268–270].
Fortunately, hydrogels have unique properties that offer many solutions to the challenges faced by cancer vaccines [141]. As a result, they are currently the most widely used vaccine delivery systems. Hydrogels enable the incorporation of antigens and various immunomodulators, such as adjuvants, cytokines, checkpoint inhibitors, medications, and nanoparticles, to enhance the efficacy of cancer vaccines against tumors [271–273]. Injectable hydrogels also have the potential to serve as immune adjuvants [274, 275]. These hydrogels can be produced in situ and injected directly into the pathological location using a syringe, thereby eliminating the need for invasive implantation procedures. This method not only improves ease of handling but also enhances patient tolerability [141, 276]. Recently, Cheng et al. [259] proposed a single-dose injectable nanovaccine and ICB-hydrogel complex called NvIH, which remodels the immune environment by a single-dose intralesional injection, reduces immunosuppression in the TME, increases the priming accumulation of tumor antigens, and triggers a systemic antitumor immune response. This study demonstrated that NvIH significantly suppressed and regressed the septic effects of large tumors, underscoring its potential as a safe and potent immunotherapy for local and distant large tumors.
Currently, the development of cancer vaccines still faces numerous challenges. The formulation of cancer vaccines is intricate, with limited treatment coverage and prolonged observation periods for therapeutic effects. Encouragingly, the utilization of biological materials in cancer vaccines holds great promise, as evidenced by ongoing clinical trials of several in situ cancer vaccines based on biological materials targeting advanced solid tumors and various types of cancer [277]. We anticipate the design of more sophisticated biological material delivery systems for imminent clinical application.
AIDS vaccines
AIDS, which is caused by the human immunodeficiency virus (HIV), is an extremely dangerous infectious disease with high morbidity and mortality rates, and its treatment is hindered by a persistent lack of effective drugs to cure HIV infection worldwide. However, biomaterials can significantly improve the efficacy of alternative HIV therapies in mouse models and rhesus monkeys [1]. For example, liposomal delivery of HIV viral envelope proteins promotes antigen uptake, prolongs antigen retention by lymphocytes, and enhances antigen capture by APCs [278]. Another emerging strategy involves attaching nanoparticles containing immunomodulatory substances to T cells or APCs during immunization or immunotherapy to alter their immunostimulatory functions [279].
In recent years, the application of nanotechnology to treat and prevent HIV infection has been extensively studied and actively investigated in clinical and large-animal trials. Long-acting injectable antiretroviral medications are at the forefront of clinical nanotechnology in HIV treatment as they reduce the need for frequent drug administration, improve patient adherence, and are widely recognized as a significant advancement in intervention [280]. Long-acting caboterivir and rilpivirine were the initial long-acting injectable therapies employed for HIV management, and they were approved in 2020 by the European Union for treating patients with controlled viral loads. Lipid nanoparticles loaded with 3–4 antiretroviral drugs have been tested in non-human primates [281, 282], and nanoencapsulation was found to enhance the circulation time of antiretroviral drugs and increase their uptake into monocytes in the peripheral blood and lymph nodes of macaques. This is particularly evident with antiretroviral drugs, as HIV persists in macrophages even in patients receiving antiretroviral therapy. These outcomes suggest that long-acting injectable therapy is a promising treatment modality for patients with HIV. Further research is needed to determine the optimal treatment strategy for application in humans.
Influenza vaccines
Influenza vaccine development is well established, and research has been initiated on biomaterials that can be used to prepare novel influenza vaccines resistant to evolving influenza viruses, which can improve patient compliance and reduce the annual redesign of influenza-specific vaccines. The significance of creating a universal influenza vaccine to address public health threats posed by pandemics and emerging strains of influenza cannot be overstated. One strategy that has recently entered clinical validation and has shown promise for widespread application is the MN vaccine delivery system. The novel approach of utilizing soluble MN patches for influenza vaccination demonstrated in the initial clinical trial the ability to maintain vaccine stability for over a year and elicit immune responses comparable to those of the current injectable vaccine, even when self-administered by patients [283–285]. In addition, Pan et al. [261] proposed a mucosal nanoparticle vaccine, HMNF, that assembles multiple conserved antigenic epitopes of influenza viruses, which when immunized via the nasal mucosa, induce potent and durable antibody and cellular immune responses and achieve cross-protection against multiple influenza A and B vaccine subtypes. Intranasal vaccination of HMNF mice results in a robust immune response, characterized by increased levels of specific antigens and T-cell reactions that demonstrate reactivity to various antigen mutations. Moreover, the HMNF nanovaccine can be rapidly produced using an E. coli system, has potential for transnasal spray self-vaccination, and is expected to be developed into a broad-spectrum anti-influenza virus vaccine in the future.
COVID-19 vaccines
The coronavirus disease (COVID-19) outbreak has spread worldwide since the end of 2019. It is an acute infectious disease caused by coronaviruses that is highly infectious and pathogenic and is associated with a high mortality rate. Therefore, studying the global pathogenic mechanism of COVID-19, rapidly developing a safe and effective vaccine, and designing a corresponding therapeutic regimen have become daunting tasks.
The first new coronavirus vaccine to enter clinical trials was an LNP-delivered mRNA vaccine; encapsulation with an advanced LNP formulation provided protection of mRNAs upon storage and injection into tissue, facilitated uptake of mRNAs into cells in vivo, and induced endosomal degradation to release nucleic acids into the cytosol of the host cell [262]. Throughout this process, precise delivery of mRNA to the cell is crucial for its function. LNPs offer several advantages over other nucleic acid vaccine delivery systems. These include a high nucleic acid encapsulation efficiency, efficient cell transfection, high tissue penetration, and minimal cytotoxic and immunogenic effects [286]. In 2018, the U.S. FDA approved Onpattro, an RNAi therapeutic vaccine delivery system that utilizes LNPs to encapsulate siRNAs for direct delivery to the liver through intravenous injection, preventing the human body from producing disease-causing proteins. This is an important milestone in the application of LNPs in vaccine delivery from small organic molecules to biomolecules.
Thanks to extensive basic research and experiments on mRNA optimization and LNPs, the development of an mRNA vaccine against the COVID-19 novel coronavirus took less than one year from the development of the viral sequence to the successful launch of the vaccine, which is a remarkable feat. Both Pfizer and Modena COVID-19 RNA vaccines, the first to be marketed in 2020, use LNP as a vector to deliver mRNA encoding the COVID-19 viral capsid protein into the cytoplasm of the host cell, where the mRNA is translated into capsid proteins that serve as antigens to induce an immune response against the virus and respond to the challenges posed by the global COVID-19 pandemic [287].
Tuberculosis vaccines
M. tuberculosis causes tuberculosis (TB), which is the deadliest bacterial infection worldwide and places a substantial burden on public health systems [288]. The situation surrounding TB has become more complicated in recent years owing to the emergence of drug-resistant M. tuberculosis and HIV-TB coinfections [289], which have also significantly worsened the prognosis and treatment of TB. Innate and adaptive immunity in humans is a powerful defense mechanism against infectious agents. However, in co-evolution with humans, M. tuberculosis has acquired multiple mechanisms to evade the immune response and maintain intracellular persistence and long-term survival within the host. One modality that has emerged in recent years for the treatment of TB is therapeutic vaccination, which, unlike prophylactic vaccination, is intended to be used as an adjunctive treatment for TB or to prevent relapse after cure [290]. Despite several years of intensive research, the Bacillus Calmette-Guérin (BCG) vaccine remains the only licensed vaccine available. It provides protection against tuberculosis in children but is not effective against tuberculosis in adults and has not been reported for use in animals.
A few new vaccine candidates are available in the clinical development stage and preclinical trials aimed at replacing or boosting BCG, which has considerable potential for TB vaccine development. For example, Magallanes et al. [263] used CS-coated Phipps BCG for primary vaccination in a goat model, and enhanced TB prevention using CFP-PLGA. The study findings suggest that the implementation of the BCG vaccination protocol combined with protein enhancement may result in a reduction in TB transmission within animal populations by mitigating pathological harm in vaccinated individuals. The vaccine has shown efficacy in reducing visible lesions and decreasing infection and bacillary counts in animals. This leads to a strong immune response [291]. Furthermore, research suggests that exosomes may be a promising option for detecting and treating TB [99]. Relevant exosomal noncoding RNAs play a crucial role in the immune response to M. tuberculosis infection and show great potential for combating bacterial infections, such as TB. The discovery of exosomal ncRNAs in response to M. tuberculosis infection provides new evidence for the potential use of exosomal ncRNAs as novel biomarkers of TB. This could aid in the development of next-generation diagnostic strategies for TB.
Malaria vaccines
Malaria is a blood-borne endemic parasitic disease with an extremely high morbidity rate, which poses a serious threat to human life, security, and socioeconomic status. Over the years, numerous drugs have been developed for the treatment of malaria. However, many of these drugs have low water solubility and poor bioavailability, which can lead to drug-resistant parasites, ultimately resulting in increased and fatal cases of malaria. Nanomaterials are attractive alternatives to conventional therapies because they can act as efficient drug carriers. They possess high loading capacity, enable targeted vaccine delivery, are biocompatible, and exhibit minimal toxicity [264]. Nanoparticles, such as liposomes, hydrogels, and nanocapsules, can be functionalized to serve as drug carriers for targeted vaccine delivery. Additionally, they can enhance the stability of the drug, overcome poor patient compliance and parasite resistance, and increase its antimalarial activity [264, 265]. Furthermore, their use as antimalarial carriers can provide sustained drug release and excellent encapsulation depending on their composition. This has the potential to improve parasite clearance, reduce relapse rates, and decrease parasite resistance [292–294].
Currently, the only malaria vaccine in the world with proven preventive efficacy is RTS S/AS01. This vaccine is a combination of repetitive T epitopes obtained from the Plasmodium falciparum cyclosporin protein (PfCSP; also known as RTS), an antigen obtained from the hepatitis B surface antigen (HBsAg), and a combination of the S antigen and AS01 adjuvant (S/AS01). The adjuvant is prepared from liposomes by combining two immunostimulants: monophosphoryl lipid (MPL) and QS-21 [295].
RTS and S/AS01 were first announced and pilot-tested in malaria endemic areas in 2019 [296]. More recently, the University of Oxford developed the R21/Matrix-M malaria vaccine. This vaccine combines the R21 virus-like protein PfCSP, linked to the N-terminus of HBsAg, with Matrix-M adjuvant [297]. The R21 vaccine is an intramuscularly injected prophase erythrocyte vaccine. It elicits similar anti-CSPs, but in a more elevated manner owing to the increase in the CSP content of individual HBsAg particles. Increased potency and duration of protection may result from an enhanced immune response to CSP. This is why it has been referred to as the “next generation RTS, S” [298, 299].
R21/Matrix-M is currently undergoing testing in Western Africa and other regions. Encouraging data have emerged from randomized phase 2b vaccine trials. These positive clinical trial results provide hope to save millions of lives.
Hepatitis B vaccines
HBV infection remains the leading cause of liver cancer, with high morbidity and mortality rates, and it poses a major global public health burden. Vaccination is the most effective approach to prevent long-term HBV infection [300, 301]. The hepatitis B vaccine is not only effective in preventing hepatitis B but is also the only vaccine that can prevent primary liver cancer. A key turning point in the fight against this virus is the isolation of HBsAg as an antigen for immunization, which effectively reduces the risk of new infections [302]. However, in patients with chronic hepatitis B (CHB) infection, HBsAg does not induce effective antibody responses, and hundreds of thousands of patients with CHB die each year from advanced complications such as liver disease, cirrhosis, and hepatocellular carcinoma. Therefore, there is an immediate need for more effective therapeutic strategies to overcome immune tolerance and promote functional immune responses against HBV infection in patients [228, 303].
The preS1 domain of the HBV large surface antigen is regarded as a highly promising candidate for therapeutic vaccine design, particularly in comparison with conventional HBsAg. Wang et al. [228] designed a ferritin NP-preS1 vaccine, a nanoparticle vaccine capable of inducing the generation of a high-level and prolonged anti-preS1 response, which effectively cleared some of the viruses in a mouse model of chronic HBV and achieved partial serological transformation, providing a promising and translatable vaccination strategy for the functional cure of CHB. In addition, several vaccine adjuvants have been developed to eliminate HBV infection in CHB patients. For example, Daisuke et al. [304] reported that the combination of cyclic guanosine monophosphate-AMP (cGAMP) and nanoparticle CpG-ODN (K3-SPG) as the HBV vaccine adjuvants with spermidine (SPD) led to a significant reduction in HBsAg levels in their livers and sera, along with an enhanced immune response by vaccination in HBV-transgenic (HBV-Tg) mice. Ernawati et al. [266] evaluated the safety and immunogenicity of chimeric HBV-HuNoV P particles in gnotobiotic (Gn) pigs, and experimentally demonstrated that intranasal injection of a strong cellular and humoral immune response was induced in Gn pigs using a chimeric HBV-HuNoV P particle vaccine.
