ABSTRACT
This study investigates the effect of Licochalcone A (Lico-A), a flavonoid from licorice roots known for its anti-inflammatory, anti-cancer, and antioxidant properties, on NMDA-induced neurotoxicity in primary cultured rat hippocampal neurons. The study measured cell survival following NMDA and Lico-A exposure, revealing that Lico-A at a 2.5 μg/ml significantly improved cell viability, countering the detrimental effects of NMDA. The study also analyzed synaptic changes by examining both postsynaptic density 95 (PSD95) and synaptophysin-targeted imaging, showing that Lico-A treatment resulted in a significant increase in synaptic puncta, contrasting with the reduction observed under NMDA exposure. Furthermore, levels of phosphorylated mixed lineage kinase domain-like pseudokinase (P-MLKL) and phosphorylated receptor-interacting serine/threonine-protein kinase 3 (P-RIP3), key necroptosis regulators, were measured using Western blotting. The results showed an increase in P-MLKL and P-RIP3 in neurons exposed to NMDA, which was reduced following Lico-A treatment. The response of astrocyte and microglia was also evaluated by immunostaining for glial fibrillary acidic protein (GFAP), ionized calcium-binding adaptor molecule 1 (IBA-1) and tumor necrosis factor alpha (TNF-α). These markers exhibited heightened expression in the NMDA group, which was substantially reduced by Lico-A treatment. These findings suggest that Lico-A has neuroprotective effects against NMDA-induced neurotoxicity, potentially contributing to synaptic preservation, inhibition of neuronal necroptosis, and modulation of glial activation. Therefore, Lico-A shows promise as a neuroprotective agent for conditions associated with NMDA-related neurotoxicity.
KEYWORDS: Licochalcone A, gliosis, neuroprotection, synapse loss, necroptosis
Introduction
Neurotoxicity is a consequence of various substances or environmental factors that detrimentally affect the nervous system, leading to alterations in the structure and function of neurons. It encompasses mechanisms such as excitotoxicity, oxidative stress, inflammation, and dysfunction of glial cell, crucial in the development of neurodegenerative diseases (Bilge 2022). This study focuses on neuronal damage due to overstimulation of glutamate receptors (Dong et al. 2009). Excess glutamate initiates enzymatic activities causing neuronal damage (Porter et al. 1997). These processes are prominent in disorders like Alzheimer's, epilepsy, multiple sclerosis, and amyotrophic lateral sclerosis (Van Den Bosch et al. 2006), highlighting the potential therapeutic value in addressing excitotoxicity. Neurodegeneration in the hippocampus, vital for memory and cognitive function, leads to substantial deficits in these functions in various neurodegenerative disorders(Anand and Dhikav 2012; Terreros-Roncal et al. 2021). However, the exact mechanisms of hippocampal neurodegeneration remain elusive.
Licochalcone A (Lico-A), a flavonoid compound extracted from licorice root and used in traditional Chinese medicine. Licorice root has applications in treating various health issues, including infections, inflammation, and cancer. Lico-A is particularly known for its anti-inflammatory, antibacterial, antiviral, and anticancer properties (Chen et al. 2017). This study investigates the effects of Lico-A on hippocampal neurons, particularly focusing on its protective role against NMDA-induced cell death.
We also examine the structural integrity of excitatory synapses and neuronal recovery by focusing on Postsynaptic density protein-95 (PSD-95) and Microtubule-associated protein 2 (MAP2) (Kornau et al. 1995; Coley and Gao 2018). PSD is critical in maintaining synaptic structure and pivotal for synaptic plasticity and neurotransmitter receptor regulation. Understanding the role of PDS95 is key to studying synaptic plasticity, neuronal development and neuronal disorders.
Another focus is neuroinflammation, involving astrocytes and microglia activation in response to central nervous system damage or disease. Overactivation of these cells can lead to chronic inflammation and cell death via the release of proinflammatory cytokines and inflammatory mediators. This process is linked to various neurological disorders, including Alzheimer's, Parkinson's, and epilepsy (Pekny and Pekna 2016; Patel et al. 2019).
Necroptosis, a programed form of cell death distinct from apoptosis, is initiated through specific molecular pathways and can trigger inflammatory responses, exacerbating cell death (Galluzzi et al. 2017). Understanding the mechanisms of necroptosis, including the roles of receptor-interacting protein kinases and MLKL, is crucial in exploring new therapeutic approaches.