Vaccines for other infectious diseases
Biomaterial-based vaccines are currently being tested against other infectious diseases. For example, for the common dengue virus (DENV), an mRNA vaccine encapsulated in LNPs was designed to induce a powerful immune response when injected into mice [305]. In addition, a vaccine for PLGA nanoparticles developed against methicillin-resistant Staphylococcus aureus is also being actively explored [306]. Simultaneously, a cationic polymer carrier with stimuli-responsive properties has been developed to augment the immune response of candidate vaccines against Zika virus and pneumococcus [307, 308]. These studies collectively highlight an emerging research trend in which biomaterials can be utilized to modulate the immune signaling environment. They also showed how biomaterials can alter the arrival of a signal to optimize immune induction.
Conclusion and prospects
Biological materials that enable the control of vaccine delivery and the spatial and temporal behavior of interacting cell populations have become highly effective tools for vaccine delivery and localization. It is a major area of intersection between chemicals, materials, biotechnological, and medical disciplines, and has huge potential applications. Dedicated efforts have been made to advance the use of biomaterials for vaccine development and immunomodulation in the fields of infectious and autoimmune diseases, regenerative medicine, and cancer.
The field of biomaterials in vaccine development is advancing at a phenomenal rate with new leads and clinical trials emerging annually. Biomaterials play an important role as key components of vaccine delivery. Some biomaterials have inherent immunostimulogenicity that facilitates the construction of vaccine vectors, acts on immune cells in the body, and regulates certain immune functions. In addition to immunogenicity, biomaterials can trigger systemic effects, reduce pain, and improve antigen stability. They can simultaneously deliver targeted signals to specific cells, organ systems, or body tissues by targeting multiple immune cell groups and cellular pathways. Vaccine development is a complex process involving multiple scientific fields and technologies, and the specific biomaterials used vary depending on research objectives, pathogen type, and development methodology. With proper development and design, biomaterials have the potential to improve the modulation of antigenic, adjuvant, or immunomodulatory responses and direct these signals to specific tissues or cell populations. In addition, biomaterials can be processed into a wide variety of dynamic delivery systems, including PNPs, LNPs, hydrogels, and MNs. These delivery systems have shown good antigen slow release and targeting capabilities, and even they can combine with each other to form new vaccine delivery platforms for research in the treatment of a variety of infectious diseases and cancers, including lipid-polymer hybrid nanoparticles, nanoparticle composite hydrogels, and hydrogel-forming MNs, which show more powerful synergistic advantages and effects.
Over all, biomaterials hold great promise for advancing vaccine development. Nevertheless, it is imperative to acknowledge the substantial challenges that must not be overlooked in their long-term advancement. For instance, the issue of vaccine transportation and preservation has always poses a significant hurdle in the field of vaccine development. Although it has been found that SiNPs, as an advanced material, can protect vaccines from being destroyed by harsh environments, its preparation process and performance stability still need to be further improved. Furthermore, despite efforts to enhance delivery methods such as polymer-based or peptide-based systems aimed at improving in vivo T cell immune response potential, their practical application effectiveness and efficiency necessitate careful consideration. Additionally, considering the safety implications and long-term effects on human health of biomaterials are paramount. The future direction is likely to be towards precision medicine, so biomaterial-based selection and safety issues need to be fully considered to accommodate the unique immune response characteristics of individual patients. Numerous unresolved issues and research directions remain that warrant comprehensive exploration before diverse biomaterials can be effectively utilized in clinical settings.
Fortunately, as our understanding of the immune system deepens, the mechanisms by which biomaterials function as vaccine adjuvants will be further elucidated. Subsequent research can focus on effectively harnessing biomaterials in the development of new adjuvants. Biomaterials possess inherent immunogenic characteristics that can be leveraged to create proactive carrier systems for vaccine responses or to modulate immune cell functions within the body. As adjuvants, they have been extensively utilized in vaccines to enhance response efficacy and quality. Through rational development and continuous exploration of improvements, it is anticipated that more efficient immune activation and broader pathogen coverage can be achieved, along with addressing current vaccine challenges such as transport difficulties, limited storage, and low delivery efficiency. We firmly believe that with ongoing breakthroughs in future medicine and biomedicine technologies, biomaterials for vaccine delivery will undoubtedly undergo further development and exploration, leading to the precise, efficient, and safe development of novel vaccine adjuvants.
Acknowledgements
The research was supported by Zhejiang Shuren University research project (2023R053).
Abbreviations
- COVID-19
Corona Virus Disease
- PNPs
Polymer-based nanoparticles
- LNPs
Liposome-based nanoparticles
- MNs
Microneedles
- PLGA
Poly (lactic-co-glycolic acid)
- PEI
Polyethyleneimine
- CS
Chitosan
- SAPs
Self-assembled peptides
- ISCOM
Immunostimulatory complexes
- FDA
Food and Drug Administration
- RES
Reticuloendothelial system
- PEG
Polyethylene glycol
- PLA
Polylactic acid
- NPs
Nanoparticles
- CpG
Cytosine-phosphate-guanine
- GPC
Glycopeptide conjugate
- CLRs
C-type lectin receptors
- APCs
Antigen-presenting cells
- DDAB
Dimethyl dioctadecylammonium bromide
- CHAL
Cholesterol
- OA
Oleic acid
- IMQ
Imiquimod
- DCs
Dendritic cells
- HPV
Human papillomavirus
- GNPs
Gold nanoparticles
- AgNPs
Silver nanoparticles
- CNPs
Carbon nanoparticles
- IgG
Immunoglobulin G
- IgA
Immunoglobulin A
- SiNPs
Silicon dioxide nanoparticles
- MSNs
Mesoporous silica nanoparticles
- VLPs
Virus-like particles
- OMVs
Outer membrane vesicles
- HER-2
Human epidermal growth factor receptor 2
- CPMV
Cowpea mosaic virus
- MenB
Meningitis B
- ncRNAs
Noncoding RNAs
- TB
Tuberculosis
- SLNs
Solid lipid nanoparticles
- TRZ
Trastuzumab
- RBD
Receptor binding domain
- CryoMNs
Cryomicroneedles
- CTLs
Cytotoxic T lymphocytes
- PS
Polystyrene
- OVA
Ovalbumin
- IM
Intramuscular injection
- ID
Needle-free injection technology-based intradermal injection
- WHO
World Health Organization
- WV
Whole-virion
- TLRs
Toll-like receptors
- PRRs
Pattern-recognition receptors
- cGAS-STING
Cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon genes
- NLRs
NOD-like receptors
- MHC
Major histocompatibility complex
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- FDCs
Follicular dendritic cells
- AIDS
Acquired immunodeficiency syndrome
- ICB
Immune checkpoint blockade
- NvIH
(Nanovaccines + ICBs)-in-hydrogel
- CFP
Culture filtrate protein
- API
Active pharmaceutical ingredients
- CSP
Cyclosporum protein
- HuNov
Human norovirus
- TME
Tumor microenvironment
- HIV
Human immunodeficiency virus
- BCG
Bacillus Calmette-Guérin
- MPL
Monophosphoryl lipid
- PfCSP
Plasmodium falciparum cyclosporin protein
- CHB
Chronic hepatitis B
- cGAMP
Cyclic guanosine monophosphate-AMP
- SPD
Spermidine
- Gn
Gnotobiotic
- DENV
Dengue virus
- INCs
Intravenous injection nanocrystals
- CDNs
Cell-derived nanovesicles
- SCCNV
Senescent cancer cell-derived nanovesicle
- HA
Hyaluronic acid
Author contributions
Y. Z. and H. Z. contributed equally to this work. Y. Z.: Writing–original draft, Software, Methodology, Investigation, Conceptualization, Visualization. H. Z.: Writing–original draft, Validation, Methodology, Investigation. C. S.: Software, Methodology, Investigation, Visualization. Q. L.: Writing–review & editing, Supervision, Conceptualization, Investigation, Project administration. T. C.: Writing–review & editing, Supervision, Formal analysis, Project administration. L. L.: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition.
Funding
The research was supported by Zhejiang Shuren University research project (2023R053).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
We give our consent for the manuscript to be published in Journal of Nanobiotechnology.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanling Zhuo and Huanxuan Zeng have contributed equally to this work.
Contributor Information
Qizhuang Lv, Email: lvqizhuang062@163.com.
Tianyin Cheng, Email: hn5368@163.com.
Lanjie Lei, Email: leilanjie1988@163.com.
References
- 1.Bookstaver ML, Tsai SJ, Bromberg JS, Jewell CM. Improving vaccine and immunotherapy design using biomaterials. Trends Immunol. 2018;39:135–50. 10.1016/j.it.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han X, Alu A, Liu H, Shi Y, Wei X, Cai L, Wei Y. Biomaterial-assisted biotherapy: a brief review of biomaterials used in drug delivery, vaccine development, gene therapy, and stem cell therapy. Bioact Mater. 2022;17:29–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu Y, Gao Y, He L, Fang B, Ge W, Yang P, Ju Y, Xie X, Lei L. Biomaterial-based gene therapy. MedComm. 2020;2023(4): e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei L, Bai Y, Qin X, Liu J, Huang W, Lv Q. Current understanding of hydrogel for drug release and tissue engineering. Gels. 2022;8:301. 10.3390/gels8050301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ju Y, Hu Y, Yang P, Xie X, Fang B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater Today Bio. 2023;18: 100522. 10.1016/j.mtbio.2022.100522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang P, Ju Y, Hu Y, Xie X, Fang B, Lei L. Emerging 3D bioprinting applications in plastic surgery. Biomater Res. 2023;27:1. 10.1186/s40824-022-00338-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su M, Ruan L, Dong X, Tian S, Lang W, Wu M, Chen Y, Lv Q, Lei L. Current state of knowledge on intelligent-response biological and other macromolecular hydrogels in biomedical engineering: a review. Int J Biol Macromol. 2023;227:472–92. 10.1016/j.ijbiomac.2022.12.148 [DOI] [PubMed] [Google Scholar]
- 8.Dykman LA, Khlebtsov NG. Immunological properties of gold nanoparticles. Chem Sci. 2017;8:1719–35. 10.1039/C6SC03631G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Li Y, Jiao J, Hu H-M. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat Nanotechnol. 2011;6:645–50. 10.1038/nnano.2011.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shichinohe S, Watanabe T. Advances in adjuvanted influenza vaccines. Vaccines (Basel). 2023;118:1391. 10.3390/vaccines11081391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu M, Wang R, Nie G. Applications of nanomaterials as vaccine adjuvants. Hum Vacc Immunother. 2014;10:2761–74. 10.4161/hv.29589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong H, Xiang J, Xu L, Song X, Dong Z, Peng R, Liu Z. Stimulation of immune systems by conjugated polymers and their potential as an alternative vaccine adjuvant. Nanoscale. 2015;7:19282–92. 10.1039/C5NR06081H [DOI] [PubMed] [Google Scholar]
- 13.Backlund C, Jalili-Firoozinezhad S, Kim B, Irvine DJ. Biomaterials-mediated engineering of the immune system. Annu Rev Immunol. 2023;41:153–79. 10.1146/annurev-immunol-101721-040259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadat Tabatabaei Mirakabad F, Nejati-Koshki K, Akbarzadeh A, Yamchi MR, Milani M, Zarghami N, Zeighamian V, Rahimzadeh A, Alimohammadi S, Hanifehpour Y, Joo SW. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac J Cancer Prev. 2014;15:517–35. 10.7314/APJCP.2014.15.2.517 [DOI] [PubMed] [Google Scholar]
- 15.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–22. 10.1016/j.jconrel.2012.01.043 [DOI] [PubMed] [Google Scholar]
- 16.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–90. 10.1016/S0142-9612(00)00115-0 [DOI] [PubMed] [Google Scholar]
- 17.Dalpiaz A, Contado C, Mari L, Perrone D, Pavan B, Paganetto G, Hanuskovà M, Vighi E, Leo E. Development and characterization of PLGA nanoparticles as delivery systems of a prodrug of zidovudine obtained by its conjugation with ursodeoxycholic acid. Drug Deliv. 2014;21:221–32. 10.3109/10717544.2013.844744 [DOI] [PubMed] [Google Scholar]
- 18.Prokop A, Davidson JM. Nanovehicular intracellular delivery systems. J Pharm Sci. 2008;97:3518–90. 10.1002/jps.21270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. 10.1016/j.ijpharm.2005.10.010 [DOI] [PubMed] [Google Scholar]
- 20.Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci. 2002;6:319–27. 10.1016/S1359-0286(02)00117-1 [DOI] [Google Scholar]
- 21.Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62:90–9. 10.1016/j.phrs.2010.03.005 [DOI] [PubMed] [Google Scholar]
- 22.Gao L, Li J, Song T. Poly lactic-co-glycolic acid-based nanoparticles as delivery systems for enhanced cancer immunotherapy. Front Chem. 2022;10: 973666. 10.3389/fchem.2022.973666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elmowafy M, Shalaby K, Elkomy MH, Alsaidan OA, Gomaa HAM, Abdelgawad MA, Mostafa EM. Polymeric nanoparticles for delivery of natural bioactive agents: recent advances and challenges. Polymers. 2023;15:1123. 10.3390/polym15051123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuomela K, Levings MK. Acidity promotes the differentiation of immunosuppressive regulatory T cells. Eur J Immunol. 2023;53: e2350511. 10.1002/eji.202350511 [DOI] [PubMed] [Google Scholar]
- 25.Horvath D, Basler M. PLGA particles in immunotherapy. Pharmaceutics. 2023;15:615. 10.3390/pharmaceutics15020615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Sahoo JK, Li Y, Xu Q, Kaplan DL. Challenges in delivering therapeutic peptides and proteins: a silk-based solution. J Control Release. 2022;345:176–89. 10.1016/j.jconrel.2022.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Listner K, Bentley L, Okonkowski J, Kistler C, Wnek R, Caparoni A, Junker B, Robinson D, Salmon P, Chartrain M. Development of a highly productive and scalable plasmid DNA production platform. Biotechnol Prog. 2006;22:1335–45. 10.1021/bp060046h [DOI] [PubMed] [Google Scholar]
- 28.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. 10.1038/nature06175 [DOI] [PubMed] [Google Scholar]
- 29.Jäger M, Schubert S, Ochrimenko S, Fischer D, Schubert US. Branched and linear poly(ethylene imine)-based conjugates: synthetic modification, characterization, and application. Chem Soc Rev. 2012;41:4755–67. 10.1039/c2cs35146c [DOI] [PubMed] [Google Scholar]
- 30.Sheppard NC, Brinckmann SA, Gartlan KH, Puthia M, Svanborg C, Krashias G, Eisenbarth SC, Flavell RA, Sattentau QJ, Wegmann F. Polyethyleneimine is a potent systemic adjuvant for glycoprotein antigens. Int Immunol. 2014;26:531–8. 10.1093/intimm/dxu055 [DOI] [PubMed] [Google Scholar]
- 31.Shen C, Li J, Zhang Y, Li Y, Shen G, Zhu J, Tao J. Polyethylenimine-based micro/nanoparticles as vaccine adjuvants. Int J Nanomed. 2017;12:5443–60. 10.2147/IJN.S137980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Guo Y, Shen M, Wang Y, Shi X. Hyperbranched polymer-based vaccines for cancer immunotherapy. Macromol Biosci. 2023;23:2300188. 10.1002/mabi.202300188 [DOI] [PubMed] [Google Scholar]
- 33.Chen X-J, Huang M-Y, Wangkahart E, Cai J, Huang Y, Jian J-C, Wang B. Immune response and protective efficacy of mannosylated polyethylenimine (PEI) as an antigen delivery vector, administered with a Streptococcus agalactiae DNA vaccine in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2023;135:108684. 10.1016/j.fsi.2023.108684 [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Zhu T, Xu S, Gu P, Cai G, Peng S, Liu Z, Yang Y, Hu Y, Liu J, Wang D. Cationic nanoparticle-stabilized vaccine delivery system for the H9N2 vaccine to promote immune response in chickens. Mol Pharm. 2023;20:1613–23. 10.1021/acs.molpharmaceut.2c00805 [DOI] [PubMed] [Google Scholar]
- 35.Nam J, Son S, Park KS, Moon JJ. Modularly programmable nanoparticle vaccine based on polyethyleneimine for personalized cancer immunotherapy. Adv Sci (Weinh). 2021;8:2002577. 10.1002/advs.202002577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dmour I, Islam N. Recent advances on chitosan as an adjuvant for vaccine delivery. Int J Biol Macromol. 2022;200:498–519. 10.1016/j.ijbiomac.2021.12.129 [DOI] [PubMed] [Google Scholar]
- 37.Islam N, Dmour I, Taha MO. Degradability of chitosan micro/nanoparticles for pulmonary drug delivery. Heliyon. 2019;5: e01684. 10.1016/j.heliyon.2019.e01684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dmour I, Taha MO. Chapter 2—natural and semisynthetic polymers in pharmaceutical nanotechnology. In: Grumezescu AM, editor. Organic materials as smart nanocarriers for drug delivery. William Andrew Publishing; 2018. p. 35–100. [Google Scholar]
- 39.Korbelik M, Banáth J, Čiplys E, Szulc Z, Bielawska A, Chen W. Activity of glycated chitosan and other adjuvants to PDT vaccines. SPIE; 2015.
- 40.Zhao K, Li SS, Li W, Yu L, Duan XT, Han JY, Wang XH, Jin Z. Quaternized chitosan nanoparticles loaded with the combined attenuated live vaccine against Newcastle disease and infectious bronchitis elicit immune response in chicken after intranasal administration. Drug Deliv. 2017;24:1574–86. 10.1080/10717544.2017.1388450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majdedin G, Mojtaba S, Jafar S, Ebrahim A, Ghader H, Hadi Esmaeili Gouvarchin G, Ali A. Biological properties the novel application of N-trimethyl chitosan nanospheres as a stabilizer and preservative in tetanus vaccine. Clin Exp Vaccine Res Clin Exp. 2021;10:24–34. 10.7774/cevr.2021.10.1.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaneko K, Miyaji EN, Gonçalves VM, Ferreira DM, Solórzano C, MacLoughlin R, Saleem I. Evaluation of polymer choice on immunogenicity of chitosan coated PLGA NPs with surface-adsorbed pneumococcal protein antigen PspA4Pro. Int J Pharm. 2021;15: 120407. 10.1016/j.ijpharm.2021.120407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaglio SC, Perduca M, Zipeto D, Bardi G. Efficiency of chitosan nanocarriers in vaccinology for mucosal immunization. Vaccines. 2023;11:1333. 10.3390/vaccines11081333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hao H, Wu S, Lin J, Zheng Z, Zhou Y, Zhang Y, Guo Q, Tian F, Zhao M, Chen Y, et al. Immunization against Zika by entrapping live virus in a subcutaneous self-adjuvanting hydrogel. Nat Biomed Eng. 2023;7:928–42. 10.1038/s41551-023-01014-4 [DOI] [PubMed] [Google Scholar]
- 45.Huo Y, Hu J, Yin Y, Liu P, Cai K, Ji W. Self-assembling peptide-based functional biomaterials. ChemBioChem. 2023;24: e202200582. 10.1002/cbic.202200582 [DOI] [PubMed] [Google Scholar]
- 46.Zhu L, Shi Y, Xiong Y, Ba L, Li Q, Qiu M, Zou Z, Peng G. Emerging self-assembling peptide nanomaterial for anti-cancer therapy. J Biomater Appl. 2021;36:882–901. 10.1177/08853282211027882 [DOI] [PubMed] [Google Scholar]
- 47.Wang B, Liu S, Li H, Dong W, Liu H, Zhang J, Tian C, Dong S. Facile preparation of carbohydrate-containing adjuvants based on self-assembling glycopeptide conjugates. Angew Chem Int Ed. 2024;63:e202309140. 10.1002/anie.202309140 [DOI] [PubMed] [Google Scholar]
- 48.Wu B, Wang H. Peptide assemblies as promising tumor vaccines: current platforms and progress. BIO Integration. 2023;4:45–51. 10.15212/bioi-2023-0005 [DOI] [Google Scholar]
- 49.Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid nanoparticles─from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15:16982–7015. 10.1021/acsnano.1c04996 [DOI] [PubMed] [Google Scholar]
- 50.Rimmelzwaan GF, Osterhaus ADME. A novel generation of viral vaccines based on the ISCOM matrix. In: Powell MF, Newman MJ, editors. Vaccine design: the subunit and adjuvant approach. Boston: Springer, US; 1995. p. 543–58. [DOI] [PubMed] [Google Scholar]
- 51.Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. 10.1038/nature18300 [DOI] [PubMed] [Google Scholar]
- 52.Simões S, Filipe A, Faneca H, Mano M, Penacho N, Düzgünes N, de Lima MP. Cationic liposomes for gene delivery. Expert Opin Drug Deliv. 2005;2:237–54. 10.1517/17425247.2.2.237 [DOI] [PubMed] [Google Scholar]
- 53.Amin M, Seynhaeve ALB, Sharifi M, Falahati M, Ten Hagen TLM. Liposomal drug delivery systems for cancer therapy: the Rotterdam experience. Pharmaceutics. 2022;14:2165. 10.3390/pharmaceutics14102165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nikolova MP, Kumar EM, Chavali MS. Updates on responsive drug delivery based on liposome vehicles for cancer treatment. Pharmaceutics. 2022;14:2195. 10.3390/pharmaceutics14102195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koynova R, Tenchov B. Cationic Lipids: molecular Structure/Transfection Activity Relationships and Interactions with Biomembranes. In: Bielke W, Erbacher C, editors. Nucleic acid transfection. Berlin, Heidelberg: Springer, Berlin Heidelberg; 2010. p. 51–93. [DOI] [PubMed] [Google Scholar]
- 56.Agallou M, Margaroni M, Tsanaktsidou E, Badounas F, Kammona O, Kiparissides C, Karagouni E. A liposomal vaccine promotes strong adaptive immune responses via dendritic cell activation in draining lymph nodes. J Control Release. 2023;356:386–401. 10.1016/j.jconrel.2023.03.006 [DOI] [PubMed] [Google Scholar]
- 57.Firdaus FZ, Bartlett S, Hussein WM, Lu L, Wright Q, Huang W, Nahar UJ, Yang J, Khongkow M, Veitch M, et al. Liposomal formulations of a polyleucine–antigen conjugate as therapeutic vaccines against cervical cancer. Pharmaceutics. 2023;15:602. 10.3390/pharmaceutics15020602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanders MT, Brown LE, Deliyannis G, Pearse MJ. ISCOM-based vaccines: the second decade. Immunol Cell Biol. 2005;83:119–28. 10.1111/j.1440-1711.2005.01319.x [DOI] [PubMed] [Google Scholar]
- 59.Pabreja S, Garg T, Rath G, Goyal AK. Mucosal vaccination against tuberculosis using Ag85A-loaded immunostimulating complexes. Artif Cells Nanomed Biotechnol. 2016;44:532–9. 10.3109/21691401.2014.966195 [DOI] [PubMed] [Google Scholar]
- 60.Pearse MJ, Drane D. ISCOMATRIX adjuvant for antigen delivery. Adv Drug Deliv Rev. 2005;57:465–74. 10.1016/j.addr.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 61.Baz Morelli A, Becher D, Koernig S, Silva A, Drane D, Maraskovsky E. ISCOMATRIX: a novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J Med Microbiol. 2012;61:935–43. 10.1099/jmm.0.040857-0 [DOI] [PubMed] [Google Scholar]
- 62.Chen Y-S, Hung Y-C, Lin W-H, Huang GS. Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology. 2010;21: 195101. 10.1088/0957-4484/21/19/195101 [DOI] [PubMed] [Google Scholar]
- 63.Wang T, Zou M, Jiang H, Ji Z, Gao P, Cheng G. Synthesis of a novel kind of carbon nanoparticle with large mesopores and macropores and its application as an oral vaccine adjuvant. Eur J Pharm Sci. 2011;44:653–9. 10.1016/j.ejps.2011.10.012 [DOI] [PubMed] [Google Scholar]
- 64.Zhou X, Zhang X, Yu X, Zha X, Fu Q, Liu B, Wang X, Chen Y, Chen Y, Shan Y. The effect of conjugation to gold nanoparticles on the ability of low molecular weight chitosan to transfer DNA vaccine. Biomaterials. 2008;29:111–7. 10.1016/j.biomaterials.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 65.Chue-Gonçalves M, Pereira GN, Faccin-Galhardi LC, Kobayashi RKT, Nakazato G. Metal Nanoparticles against viruses: possibilities to fight SARS-CoV-2. Nanomaterials (Basel). 2021;11:3118. 10.3390/nano11113118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bansal SA, Kumar V, Karimi J, Singh AP, Kumar S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020;2:3764–87. 10.1039/D0NA00472C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanna SJ, Thayer TC, Robinson EJS, Vinh NN, Williams N, Landry LG, Andrews R, Siah QZ, Leete P, Wyatt R, et al. Single-cell RNAseq identifies clonally expanded antigen-specific T-cells following intradermal injection of gold nanoparticles loaded with diabetes autoantigen in humans. Front Immunol. 2023;14:1276255. 10.3389/fimmu.2023.1276255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sengupta A, Azharuddin M, Al-Otaibi N, Hinkula J. Efficacy and immune response elicited by gold nanoparticle- based nanovaccines against infectious diseases. Vaccines (Basel). 2022;10:505. 10.3390/vaccines10040505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdalhamed AM, Naser SM, Mohamed AH, Zeedan GSG. Development of gold nanoparticles-lateral flow test as a novel field diagnostic assay for detecting foot-and-mouth disease and lumpy skin disease viruses. Iran J Microbiol. 2022;14:574–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yen HJ, Hsu SH, Tsai CL. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small. 2009;5:1553–61. 10.1002/smll.200900126 [DOI] [PubMed] [Google Scholar]