Natural substances have been increasingly recognized for their therapeutic potential in various conditions, including necrosis. Compounds such as Resveratrol from grapes (Hu et al. 2021), Curcumin from turmeric (Lee et al. 2021), Quercetin from fruits and vegetables (Fan et al. 2019), EGCG from green tea (Machin et al. 2020), and Apigenin from parsley (Lee et al. 2020) have shown promise in inhibiting necrotic processes. However, the therapeutic potential of Lico-A in mitigating necroptosis, especially in relation to central nervous system damage, remains insufficiently explored. Therefore, this study aims to investigate the effects of Lico-A on the hippocampal neurons exposed to NMDA-induced necroptosis, exploring its potential as a neuroprotective agent in neurodegenerative diseases.
Materials and methods
Cell culture
Primary hippocampal neurons from embryonic Sprague–Dawley rats (gestational day 19) were cultured in HEPES-buffered Hanks salt solution, excluding Ca2+ and Mg2+ (HHSS), maintaining a pH of 7.45 (Hong et al. 2022). Neurons were dissociated using a flame-polished Pasteur pipette and incubated either in Neurobasal medium supplemented with 2% B27, 0.25% Glutamax I, and antibiotics (penicillin, streptomycin, amphotericin B), or DMEM containing phenol red, 10% FBS, and the same antibiotic mixture. The neurons were plated on 60 mm ECM Gel-coated (Sigma-Aldrich, USA) cover glasses at 75×104 cells/ml and incubated at 37°C in an atmosphere of 10% CO2 and 95% humidity, with three-quarters of the medium refreshed every three days.
MTT assay
Cultured hippocampal neurons were seeded at 66×104 cells/ml in 96-well plates and cultured for 11 days. Before 100 μM NMDA exposure, cells were pre-treated with Lico-A at 1.25 and 2.5 μg/ml for one hour. Following this, cells were incubated for an additional 24 hours with Lico-A at the same concentrations. Cell viability was assessed using an MTT assay, measuring absorbance at 570 nm after a four-hour incubation with MTT solution, using a microplate reader (Kim et al. 2023).
Immunocytochemistry
After 11 days of culture and Lico-A treatment, hippocampal neurons were prepared for immunocytochemical analysis. Cells were fixed in methanol at −20°C for 8 minutes and washed. Permeabilization was achieved using 0.3% Triton X-100 for 5 minutes, followed by blocking with 5% Bovine Serum Albumin (BSA, Sigma Aldrich, USA) for 90 minutes at room temperature (Yun et al. 2023). Overnight incubation at 4°C was done with primary antibodies including mouse anti-MAP2 (1:200, Sigma-Aldrich, USA), rabbit anti-PSD95 (1:200, Invitrogen, USA), mouse anti-GFAP (1:200, Sigma-Aldrich, USA), rabbit anti-IBA-1 (1:500, Wako, Japan) and mouse anti-TNF-α (1:200, Santa Cruz, USA). This is followed by incubation with Alexa Fluor 488 anti-rabbit IgG (1:500, Invitrogen, USA), and Alexa Fluor 555 anti-mouse IgG (1:1000, Invitrogen, USA) secondary antibodies. Cells were then mounted on slides using VECTASHIELD Mounting Medium (Vector Laboratories, Inc., Burlingame, USA) for confocal microscopy.
Confocal imaging and morphological analysis
The immunostained cells were examined using a LSM 700 confocal microscope (Carl Zeiss, Germany) equipped with a 40x objective lens. Z-stack imaging was utilized, capturing 8 sectional images at 1 μm intervals. GFP and RFP fluorescence were visualized with an argon ion laser (for GFP) and a HeNe laser (for RFP), respectively, for exciation and emission wavelengths specific to each flurophore. Quantification of puncta labeled by PSD95 and synaptophysin followed the methods outlined in prior studies (Kim and Thayer 2009; Hong et al. 2022). Measurements were based on randomly selected image fields per coverslip (n), where n represents the number of image fields per coverslip. The intensity of GFAP, IBA1, and TNF-α was expressed as the mean ± SEM, with n indicating the number of image fields analyzed.