- 71.Alavi M, Kamarasu P, McClements DJ, Moore MD. Metal and metal oxide-based antiviral nanoparticles: properties, mechanisms of action, and applications. Adv Colloid Interface Sci. 2022;306: 102726. 10.1016/j.cis.2022.102726 [DOI] [PubMed] [Google Scholar]
- 72.Sarkar J, Das S, Aich S, Bhattacharyya P, Acharya K. Antiviral potential of nanoparticles for the treatment of Coronavirus infections. J Trace Elem Med Biol. 2022;72: 126977. 10.1016/j.jtemb.2022.126977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Faria PC, dos Santos LI, Coelho JP, Ribeiro HB, Pimenta MA, Ladeira LO, Gomes DA, Furtado CA, Gazzinelli RT. Oxidized multiwalled carbon nanotubes as antigen delivery system to promote superior CD8(+) T cell response and protection against cancer. Nano Lett. 2014;14:5458–70. 10.1021/nl502911a [DOI] [PubMed] [Google Scholar]
- 74.Huang Y, Li P, Zhao R, Zhao L, Liu J, Peng S, Fu X, Wang X, Luo R, Wang R, Zhang Z. Silica nanoparticles: biomedical applications and toxicity. Biomed Pharmacother. 2022;151: 113053. 10.1016/j.biopha.2022.113053 [DOI] [PubMed] [Google Scholar]
- 75.Nafisi S, Schäfer-Korting M, Maibach HI. Perspectives on percutaneous penetration: silica nanoparticles. Nanotoxicology. 2015;9:643–57. 10.3109/17435390.2014.958115 [DOI] [PubMed] [Google Scholar]
- 76.Carvalho LV, Ruiz Rde C, Scaramuzzi K, Marengo EB, Matos JR, Tambourgi DV, Fantini MC, Sant’Anna OA. Immunological parameters related to the adjuvant effect of the ordered mesoporous silica SBA-15. Vaccine. 2010;28:7829–36. 10.1016/j.vaccine.2010.09.087 [DOI] [PubMed] [Google Scholar]
- 77.Wang T, Jiang H, Zhao Q, Wang S, Zou M, Cheng G. Enhanced mucosal and systemic immune responses obtained by porous silica nanoparticles used as an oral vaccine adjuvant: effect of silica architecture on immunological properties. Int J Pharm. 2012;436:351–8. 10.1016/j.ijpharm.2012.06.028 [DOI] [PubMed] [Google Scholar]
- 78.Mahony D, Cavallaro AS, Stahr F, Mahony TJ, Qiao SZ, Mitter N. Mesoporous silica nanoparticles act as a self-adjuvant for ovalbumin model antigen in mice. Small. 2013;9:3138–46. 10.1002/smll.201300012 [DOI] [PubMed] [Google Scholar]
- 79.Li AW, Sobral MC, Badrinath S, Choi Y, Graveline A, Stafford AG, Weaver JC, Dellacherie MO, Shih TY, Ali OA, et al. A facile approach to enhance antigen response for personalized cancer vaccination. Nat Mater. 2018;17:528–34. 10.1038/s41563-018-0028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Y-C, Smith T, Hicks RH, Doekhie A, Koumanov F, Wells SA, Edler KJ, van den Elsen J, Holman GD, Marchbank KJ, Sartbaeva A. Thermal stability, storage and release of proteins with tailored fit in silica. Sci Rep. 2017;7:46568. 10.1038/srep46568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo J, De May H, Franco S, Noureddine A, Tang L, Brinker CJ, Kusewitt DF, Adams SF, Serda RE. Cancer vaccines from cryogenically silicified tumour cells functionalized with pathogen-associated molecular patterns. Nat Biomed Eng. 2022;6:19–31. 10.1038/s41551-021-00795-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang N, Mei K, Guan P, Hu X, Zhao Y. Protein-based artificial nanosystems in cancer therapy. Small. 2020;16: e1907256. 10.1002/smll.201907256 [DOI] [PubMed] [Google Scholar]
- 83.Jennings GT, Bachmann MF. The coming of age of virus-like particle vaccines. Biol Chem. 2008;389:521–36. 10.1515/BC.2008.064 [DOI] [PubMed] [Google Scholar]
- 84.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–96. 10.1038/nri2868 [DOI] [PubMed] [Google Scholar]
- 85.Mohsen MO, Zha L, Cabral-Miranda G, Bachmann MF. Major findings and recent advances in virus–like particle (VLP)-based vaccines. In Semin Immunopathol. Elsevier; 2017: 123–132. [DOI] [PubMed]
- 86.Adepoju P. RTS, S malaria vaccine pilots in three African countries. The Lancet. 2019;393:1685. 10.1016/S0140-6736(19)30937-7 [DOI] [PubMed] [Google Scholar]
- 87.Shukla S, Myers JT, Woods SE, Gong X, Czapar AE, Commandeur U, Huang AY, Levine AD, Steinmetz NF. Plant viral nanoparticles-based HER2 vaccine: Immune response influenced by differential transport, localization and cellular interactions of particulate carriers. Biomaterials. 2017;121:15–27. 10.1016/j.biomaterials.2016.12.030 [DOI] [PubMed] [Google Scholar]
- 88.Coates EE, Edupuganti S, Chen GL, Happe M, Strom L, Widge A, Florez MB, Cox JH, Gordon I, Plummer S. Safety and immunogenicity of a trivalent virus-like particle vaccine against western, eastern, and Venezuelan equine encephalitis viruses: a phase 1, open-label, dose-escalation, randomised clinical trial. Lancet Infect Dis. 2022;22:1210–20. 10.1016/S1473-3099(22)00052-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15:375–87. 10.1038/nri3837 [DOI] [PubMed] [Google Scholar]
- 90.Arigita C, Jiskoot W, Westdijk J, van Ingen C, Hennink WE, Crommelin DJ, Kersten GF. Stability of mono- and trivalent meningococcal outer membrane vesicle vaccines. Vaccine. 2004;22:629–42. 10.1016/j.vaccine.2003.08.027 [DOI] [PubMed] [Google Scholar]
- 91.Baker JL, Chen L, Rosenthal JA, Putnam D, DeLisa MP. Microbial biosynthesis of designer outer membrane vesicles. Curr Opin Biotechnol. 2014;29:76–84. 10.1016/j.copbio.2014.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74:81–94. 10.1128/MMBR.00031-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hong J, Jung M, Kim C, Kang M, Go S, Sohn H, Moon S, Kwon S, Song SY, Kim BS. Senescent cancer cell-derived nanovesicle as a personalized therapeutic cancer vaccine. Exp Mol Med. 2023;55:541–54. 10.1038/s12276-023-00951-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Xia Q, Wu T, He Z, Li Y, Li Z, Hou X, He Y, Ruan S, Wang Z, et al. A novel multi-functionalized multicellular nanodelivery system for non-small cell lung cancer photochemotherapy. J Nanobiotechnology. 2021;19:245. 10.1186/s12951-021-00977-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O’Ryan M, Stoddard J, Toneatto D, Wassil J, Dull PM. A multi-component meningococcal serogroup B vaccine (4CMenB): the clinical development program. Drugs. 2014;74:15–30. 10.1007/s40265-013-0155-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, Nilsson J, Lötvall J, Kim YK, Gho YS. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698–710. 10.1021/nn402232g [DOI] [PubMed] [Google Scholar]
- 97.Liang S, Ma J, Gong H, Shao J, Li J, Zhan Y, Wang Z, Wang C, Li W. Immune regulation and emerging roles of noncoding RNAs in Mycobacterium tuberculosis infection. Front Immunol. 2022;13: 987018. 10.3389/fimmu.2022.987018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang XH, Guo T, Gao XY, Wu XL, Xing XF, Ji JF, Li ZY. Exosome-derived noncoding RNAs in gastric cancer: functions and clinical applications. Mol Cancer. 2021;20:99. 10.1186/s12943-021-01396-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang J, Li Y, Wang N, Wu J, Ye X, Jiang Y, Tang L. Functions of exosomal non-coding RNAs to the infection with Mycobacterium tuberculosis. Front Immunol. 2023;14:1127214. 10.3389/fimmu.2023.1127214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang R, Jia B, Su D, Li M, Xu Z, He C, Huang Y, Fan H, Chen H, Cheng F. Plant exosomes fused with engineered mesenchymal stem cell-derived nanovesicles for synergistic therapy of autoimmune skin disorders. J Extracell Vesicles. 2023;12: e12361. 10.1002/jev2.12361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jo W, Kim J, Yoon J, Jeong D, Cho S, Jeong H, Yoon YJ, Kim SC, Gho YS, Park J. Large-scale generation of cell-derived nanovesicles. Nanoscale. 2014;6:12056–64. 10.1039/C4NR02391A [DOI] [PubMed] [Google Scholar]
- 102.Cha H, Hong S, Park JH, Park HH. Stem cell-derived exosomes and nanovesicles: promotion of cell proliferation, migration, and anti-senescence for treatment of wound damage and skin ageing. Pharmaceutics. 2020;12:1135. 10.3390/pharmaceutics12121135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ou YH, Zou S, Goh WJ, Wang JW, Wacker M, Czarny B, Pastorin G. Cell-derived nanovesicles as exosome-mimetics for drug delivery purposes: uses and recommendations. Methods Mol Biol. 2021;2211:147–70. 10.1007/978-1-0716-0943-9_11 [DOI] [PubMed] [Google Scholar]
- 104.Kelly HG, Kent SJ, Wheatley AK. Immunological basis for enhanced immunity of nanoparticle vaccines. Expert Rev Vaccines. 2019;18:269–80. 10.1080/14760584.2019.1578216 [DOI] [PubMed] [Google Scholar]
- 105.Cunha A, Gaubert A, Latxague L, Dehay B. PLGA-based nanoparticles for neuroprotective drug delivery in neurodegenerative diseases. Pharmaceutics. 2021;13:1042. 10.3390/pharmaceutics13071042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qin X, Yu C, Wei J, Li L, Zhang C, Wu Q, Liu J, Yao SQ, Huang W. Rational design of nanocarriers for intracellular protein delivery. Adv Mater. 2019;31: e1902791. 10.1002/adma.201902791 [DOI] [PubMed] [Google Scholar]
- 107.Chen S, Hao X, Liang X, Zhang Q, Zhang C, Zhou G, Shen S, Jia G, Zhang J. Inorganic nanomaterials as carriers for drug delivery. J Biomed Nanotechnol. 2016;12:1–27. 10.1166/jbn.2016.2122 [DOI] [PubMed] [Google Scholar]
- 108.Mody KT, Popat A, Mahony D, Cavallaro AS, Yu C, Mitter N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale. 2013;5:5167–79. 10.1039/c3nr00357d [DOI] [PubMed] [Google Scholar]
- 109.Nguyen B, Tolia NH. Protein-based antigen presentation platforms for nanoparticle vaccines. NPJ Vaccines. 2021;6:70. 10.1038/s41541-021-00330-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao X, Zhao R, Nie G. Nanocarriers based on bacterial membrane materials for cancer vaccine delivery. Nat Protoc. 2022;17:2240–74. 10.1038/s41596-022-00713-7 [DOI] [PubMed] [Google Scholar]
- 111.van der Ley PA, Zariri A, van Riet E, Oosterhoff D, Kruiswijk CP. An intranasal OMV-based vaccine induces high mucosal and systemic protecting immunity against a SARS-CoV-2 infection. Front Immunol. 2021;12: 781280. 10.3389/fimmu.2021.781280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li S, Li X, Meng J, Bao W, Wang S, Ye P, Gao X-D, Wei W. Dynamic shielding of bacterial outer membrane vesicles for safe and efficient chemo-immunotherapy against tumors. Nano Res. 2024;17:836–47. 10.1007/s12274-023-6225-6 [DOI] [Google Scholar]
- 113.Kearney CJ, Mooney DJ. Macroscale delivery systems for molecular and cellular payloads. Nat Mater. 2013;12:1004–17. 10.1038/nmat3758 [DOI] [PubMed] [Google Scholar]
- 114.Goldberg MS. Immunoengineering: how nanotechnology can enhance cancer immunotherapy. Cell. 2015;161:201–4. 10.1016/j.cell.2015.03.037 [DOI] [PubMed] [Google Scholar]
- 115.Dristant U, Mukherjee K, Saha S, Maity D. An overview of polymeric nanoparticles-based drug delivery system in cancer treatment. Technol Cancer Res Treat. 2023;22:15330338231152084. 10.1177/15330338231152083 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 116.Kim S, Shi Y, Kim JY, Park K, Cheng JX. Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin Drug Deliv. 2010;7:49–62. 10.1517/17425240903380446 [DOI] [PubMed] [Google Scholar]
- 117.Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A, Anderson DG, Langer R, Blankschtein D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17:1326–35. 10.1021/acs.nanolett.6b03329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sivadasan D, Ramakrishnan K, Mahendran J, Ranganathan H, Karuppaiah A, Rahman H. Solid lipid nanoparticles: applications and prospects in cancer treatment. Int J Mol Sci. 2023;24:6199. 10.3390/ijms24076199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wei W, Zhang Q, Zhou W, Liu Z, Wang Y, Alakpa EV, Ouyang H, Liu H. Immunomodulatory application of engineered hydrogels in regenerative medicine. Appl Mater Today. 2019;14:126–36. 10.1016/j.apmt.2018.11.013 [DOI] [Google Scholar]