Western blot
After 11 days in primary culture and 24 hours following Lico-A treatment, hippocampal neurons in 6-well plates were harvested. Cells were dissociated using Accutase (Sigma-Aldrich, USA) and lysed using ProPrep™ lysis buffer (iNtRON Biotechnology, Seongnam, Korea) (Roh et al. 2023). Protein concentrations were determined using a BCA protein assay. 30 µg protein was loaded onto 12% SDS-polyacrylamide gels. Western blotting involved incubation with anti-P-MLKL and anti-P-RIP3 primary antibody, followed by an anti-rabbit IgG secondary antibody. Bands were visualized using Pierce™ ECL Western Blotting Substrate (Thermo Scientific, USA) and subsequently re-probed for β-actin for normalization.
Statistical analysis
Data were presented as mean ± SEM. Statistical comparisons for multiple samples were conducted using ANOVA, followed by Bonferroni’s post-hoc test (GraphPad Prism8, GraphPad, California, USA). Significance levels were set at P<0.05 for 95% confidence and P<0.01 for 99% confidence, as appropriate for each experimental condition.
Results
Neuroprotective effects of Lico-A against NMDA-induced neurotoxicity
Our study investigated the protective effects of Lico-A on hippocampal neurons under NMDA-induced neurotoxicity. Cell viability, assessed using the MTT assays, indicated a decline in neuron survival (79.1±1.4%, n = 7, p<0.001) following NMDA exposure, contrasted with untreated controls (100±0%, n = 7). Lico-A treatment alone did not alter cell viability significantly. However, neurons treated with Lico-A post-NMDA exposure at 1.25 and 2.5 μg/ml concentrations exhibited increased survival rates of 85.9±3.8% (n = 7) and 92.7±4.2% (n = 7, p<0.01), respectively. A dose-dependent increase in cell survival with the 2.5 μg/ml dose nearly restoring cell viability control levels (Figure 1C).
Figure 1.
Neuroprotective effects of Lico-A in NMDA-induced neurotoxicity. Cell viability was assessed using the MTT assay, involving a 4-hour incubation with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. A, Chemical structures of the Lico-A compound. The MTT assay results indicate that Lico-A does not adversely affect neuronal survival. B, Experimental design of NMDA-induced neurotoxicity model is shown as a timeline. The pretreatment group was treated with Lico-A (1.25 and 2.5 μg/ml) for one hour before exposure to NMDA (100 μM) and continued with Lico-A for an additional 24 hours. C, Quantification of neuronal survival over 24 hours following NMDA-induced damage in cultured rat hippocampal neurons. Notably, Lico-A at concentrations of 1.25 μg /ml and 2.5 μg /ml demonstrated a dose-dependent improvement in cell viability, effectively reducing the neuronal damage caused by NMDA. One-way ANOVA analysis with Bonferroni post-hoc test, p < 0.0001, F(5, 36) = 9.509. Data are expressed as means ± SEM. ***p < 0.001 compared to the control; ##p < 0.01 compared to the NMDA group.
Effect of Lico-A on NMDA-induced synaptic loss
We analyzed the expression of PSD-95, integral for synaptic stability, in neurons treated with NMDA. Immunocytochemistry targeting PSD-95 and MAP2 revealed a significant reduction in synaptic puncta in the NMDA-treated group (43.0±4.0%, n = 7, p<0.001) compared to the control (100±7.5%, n = 7). Treatment with Lico-A at both tested concentrations resulted in maintained synaptic numbers (86.5±5.1%, n = 5 for 1.25 μg/ml and 88.9±5.4%, n = 3 for 2.5 μg/ml), with significant synaptic preservation at both concentration (68.0±5.4%, n = 5, p<0.05 for 1.25 μg/ml and 74.6±5.4%, n = 5, p<0.01 for 2.5 μg/ml) (Figure 2B). We also examined synaptic puncta using the presynaptic marker synaptophysin (Figure 2C). The NMDA-treated group showed a significant reduction in puncta (67.3±6.1%, n = 15, p<0.01) compared to the control group (100±5.7%, n = 17, p<0.01). This reduction in puncta was significantly reversed by treatment with both concentrations of Lico-A, with puncta levels increasing to 101.0±8.9% (n = 12, p<0.01) for 1.25 μg/ml and 107.5±8.9% (n = 13, p<0.001) for 2.5 μg/ml (Figure 2D). These findings indicate that Lico-A can facilitate synaptic recovery in hippocampal neurons affected by NMDA-induced neurodegeneration.
Figure 2.