- 120.Wang Y, Malcolm DW, Benoit DSW. Controlled and sustained delivery of siRNA/NPs from hydrogels expedites bone fracture healing. Biomaterials. 2017;139:127–38. 10.1016/j.biomaterials.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McMillan A, Nguyen MK, Huynh CT, Sarett SM, Ge P, Chetverikova M, Nguyen K, Grosh D, Duvall CL, Alsberg E. Hydrogel microspheres for spatiotemporally controlled delivery of RNA and silencing gene expression within scaffold-free tissue engineered constructs. Acta Biomater. 2021;124:315–26. 10.1016/j.actbio.2021.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vora LK, Moffatt K, Tekko IA, Paredes AJ, Volpe-Zanutto F, Mishra D, Peng K, Raj Singh Thakur R, Donnelly RF. Microneedle array systems for long-acting drug delivery. Eur J Pharm Biopharm. 2021;159:44–76. 10.1016/j.ejpb.2020.12.006 [DOI] [PubMed] [Google Scholar]
- 123.Waghule T, Singhvi G, Dubey SK, Pandey MM, Gupta G, Singh M, Dua K. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–58. 10.1016/j.biopha.2018.10.078 [DOI] [PubMed] [Google Scholar]
- 124.Hou C, Yi B, Jiang J, Chang Y-F, Yao X. Up-to-date vaccine delivery systems: robust immunity elicited by multifarious nanomaterials upon administration through diverse routes. Biomater Sci. 2019;7:822–35. 10.1039/C8BM01197D [DOI] [PubMed] [Google Scholar]
- 125.Pati R, Shevtsov M, Sonawane A. Nanoparticle vaccines against infectious diseases. Front Immunol. 2018;9:2224. 10.3389/fimmu.2018.02224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wibowo D, Jorritsma SHT, Gonzaga ZJ, Evert B, Chen S, Rehm BHA. Polymeric nanoparticle vaccines to combat emerging and pandemic threats. Biomaterials. 2021;268: 120597. 10.1016/j.biomaterials.2020.120597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Y, Chen J, Shi L, Ma F. Polymeric nanoparticle-based nanovaccines for cancer immunotherapy. Mater Horiz. 2023;10:361–92. 10.1039/D2MH01358D [DOI] [PubMed] [Google Scholar]
- 128.Cheng CJ, Tietjen GT, Saucier-Sawyer JK, Saltzman WM. A holistic approach to targeting disease with polymeric nanoparticles. Nat Rev Drug Discov. 2015;14:239–47. 10.1038/nrd4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Comberlato A, Paloja K, Bastings MM. Nucleic acids presenting polymer nanomaterials as vaccine adjuvants. J Mater Chem B. 2019;7:6321–46. 10.1039/C9TB01222B [DOI] [PubMed] [Google Scholar]
- 130.Ben-Akiva E, Witte SE, Meyer RA, Rhodes KR, Green JJ. Polymeric micro-and nanoparticles for immune modulation. Biomater Sci. 2019;7:14–30. 10.1039/C8BM01285G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moran HB, Turley JL, Andersson M, Lavelle EC. Immunomodulatory properties of chitosan polymers. Biomaterials. 2018;184:1–9. 10.1016/j.biomaterials.2018.08.054 [DOI] [PubMed] [Google Scholar]
- 132.Gonzalez-Miro M, Chen S, Gonzaga ZJ, Evert B, Wibowo D, Rehm BH. Polyester as antigen carrier toward particulate vaccines. Biomacromol. 2019;20:3213–32. 10.1021/acs.biomac.9b00509 [DOI] [PubMed] [Google Scholar]
- 133.Alameh M-G, Tombácz I, Bettini E, Lederer K, Sittplangkoon C, Wilmore JR, Gaudette BT, Soliman OY, Pine M, Hicks P. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2022;55:1136–8. 10.1016/j.immuni.2022.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. NEJM. 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. NEJM. 2021;384:403–16. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rajpoot K. Solid lipid nanoparticles: a promising nanomaterial in drug delivery. Curr Pharm Des. 2019;25:3943–59. 10.2174/1381612825666190903155321 [DOI] [PubMed] [Google Scholar]
- 137.Mo K, Kim A, Choe S, Shin M, Yoon H. Overview of solid lipid nanoparticles in breast cancer therapy. Pharmaceutics. 2023;15:2065. 10.3390/pharmaceutics15082065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aly S, El-Kamel AH, Sheta E, El-Habashy SE. Chondroitin/Lactoferrin-dual functionalized pterostilbene-solid lipid nanoparticles as targeted breast cancer therapy. Int J Pharm. 2023;642: 123163. 10.1016/j.ijpharm.2023.123163 [DOI] [PubMed] [Google Scholar]
- 139.Ozgenc E, Karpuz M, Arzuk E, Gonzalez-Alvarez M, Sanz MB, Gundogdu E, Gonzalez-Alvarez I. Radiolabeled trastuzumab solid lipid nanoparticles for breast cancer cell: in vitro and in vivo studies. ACS Omega. 2022;7:30015–27. 10.1021/acsomega.2c03023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bernhard S, Tibbitt MW. Supramolecular engineering of hydrogels for drug delivery. Adv Drug Deliv Rev. 2021;171:240–56. 10.1016/j.addr.2021.02.002 [DOI] [PubMed] [Google Scholar]
- 141.Correa S, Grosskopf AK, Lopez Hernandez H, Chan D, Yu AC, Stapleton LM, Appel EA. Translational applications of hydrogels. Chem Rev. 2021;121:11385–457. 10.1021/acs.chemrev.0c01177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Narayanan KB, Bhaskar R, Han SS. Recent advances in the biomedical applications of functionalized nanogels. Pharmaceutics. 2022;14:2832. 10.3390/pharmaceutics14122832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mei X, Li J, Wang Z, Zhu D, Huang K, Hu S, Popowski KD, Cheng K. An inhaled bioadhesive hydrogel to shield non-human primates from SARS-CoV-2 infection. Nat Mater. 2023;22:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nkanga CI, Ortega-Rivera OA, Shin MD, Moreno-Gonzalez MA, Steinmetz NF. Injectable slow-release hydrogel formulation of a plant virus-based COVID-19 vaccine candidate. Biomacromol. 2022;23:1812–25. 10.1021/acs.biomac.2c00112 [DOI] [PubMed] [Google Scholar]
- 145.Chen J, Wang B, Caserto JS, Shariati K, Cao P, Pan Y, Xu Q, Ma M. Sustained delivery of SARS-CoV-2 RBD subunit vaccine using a high affinity injectable hydrogel scaffold. Adv Healthc Mater. 2022;11:2101714. 10.1002/adhm.202101714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Marco-Dufort B, Janczy JR, Hu T, Lütolf M, Gatti F, Wolf M, Woods A, Tetter S, Sridhar BV, Tibbitt MW. Thermal stabilization of diverse biologics using reversible hydrogels. Sci Adv. 2022;8:eabo0502. 10.1126/sciadv.abo0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pacifici N, Bolandparvaz A, Lewis JS. Stimuli-responsive biomaterials for vaccines and immunotherapeutic applications. Adv Ther. 2020;3:2000129. 10.1002/adtp.202000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Donnelly R, Douroumis D. Microneedles for drug and vaccine delivery and patient monitoring. Drug Deliv Transl Res. 2015;5:311–2. 10.1007/s13346-015-0250-2 [DOI] [PubMed] [Google Scholar]
- 149.Hou X, Li J, Hong Y, Ruan H, Long M, Feng N, Zhang Y. Advances and prospects for hydrogel-forming microneedles in transdermal drug delivery. Biomedicines. 2023;11:2119. 10.3390/biomedicines11082119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mitragotri S. Bioengineering & translational medicine: year 2020 in review. Bioeng Transl Med. 2020;5: e10178. 10.1002/btm2.10178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chang H, Chew SWT, Zheng M, Lio DCS, Wiraja C, Mei Y, Ning X, Cui M, Than A, Shi P, et al. Cryomicroneedles for transdermal cell delivery. Nat Biomed Eng. 2021;5:1008–18. 10.1038/s41551-021-00720-1 [DOI] [PubMed] [Google Scholar]
- 152.Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017;114:206–21. 10.1016/j.addr.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Meyer RA, Sunshine JC, Perica K, Kosmides AK, Aje K, Schneck JP, Green JJ. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small. 2015;11:1519–25. 10.1002/smll.201402369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wibroe PP, Anselmo AC, Nilsson PH, Sarode A, Gupta V, Urbanics R, Szebeni J, Hunter AC, Mitragotri S, Mollnes TE, Moghimi SM. Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes. Nat Nanotechnol. 2017;12:589–94. 10.1038/nnano.2017.47 [DOI] [PubMed] [Google Scholar]
- 155.Ben-Akiva E, Hickey JW, Meyer RA, Isser A, Shannon SR, Livingston NK, Rhodes KR, Kosmides AK, Warren TR, Tzeng SY, et al. Shape matters: biodegradable anisotropic nanoparticle artificial antigen presenting cells for cancer immunotherapy. Acta Biomater. 2023;160:187–97. 10.1016/j.actbio.2023.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li Z, Sun L, Zhang Y, Dove AP, O’Reilly RK, Chen G. Shape effect of Glyco-nanoparticles on macrophage cellular uptake and immune response. ACS Macro Lett. 2016;5:1059–64. 10.1021/acsmacrolett.6b00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cooley M, Sarode A, Hoore M, Fedosov DA, Mitragotri S, Sen GA. Influence of particle size and shape on their margination and wall-adhesion: implications in drug delivery vehicle design across nano-to-micro scale. Nanoscale. 2018;10:15350–64. 10.1039/C8NR04042G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20:101–24. 10.1038/s41573-020-0090-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Andorko JI, Hess KL, Jewell CM. Harnessing biomaterials to engineer the lymph node microenvironment for immunity or tolerance. Aaps J. 2015;17:323–38. 10.1208/s12248-014-9708-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12:978–90. 10.1038/nmat3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hardy CL, Lemasurier JS, Mohamud R, Yao J, Xiang SD, Rolland JM, O’Hehir RE, Plebanski M. Differential uptake of nanoparticles and microparticles by pulmonary APC subsets induces discrete immunological imprints. J Immunol. 2013;191:5278–90. 10.4049/jimmunol.1203131 [DOI] [PubMed] [Google Scholar]
- 162.Kumar S, Anselmo AC, Banerjee A, Zakrewsky M, Mitragotri S. Shape and size-dependent immune response to antigen-carrying nanoparticles. J Control Release. 2015;220:141–8. 10.1016/j.jconrel.2015.09.069 [DOI] [PubMed] [Google Scholar]
- 163.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20. 10.1016/S0168-3659(00)00339-4 [DOI] [PubMed] [Google Scholar]
- 164.Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298:315–22. 10.1016/j.ijpharm.2005.03.035 [DOI] [PubMed] [Google Scholar]
- 165.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–51. 10.1038/nbt.3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gu P, Wusiman A, Zhang Y, Liu Z, Bo R, Hu Y, Liu J, Wang D. Rational design of PLGA nanoparticle vaccine delivery systems to improve immune responses. Mol Pharm. 2019;16:5000–12. 10.1021/acs.molpharmaceut.9b00860 [DOI] [PubMed] [Google Scholar]
- 167.Roldán-Chicano MT, Rodríguez-Tello J, Cebrián-López R, Moore JR, Del Mar García-López M. Adverse effects of dorsogluteal intramuscular injection versus ventrogluteal intramuscular injection: a systematic review and meta-analysis. Nurs Open. 2023;10:5975–88. 10.1002/nop2.1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McCartan AJS, Curran DW, Mrsny RJ. Evaluating parameters affecting drug fate at the intramuscular injection site. J Control Release. 2021;336:322–35. 10.1016/j.jconrel.2021.06.023 [DOI] [PubMed] [Google Scholar]
- 169.Zhao H, Li P, Bian L, Zhang W, Jiang C, Chen Y, Kong W, Zhang Y. Immune response of inactivated rabies vaccine inoculated via intraperitoneal, intramuscular, subcutaneous and needle-free injection technology-based intradermal routes in mice. Int J Mol Sci. 2023;24:13587. 10.3390/ijms241713587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xia Q, Shen J, Ding H, Liu S, Li F, Li F, Feng N. Intravenous nanocrystals: fabrication, solidification, in vivo fate, and applications for cancer therapy. Expert Opin Drug Deliv. 2023;20:1467–88. 10.1080/17425247.2023.2268512 [DOI] [PubMed] [Google Scholar]
- 171.Cherian V, Joice S, Stephenson B. Mitigating painful vaccination. Indian J Pediatr. 2019;86:410–1. 10.1007/s12098-019-02862-2 [DOI] [PubMed] [Google Scholar]
- 172.Zheng Y, Ling Z, Li Z, Zhao P, Wen X, Qu F, Yu H, Chang H. A rapidly dissolvable microneedle patch with tip-accumulated antigens for efficient transdermal vaccination. Macromol Biosci. 2023;23: e2300253. 10.1002/mabi.202300253 [DOI] [PubMed] [Google Scholar]
- 173.Ning X, Seeni RZ, Xu C. Iontophoresis enhanced transdermal drug delivery. In Imaging Technologies and Transdermal Delivery in Skin Disorders. 2019: 291–307.
- 174.Wang M, Yan G, Xiao Q, Zhou N, Chen H-R, Xia W, Peng L. Iontophoresis-driven microneedle arrays delivering transgenic outer membrane vesicles in program that stimulates transcutaneous vaccination for cancer immunotherapy. Small Science. 2023;3:2300126. 10.1002/smsc.202300126 [DOI] [Google Scholar]
- 175.Brekke K, Lind A, Holm-Hansen C, Haugen IL, Sørensen B, Sommerfelt M, Kvale D. Intranasal administration of a therapeutic HIV vaccine (Vacc-4x) induces dose-dependent systemic and mucosal immune responses in a randomized controlled trial. PLoS ONE. 2014;9: e112556. 10.1371/journal.pone.0112556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Riese P, Sakthivel P, Trittel S, Guzmán CA. Intranasal formulations: promising strategy to deliver vaccines. Expert Opin Drug Deliv. 2014;11:1619–34. 10.1517/17425247.2014.931936 [DOI] [PubMed] [Google Scholar]
- 177.Su K, Wang Y, Yuan C, Zhang Y, Li Y, Li T, Song Q. Intranasally inoculated bacterium-like particles displaying porcine epidemic diarrhea virus S1 protein induced intestinal mucosal immune response in mice. Front Immunol. 2023;14:1269409. 10.3389/fimmu.2023.1269409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tokunoh N, Tamiya S, Watanabe M, Okamoto T, Anindita J, Tanaka H, Ono C, Hirai T, Akita H, Matsuura Y, Yoshioka Y. A nasal vaccine with inactivated whole-virion elicits protective mucosal immunity against SARS-CoV-2 in mice. Front Immunol. 2023;14:1224634. 10.3389/fimmu.2023.1224634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ruan S, Li J, Ruan H, Xia Q, Hou X, Wang Z, Guo T, Zhu C, Feng N, Zhang Y. Microneedle-mediated nose-to-brain drug delivery for improved Alzheimer’s disease treatment. J Control Release. 2024;366:712–31. 10.1016/j.jconrel.2024.01.013 [DOI] [PubMed] [Google Scholar]
- 180.Baskin J, Jeon JE, Lewis SJG. Nanoparticles for drug delivery in Parkinson’s disease. J Neurol. 2021;268:1981–94. 10.1007/s00415-020-10291-x [DOI] [PubMed] [Google Scholar]
- 181.Tabynov K, Solomadin M, Turebekov N, Babayeva M, Fomin G, Yadagiri G, Renu S, Yerubayev T, Petrovsky N, Renukaradhya GJ, Tabynov K. An intranasal vaccine comprising SARS-CoV-2 spike receptor-binding domain protein entrapped in mannose-conjugated chitosan nanoparticle provides protection in hamsters. Sci Rep. 2023;13:12115. 10.1038/s41598-023-39402-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dietrich G, Griot-Wenk M, Metcalfe IC, Lang AB, Viret JF. Experience with registered mucosal vaccines. Vaccine. 2003;21:678–83. 10.1016/S0264-410X(02)00579-0 [DOI] [PubMed] [Google Scholar]
- 183.Marasini N, Skwarczynski M, Toth I. Oral delivery of nanoparticle-based vaccines. Expert Rev Vaccines. 2014;13:1361–76. 10.1586/14760584.2014.936852 [DOI] [PubMed] [Google Scholar]
- 184.Pavot V, Rochereau N, Genin C, Verrier B, Paul S. New insights in mucosal vaccine development. Vaccine. 2012;30:142–54. 10.1016/j.vaccine.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 185.Kamble NM, Hajam IA, Lee JH. Orally administered live attenuated Salmonella Typhimurium protects mice against lethal infection with H1N1 influenza virus. Vet Microbiol. 2017;201:1–6. 10.1016/j.vetmic.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 186.Azegami T, Itoh H, Kiyono H, Yuki Y. Novel transgenic rice-based vaccines. Arch Immunol Ther Exp (Warsz). 2015;63:87–99. 10.1007/s00005-014-0303-0 [DOI] [PubMed] [Google Scholar]
- 187.Fukuda K, Ishida W, Harada Y, Wakasa Y, Takagi H, Takaiwa F, Fukushima A. Efficacy of oral immunotherapy with a rice-based edible vaccine containing hypoallergenic Japanese cedar pollen allergens for treatment of established allergic conjunctivitis in mice. Allergol Int. 2018;67:119–23. 10.1016/j.alit.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 188.Ogawa T, Kashima K, Yuki Y, Mejima M, Kurokawa S, Kuroda M, Okazawa A, Kiyono H, Ohta D. Seed metabolome analysis of a transgenic rice line expressing cholera toxin B-subunit. Sci Rep. 2017;7:5196. 10.1038/s41598-017-04701-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Takeyama N, Yuki Y, Tokuhara D, Oroku K, Mejima M, Kurokawa S, Kuroda M, Kodama T, Nagai S, Ueda S, Kiyono H. Oral rice-based vaccine induces passive and active immunity against enterotoxigenic E. coli-mediated diarrhea in pigs. Vaccine. 2015;33:5204–11. 10.1016/j.vaccine.2015.07.074 [DOI] [PubMed] [Google Scholar]
- 190.Oliveira CR, Rezende CM, Silva MR, Borges OM, Pêgo AP, Goes AM. Oral vaccination based on DNA-chitosan nanoparticles against Schistosoma mansoni infection. ScientificWorldJournal. 2012;2012: 938457. 10.1100/2012/938457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yagnik B, Sharma D, Padh H, Desai P. Immunization with r-Lactococcus lactis expressing outer membrane protein A of Shigella dysenteriae type-1: evaluation of oral and intranasal route of administration. J Appl Microbiol. 2017;122:493–505. 10.1111/jam.13353 [DOI] [PubMed] [Google Scholar]
- 192.Gammon JM, Jewell CM. Engineering immune tolerance with biomaterials. Adv Healthc Mater. 2019;8: e1801419. 10.1002/adhm.201801419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. 10.1016/j.immuni.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jones KS. Biomaterials as vaccine adjuvants. Biotechnol Prog. 2008;24:807–14. 10.1002/btpr.10 [DOI] [PubMed] [Google Scholar]
- 195.Xia Y, Fu S, Ma Q, Liu Y, Zhang N. Application of nano-delivery systems in lymph nodes for tumor immunotherapy. Nanomicro Lett. 2023;15:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mandal B, Kempf M, Merkle HP, Walter E. Immobilisation of GM-CSF onto particulate vaccine carrier systems. Int J Pharm. 2004;269:259–65. 10.1016/j.ijpharm.2003.09.023 [DOI] [PubMed] [Google Scholar]
- 197.Palmer K, Hitt M, Emtage PC, Gyorffy S, Gauldie J. Combined CXC chemokine and interleukin-12 gene transfer enhances antitumor immunity. Gene Ther. 2001;8:282–90. 10.1038/sj.gt.3301386 [DOI] [PubMed] [Google Scholar]
- 198.van Broekhoven CL, Parish CR, Demangel C, Britton WJ, Altin JG. Targeting dendritic cells with antigen-containing liposomes: a highly effective procedure for induction of antitumor immunity and for tumor immunotherapy. Cancer Res. 2004;64:4357–65. 10.1158/0008-5472.CAN-04-0138 [DOI] [PubMed] [Google Scholar]
- 199.Altin JG, van Broekhoven CL, Parish CR. Targeting dendritic cells with antigen-containing liposomes: antitumour immunity. Expert Opin Biol Ther. 2004;4:1735–47. 10.1517/14712598.4.11.1735 [DOI] [PubMed] [Google Scholar]
- 200.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–90. 10.1038/nri2038 [DOI] [PubMed] [Google Scholar]
- 201.Blander JM. Coupling Toll-like receptor signaling with phagocytosis: potentiation of antigen presentation. Trends Immunol. 2007;28:19–25. 10.1016/j.it.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 202.Ehlers M, Ravetch JV. Opposing effects of Toll-like receptor stimulation induce autoimmunity or tolerance. Trends Immunol. 2007;28:74–9. 10.1016/j.it.2006.12.006 [DOI] [PubMed] [Google Scholar]
- 203.Zhao T, Cai Y, Jiang Y, He X, Wei Y, Yu Y, Tian X. Vaccine adjuvants: mechanisms and platforms. Signal Transduct Target Ther. 2023;8:283. 10.1038/s41392-023-01557-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Turley JL, Lavelle EC. Resolving adjuvant mode of action to enhance vaccine efficacy. Curr Opin Immunol. 2022;77: 102229. 10.1016/j.coi.2022.102229 [DOI] [PubMed] [Google Scholar]
- 205.Walsh KP, Mills KH. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013;34:521–30. 10.1016/j.it.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 206.Fritz JH, Girardin SE. How Toll-like receptors and Nod-like receptors contribute to innate immunity in mammals. J Endotoxin Res. 2005;11:390–4. 10.1177/09680519050110060301 [DOI] [PubMed] [Google Scholar]
- 207.Reddy ST, Swartz MA, Hubbell JA. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 2006;27:573–9. 10.1016/j.it.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 208.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol. 2007;19:3–10. 10.1016/j.smim.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 209.Kaparakis M, Philpott DJ, Ferrero RL. Mammalian NLR proteins; discriminating foe from friend. Immunol Cell Biol. 2007;85:495–502. 10.1038/sj.icb.7100105 [DOI] [PubMed] [Google Scholar]
- 210.Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. 2015;15:203–16. 10.1038/nri3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Roth GA, Picece V, Ou BS, Luo W, Pulendran B, Appel EA. Designing spatial and temporal control of vaccine responses. Nat Rev Mater. 2022;7:174–95. 10.1038/s41578-021-00372-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cirelli KM, Carnathan DG, Nogal B, Martin JT, Rodriguez OL, Upadhyay AA, Enemuo CA, Gebru EH, Choe Y, Viviano F, et al. Slow delivery immunization enhances HIV neutralizing antibody and germinal center responses via modulation of immunodominance. Cell. 2019;177:1153-1171.e1128. 10.1016/j.cell.2019.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lee JH, Sutton HJ, Cottrell CA, Phung I, Ozorowski G, Sewall LM, Nedellec R, Nakao C, Silva M, Richey ST, et al. Long-primed germinal centres with enduring affinity maturation and clonal migration. Nature. 2022;609:998–1004. 10.1038/s41586-022-05216-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chen C, Zhai S, Zhang L, Chen J, Long X, Qin J, Li J, Huo R, Wang X. Uhrf1 regulates germinal center B cell expansion and affinity maturation to control viral infection. J Exp Med. 2018;215:1437–48. 10.1084/jem.20171815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016; 1. [DOI] [PMC free article] [PubMed]
- 216.Kipper MJ, Shen E, Determan A, Narasimhan B. Design of an injectable system based on bioerodible polyanhydride microspheres for sustained drug delivery. Biomaterials. 2002;23:4405–12. 10.1016/S0142-9612(02)00181-3 [DOI] [PubMed] [Google Scholar]
- 217.Yue H, Ma G. Polymeric micro/nanoparticles: particle design and potential vaccine delivery applications. Vaccine. 2015;33:5927–36. 10.1016/j.vaccine.2015.07.100 [DOI] [PubMed] [Google Scholar]
- 218.Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G, Mooney DJ. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat Biotechnol. 2015;33:64–72. 10.1038/nbt.3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Adu-Berchie K, Mooney DJ. Biomaterials as local niches for immunomodulation. Acc Chem Res. 2020;53:1749–60. 10.1021/acs.accounts.0c00341 [DOI] [PubMed] [Google Scholar]
- 220.Sun Z, Liang J, Dong X, Wang C, Kong D, Lv F. Injectable hydrogels coencapsulating granulocyte-macrophage colony-stimulating factor and ovalbumin nanoparticles to enhance antigen uptake efficiency. ACS Appl Mater Interfaces. 2018;10:20315–25. 10.1021/acsami.8b04312 [DOI] [PubMed] [Google Scholar]
- 221.Li WA, Lu BY, Gu L, Choi Y, Kim J, Mooney DJ. The effect of surface modification of mesoporous silica micro-rod scaffold on immune cell activation and infiltration. Biomaterials. 2016;83:249–56. 10.1016/j.biomaterials.2016.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–31. 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lorentzen CL, Haanen JB, Met Ö, Svane IM. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 2022;23:e450–8. 10.1016/S1470-2045(22)00372-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tokatlian T, Kulp DW, Mutafyan AA, Jones CA, Menis S, Georgeson E, Kubitz M, Zhang MH, Melo MB, Silva M, et al. Enhancing humoral responses against HIV envelope trimers via nanoparticle delivery with stabilized synthetic liposomes. Sci Rep. 2018;8:16527. 10.1038/s41598-018-34853-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Babapoor S, Neef T, Mittelholzer C, Girshick T, Garmendia A, Shang H, Khan MI, Burkhard P. A novel vaccine using nanoparticle platform to present immunogenic M2e against avian influenza infection. Influenza Res Treat. 2011;2011: 126794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tokatlian T, Read BJ, Jones CA, Kulp DW, Menis S, Chang JYH, Steichen JM, Kumari S, Allen JD, Dane EL, et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science. 2019;363:649–54. 10.1126/science.aat9120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Brouwer PJM, Antanasijevic A, Berndsen Z, Yasmeen A, Fiala B, Bijl TPL, Bontjer I, Bale JB, Sheffler W, Allen JD, et al. Enhancing and shaping the immunogenicity of native-like HIV-1 envelope trimers with a two-component protein nanoparticle. Nat Commun. 2019;10:4272. 10.1038/s41467-019-12080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang W, Zhou X, Bian Y, Wang S, Chai Q, Guo Z, Wang Z, Zhu P, Peng H, Yan X, et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat Nanotechnol. 2020;15:406–16. 10.1038/s41565-020-0648-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Soleimanpour S, Farsiani H, Mosavat A, Ghazvini K, Eydgahi MR, Sankian M, Sadeghian H, Meshkat Z, Rezaee SA. APC targeting enhances immunogenicity of a novel multistage Fc-fusion tuberculosis vaccine in mice. Appl Microbiol Biotechnol. 2015;99:10467–80. 10.1007/s00253-015-6952-z [DOI] [PubMed] [Google Scholar]
- 230.Lu L, Palaniyandi S, Zeng R, Bai Y, Liu X, Wang Y, Pauza CD, Roopenian DC, Zhu X. A neonatal Fc receptor-targeted mucosal vaccine strategy effectively induces HIV-1 antigen-specific immunity to genital infection. J Virol. 2011;85:10542–53. 10.1128/JVI.05441-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Levin D, Golding B, Strome SE, Sauna ZE. Fc fusion as a platform technology: potential for modulating immunogenicity. Trends Biotechnol. 2015;33:27–34. 10.1016/j.tibtech.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 232.Shafifar M, Mozhgani SH, Razavi Pashabayg K, Mosavat A, Karbalaei M, Norouzi M, Rezaee SA. Selective APC-targeting of a novel Fc-fusion multi-immunodominant recombinant protein ((t)Tax-(t)Env:mFcγ2a) for HTLV-1 vaccine development. Life Sci. 2022;308: 120920. 10.1016/j.lfs.2022.120920 [DOI] [PubMed] [Google Scholar]
- 233.Macri C, Jenika D, Ouslinis C, Mintern JD. Targeting dendritic cells to advance cross-presentation and vaccination outcomes. Semin Immunol. 2023;68: 101762. 10.1016/j.smim.2023.101762 [DOI] [PubMed] [Google Scholar]
- 234.Zhang YN, Lazarovits J, Poon W, Ouyang B, Nguyen LNM, Kingston BR, Chan WCW. Nanoparticle size influences antigen retention and presentation in lymph node follicles for humoral immunity. Nano Lett. 2019;19:7226–35. 10.1021/acs.nanolett.9b02834 [DOI] [PubMed] [Google Scholar]
- 235.Heesters BA, Myers RC, Carroll MC. Follicular dendritic cells: dynamic antigen libraries. Nat Rev Immunol. 2014;14:495–504. 10.1038/nri3689 [DOI] [PubMed] [Google Scholar]
- 236.Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008;20:14–25. 10.1016/j.smim.2007.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ding Y, Li Z, Jaklenec A, Hu Q. Vaccine delivery systems toward lymph nodes. Adv Drug Deliv Rev. 2021;179: 113914. 10.1016/j.addr.2021.113914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Moyer TJ, Zmolek AC, Irvine DJ. Beyond antigens and adjuvants: formulating future vaccines. J Clin Invest. 2016;126:799–808. 10.1172/JCI81083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jiang H, Wang Q, Sun X. Lymph node targeting strategies to improve vaccination efficacy. J Control Release. 2017;267:47–56. 10.1016/j.jconrel.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 240.Rao DA, Forrest ML, Alani AW, Kwon GS, Robinson JR. Biodegradable PLGA based nanoparticles for sustained regional lymphatic drug delivery. J Pharm Sci. 2010;99:2018–31. 10.1002/jps.21970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Famta P, Shah S, Jain N, Srinivasarao DA, Murthy A, Ahmed T, Vambhurkar G, Shahrukh S, Singh SB, Srivastava S. Albumin-hitchhiking: fostering the pharmacokinetics and anticancer therapeutics. J Control Release. 2023;353:166–85. 10.1016/j.jconrel.2022.11.034 [DOI] [PubMed] [Google Scholar]
- 242.Abdallah M, Müllertz OO, Styles IK, Mörsdorf A, Quinn JF, Whittaker MR, Trevaskis NL. Lymphatic targeting by albumin-hitchhiking: applications and optimisation. J Control Release. 2020;327:117–28. 10.1016/j.jconrel.2020.07.046 [DOI] [PubMed] [Google Scholar]
- 243.Linciano S, Moro G, Zorzi A, Angelini A. Molecular analysis and therapeutic applications of human serum albumin-fatty acid interactions. J Control Release. 2022;348:115–26. 10.1016/j.jconrel.2022.05.038 [DOI] [PubMed] [Google Scholar]
- 244.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–22. 10.1038/nature12978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tsopelas C, Sutton R. Why certain dyes are useful for localizing the sentinel lymph node. J Nucl Med. 2002;43:1377–82. [PubMed] [Google Scholar]
- 246.Zhu G, Lynn GM, Jacobson O, Chen K, Liu Y, Zhang H, Ma Y, Zhang F, Tian R, Ni Q, et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat Commun. 2017;8:1954. 10.1038/s41467-017-02191-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Cruz FM, Colbert JD, Merino E, Kriegsman BA, Rock KL. The biology and underlying mechanisms of cross-presentation of exogenous antigens on MHC-I molecules. Annu Rev Immunol. 2017;35:149–76. 10.1146/annurev-immunol-041015-055254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Embgenbroich M, Burgdorf S. Current concepts of antigen cross-presentation. Front Immunol. 2018;9:1643. 10.3389/fimmu.2018.01643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol Ther. 2013;21:149–57. 10.1038/mt.2012.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chen J, Li Z, Huang H, Yang Y, Ding Q, Mai J, Guo W, Xu Y. Improved antigen cross-presentation by polyethyleneimine-based nanoparticles. Int J Nanomedicine. 2011;6:77–84. 10.2147/IJN.S15457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Xu J, Wang H, Xu L, Chao Y, Wang C, Han X, Dong Z, Chang H, Peng R, Cheng Y, Liu Z. Nanovaccine based on a protein-delivering dendrimer for effective antigen cross-presentation and cancer immunotherapy. Biomaterials. 2019;207:1–9. 10.1016/j.biomaterials.2019.03.037 [DOI] [PubMed] [Google Scholar]
- 252.Zhang S, Jiang H, Huang S, Li P, Wang F. Curdlan sulfate/O-linked quaternized chitosan nanoparticles acting as potential adjuvants promote multiple arms of immune responses. Carbohydr Polym. 2019;213:100–11. 10.1016/j.carbpol.2019.02.093 [DOI] [PubMed] [Google Scholar]
- 253.Zhang L, Wu S, Qin Y, Fan F, Zhang Z, Huang C, Ji W, Lu L, Wang C, Sun H, et al. Targeted codelivery of an antigen and dual agonists by hybrid nanoparticles for enhanced cancer immunotherapy. Nano Lett. 2019;19:4237–49. 10.1021/acs.nanolett.9b00030 [DOI] [PubMed] [Google Scholar]
- 254.Du G, Sun X. Engineering nanoparticulate vaccines for enhancing antigen cross-presentation. Curr Opin Biotechnol. 2020;66:113–22. 10.1016/j.copbio.2020.06.015 [DOI] [PubMed] [Google Scholar]
- 255.Jerjes W, Theodossiou TA, Hirschberg H, Høgset A, Weyergang A, Selbo PK, Hamdoon Z, Hopper C, Berg K. Photochemical internalization for intracellular drug delivery from basic mechanisms to clinical research. J Clin Med. 2020;9:528. 10.3390/jcm9020528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Otterhaug T, Janetzki S, Welters MJP, Håkerud M, Nedberg AG, Edwards VT, Boekestijn S, Loof NM, Selbo PK, Olivecrona H, et al. Photochemical internalization enhanced vaccination is safe, and gives promising cellular immune responses to an HPV peptide-based vaccine in a phase I clinical study in healthy volunteers. Front Immunol. 2020;11: 576756. 10.3389/fimmu.2020.576756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Šošić L, Selbo PK, Kotkowska ZK, Kündig TM, Høgset A, Johansen P. Photochemical internalization: light paves way for new cancer chemotherapies and vaccines. Cancers (Basel). 2020;12:165. 10.3390/cancers12010165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ji Y, Zhao J, Chu CC. Enhanced MHC-I antigen presentation from the delivery of ovalbumin by light-facilitated biodegradable poly(ester amide)s nanoparticles. J Mater Chem B. 2018;6:1930–42. 10.1039/C7TB03233A [DOI] [PubMed] [Google Scholar]
- 259.Cheng F, Su T, Zhou S, Liu X, Yang S, Lin S, Guo W, Zhu G. Single-dose injectable nanovaccine-in-hydrogel for robust immunotherapy of large tumors with abscopal effect. Sci Adv. 2023;9:eade6257. 10.1126/sciadv.ade6257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Mu Z, Wiehe K, Saunders KO, Henderson R, Cain DW, Parks R, Martik D, Mansouri K, Edwards RJ, Newman A, et al. mRNA-encoded HIV-1 Env trimer ferritin nanoparticles induce monoclonal antibodies that neutralize heterologous HIV-1 isolates in mice. Cell Rep. 2022;38: 110514. 10.1016/j.celrep.2022.110514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Pan J, Wang Q, Qi M, Chen J, Wu X, Zhang X, Li W, Zhang X-E, Cui Z. An intranasal multivalent epitope-based nanoparticle vaccine confers broad protection against divergent influenza viruses. ACS Nano. 2023;17:13474–87. 10.1021/acsnano.3c01829 [DOI] [PubMed] [Google Scholar]
- 262.Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6:1078–94. 10.1038/s41578-021-00358-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Contreras-Magallanes YG, Durán-Aguilar M, Sosa-Gallegos SL, Álvarez ÁH, Andrade-Santillán FA, Bárcenas-Reyes I, González-Ruíz S, Rodríguez-Hernández E, Cantó-Alarcón GJ, Milián-Suazo F. Prime vaccination with chitosan-coated phipps BCG and boosting with CFP-PLGA against tuberculosis in a goat model. Animals (Basel). 2021;11:1046. 10.3390/ani11041046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kekani LN, Witika BA. Current advances in nanodrug delivery systems for malaria prevention and treatment. Discov Nano. 2023;18:66. 10.1186/s11671-023-03849-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Marques J, Valle-Delgado JJ, Urbán P, Baró E, Prohens R, Mayor A, Cisteró P, Delves M, Sinden RE, Grandfils C, et al. Adaptation of targeted nanocarriers to changing requirements in antimalarial drug delivery. Nanomedicine. 2017;13:515–25. 10.1016/j.nano.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Giri-Rachman EA, Irasonia Tan M, Ramesh A, Fajar PA, Nurul Ilmi A, Retnoningrum DS, Hertadi R, Irawan A, Wojciechowska GEP, Yuan L. Development of Chimeric Hepatitis B (HBV)—Norovirus (NoV) P particle as candidate vaccine against Hepatitis B and norovirus infection. Vaccine X. 2023;14: 100354. 10.1016/j.jvacx.2023.100354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21:360–78. 10.1038/s41568-021-00346-0 [DOI] [PubMed] [Google Scholar]
- 268.Qin L, Zhang H, Zhou Y, Umeshappa CS, Gao H. Nanovaccine-based strategies to overcome challenges in the whole vaccination cascade for tumor immunotherapy. Small. 2021;17: e2006000. 10.1002/smll.202006000 [DOI] [PubMed] [Google Scholar]
- 269.Liu J, Liew SS, Wang J, Pu K. Bioinspired and biomimetic delivery platforms for cancer vaccines. Adv Mater. 2021;34: e2103790. 10.1002/adma.202103790 [DOI] [PubMed] [Google Scholar]
- 270.Mei H, Cai S, Huang D, Gao H, Cao J, He B. Carrier-free nanodrugs with efficient drug delivery and release for cancer therapy: from intrinsic physicochemical properties to external modification. Bioact Mater. 2022;8:220–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Fan M, Jia L, Pang M, Yang X, Yang Y, Kamel Elyzayati S, Liao Y, Wang H, Zhu Y, Wang Q. Injectable adhesive hydrogel as photothermal-derived antigen reservoir for enhanced anti-tumor immunity. Adv Funct Mater. 2021;20:10587. [Google Scholar]
- 272.Yang F, Shi K, Jia Y, Hao Y, Peng J, Yuan L, Chen Y, Pan M, Qian Z. A biodegradable thermosensitive hydrogel vaccine for cancer immunotherapy. Appl Mater Today. 2020;19: 100608. 10.1016/j.apmt.2020.100608 [DOI] [Google Scholar]
- 273.Ji G, Zhang Y, Si X, Yao H, Ma S, Xu Y, Zhao J, Ma C, He C, Tang Z, et al. Biopolymer immune implants’ sequential activation of innate and adaptive immunity for colorectal cancer postoperative immunotherapy. Adv Mater. 2020;33: e2004559. 10.1002/adma.202004559 [DOI] [PubMed] [Google Scholar]
- 274.Wang Y, Jiang Z, Xu W, Yang Y, Zhuang X, Ding J, Chen X. Chiral polypeptide thermogels induce controlled inflammatory response as potential immunoadjuvants. ACS Appl Mater Interfaces. 2019;11:8725–30. 10.1021/acsami.9b01872 [DOI] [PubMed] [Google Scholar]
- 275.Luo Z, Wu Q, Yang C, Wang H, He T, Wang Y, Wang Z, Chen H, Li X, Gong C, Yang Z. A powerful CD8+ T-cell stimulating D-tetra-peptide hydrogel as a very promising vaccine adjuvant. Adv Mater. 2016;29:01776. [DOI] [PubMed] [Google Scholar]
- 276.Chao Y, Chen Q, Liu Z. Smart injectable hydrogels for cancer immunotherapy. Adv Func Mater. 2019;30:1902785. 10.1002/adfm.201902785 [DOI] [Google Scholar]
- 277.Bo Y, Wang H. Biomaterial-based in situ cancer vaccines. Adv Mater; n/a:2210452. [DOI] [PMC free article] [PubMed]
- 278.Hanson MC, Abraham W, Crespo MP, Chen SH, Liu H, Szeto GL, Kim M, Reinherz EL, Irvine DJ. Liposomal vaccines incorporating molecular adjuvants and intrastructural T-cell help promote the immunogenicity of HIV membrane-proximal external region peptides. Vaccine. 2015;33:861–8. 10.1016/j.vaccine.2014.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Jones RB, Mueller S, Kumari S, Vrbanac V, Genel S, Tager AM, Allen TM, Walker BD, Irvine DJ. Antigen recognition-triggered drug delivery mediated by nanocapsule-functionalized cytotoxic T-cells. Biomaterials. 2017;117:44–53. 10.1016/j.biomaterials.2016.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kirtane AR, Verma M, Karandikar P, Furin J, Langer R, Traverso G. Nanotechnology approaches for global infectious diseases. Nat Nanotechnol. 2021;16:369–84. 10.1038/s41565-021-00866-8 [DOI] [PubMed] [Google Scholar]
- 281.Freeling JP, Koehn J, Shu C, Sun J, Ho RJ. Anti-HIV drug-combination nanoparticles enhance plasma drug exposure duration as well as triple-drug combination levels in cells within lymph nodes and blood in primates. AIDS Res Hum Retroviruses. 2015;31:107–14. 10.1089/aid.2014.0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Freeling JP, Koehn J, Shu C, Sun J, Ho RJ. Long-acting three-drug combination anti-HIV nanoparticles enhance drug exposure in primate plasma and cells within lymph nodes and blood. AIDS. 2014;28:2625–7. 10.1097/QAD.0000000000000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Rouphael NG, Paine M, Mosley R, Henry S, McAllister DV, Kalluri H, Pewin W, Frew PM, Yu T, Thornburg NJ, et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet. 2017;390:649–58. 10.1016/S0140-6736(17)30575-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Mistilis MJ, Joyce JC, Esser ES, Skountzou I, Compans RW, Bommarius AS, Prausnitz MR. Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv Transl Res. 2017;7:195–205. 10.1007/s13346-016-0282-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Arya J, Henry S, Kalluri H, McAllister DV, Pewin WP, Prausnitz MR. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials. 2017;128:1–7. 10.1016/j.biomaterials.2017.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Schoenmaker L, Witzigmann D, Kulkarni JA, Verbeke R, Kersten G, Jiskoot W, Crommelin DJA. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharm. 2021;601: 120586. 10.1016/j.ijpharm.2021.120586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Knezevic I, Liu MA, Peden K, Zhou T, Kang HN. Development of mRNA vaccines: scientific and regulatory issues. Vaccines (Basel). 2021;9:81. 10.3390/vaccines9020081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Bouzeyen R, Javid B. Therapeutic vaccines for tuberculosis: an overview. Front Immunol. 2022;13: 878471. 10.3389/fimmu.2022.878471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Fatima S, Kumari A, Das G, Dwivedi VP. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252: 117594. 10.1016/j.lfs.2020.117594 [DOI] [PubMed] [Google Scholar]
- 290.Hatherill M, White RG, Hawn TR. Clinical development of new TB vaccines: recent advances and next steps. Front Microbiol. 2019;10:3154. 10.3389/fmicb.2019.03154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Milián-Suazo F, González-Ruiz S, Contreras-Magallanes YG, Sosa-Gallegos SL, Bárcenas-Reyes I, Cantó-Alarcón GJ, Rodríguez-Hernández E. Vaccination strategies in a potential use of the vaccine against bovine tuberculosis in infected herds. Animals (Basel). 2022;12:3377. 10.3390/ani12233377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Thakkar M, Brijesh S. Physicochemical investigation and in vivo activity of anti-malarial drugs co-loaded in Tween 80 niosomes. J Liposome Res. 2018;28:315–21. 10.1080/08982104.2017.1376684 [DOI] [PubMed] [Google Scholar]
- 293.Shakeel K, Raisuddin S, Ali S, Imam SS, Rahman MA, Jain GK, Ahmad FJ. Development and in vitro/in vivo evaluation of artemether and lumefantrine co-loaded nanoliposomes for parenteral delivery. J Liposome Res. 2019;29:35–43. 10.1080/08982104.2017.1410173 [DOI] [PubMed] [Google Scholar]
- 294.Ouji M, Augereau JM, Paloque L, Benoit-Vical F. Plasmodium falciparum resistance to artemisinin-based combination therapies: a sword of Damocles in the path toward malaria elimination. Parasite. 2018;25:24. 10.1051/parasite/2018021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Siddiqui AJ, Bhardwaj J, Saxena J, Jahan S, Snoussi M, Bardakci F, Badraoui R, Adnan M. A critical review on human malaria and schistosomiasis vaccines: current state, recent advancements, and developments. Vaccines (Basel). 2023;11:792. 10.3390/vaccines11040792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hailegebriel T, Nibret E, Munshea A. Prevalence of Schistosoma mansoni and S. haematobium in snail intermediate hosts in Africa: a systematic review and meta-analysis. J Trop Med. 2020;2020:8850840. 10.1155/2020/8850840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Datoo MS, Natama HM, Somé A, Bellamy D, Traoré O, Rouamba T, Tahita MC, Ido NFA, Yameogo P, Valia D, et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. Lancet Infect Dis. 2022;22:1728–36. 10.1016/S1473-3099(22)00442-X [DOI] [PubMed] [Google Scholar]
- 298.Laurens MB. RTS, S/AS01 vaccine (Mosquirix™): an overview. Hum Vaccin Immunother. 2020;16:480–9. 10.1080/21645515.2019.1669415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Datoo MS, Natama MH, Somé A, Traoré O, Rouamba T, Bellamy D, Yameogo P, Valia D, Tegneri M, Ouedraogo F, et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. Lancet. 2021;397:1809–18. 10.1016/S0140-6736(21)00943-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Pattyn J, Hendrickx G, Vorsters A, Van Damme P. Hepatitis B vaccines. J Infect Dis. 2021;224:S343-s351. 10.1093/infdis/jiaa668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Jeng WJ, Papatheodoridis GV, Lok ASF. Hepatitis B. Lancet. 2023;401:1039–52. 10.1016/S0140-6736(22)01468-4 [DOI] [PubMed] [Google Scholar]
- 302.Ho JK, Jeevan-Raj B, Netter HJ. Hepatitis B virus (HBV) subviral particles as protective vaccines and vaccine platforms. Viruses. 2020;12:126. 10.3390/v12020126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Su J, Brunner L, Ates Oz E, Sacherl J, Frank G, Kerth HA, Thiele F, Wiegand M, Mogler C, Aguilar JC, et al. Activation of CD4 T cells during prime immunization determines the success of a therapeutic hepatitis B vaccine in HBV-carrier mouse models. J Hepatol. 2023;78:717–30. 10.1016/j.jhep.2022.12.013 [DOI] [PubMed] [Google Scholar]
- 304.Ito D, Ito H, Ando T, Sakai Y, Ideta T, Ishii KJ, Ishikawa T, Shimizu M. Spermidine enhances the efficacy of adjuvant in HBV vaccination in mice. Hepatol Commun. 2023;7: e0104. 10.1097/HC9.0000000000000104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Wollner CJ, Richner M, Hassert MA, Pinto AK, Brien JD, Richner JM. A dengue virus serotype 1 mRNA-LNP vaccine elicits protective immune responses. J Virol. 2021;95:e02482-e2520. 10.1128/JVI.02482-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wang F, Fang RH, Luk BT, Hu CJ, Thamphiwatana S, Dehaini D, Angsantikul P, Kroll AV, Pang Z, Gao W, et al. Nanoparticle-based antivirulence vaccine for the management of methicillin-resistant Staphylococcus aureus skin infection. Adv Funct Mater. 2016;26:1628–35. 10.1002/adfm.201505231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Chahal JS, Fang T, Woodham AW, Khan OF, Ling J, Anderson DG, Ploegh HL. An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci Rep. 2017;7:252. 10.1038/s41598-017-00193-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Li Y, Beitelshees M, Fang L, Hill A, Ahmadi MK, Chen M, Davidson BA, Knight P 3rd, Smith RJ Jr, Andreadis ST, et al. In situ pneumococcal vaccine production and delivery through a hybrid biological-biomaterial vector. Sci Adv. 2016;2: e1600264. 10.1126/sciadv.1600264 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.