Effect of Lico-A on synaptic punctate pattern in NMDA-induced synaptic loss. Confocal imaging was used to analyze the punctate patterns of PSD95 (A) and synaptophysin (C), demonstrating that Lico-A significantly reduced the neurodegeneration induced by NMDA. The graph displays a quantitative analysis of PSD95 (B) and synaptophysin (D) puncta, highlighting the neuroprotective effect of Licochalcone A in mitigating synapse loss caused by NMDA. One-way ANOVA was performed with Bonferroni post-hoc test, (B) p < 0.0001, F(5,26) = 13.80; (D) p = 0.0023, F(5,88) = 4.071. Scale bar represents 10 μm for low magnified images, 2 μm for high magnified images. Data are presented as means ± SEM; **p < 0.01, ***p < 0.001 compared to the control group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared to the NMDA-treated group.
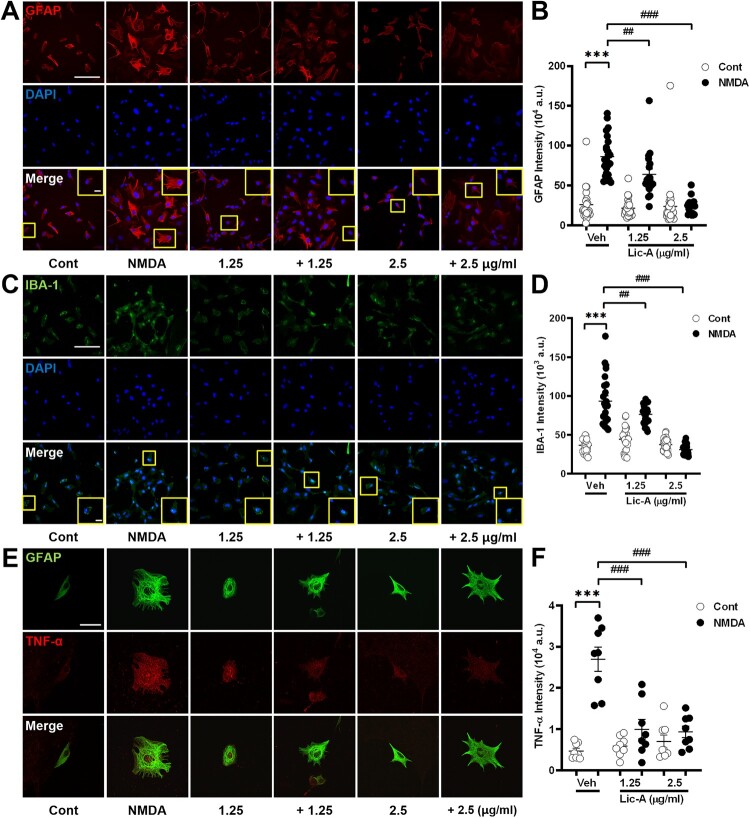
Influence of Lico-A on glial cell responses in NMDA-induced neurotoxicity
This study evaluated the effects of Lico-A on glial cells under NMDA-induced neurotoxicity using immunocytochemistry (Figure 3). In the NMDA-treated group, astrocytes exhibited increased intensity (86.38±5.2, n = 24, p<0.001) in contrast to the control group (26.00±4.6, n = 22) (Figure 3B). Treatment with Lico-A at concentrations of 1.25 and 2.5 μg/ml demonstrated a dose-dependent reduction of GFAP intensity in astrocyte (63.66±7.0, n = 18, p<0.01 for 1.25 μg/ml and 23.63±2.3, n = 19, p<0.001 for 2.5 μg/ml) and returned expression levels to near normal (21.3±2.2, n = 25 for 1.25 μg/ml and 23.4±5.0m = , n = 33 for 2.5 μg/ml). Similarly, microglial cells exhibited a notable decrease in intensity following Lico-A treatment (76.52±2.69, n = 22, p<0.01 for 1.25 μg/ml and 30.96±1.25, n = 26, p<0.001 for 2.5 μg/ml) compared to the NMDA-treated group (93.56±6.53, n = 24, p<0.001), indicating a dose-responsive reduction (Figure 3D).
Figure 3.
The impact of Lico-A on gliosis in NMDA-induced neurotoxicity of hippocampal neurons. A, C and E, Confocal images depict changes in astrocytes in response to NMDA-induced neurotoxicity and subsequent treatment with Lico-A. The graph illustrated the quantitative analysis of GFAP (B), IBA-1 (D) and TNF-α (F) intensity. One-way ANOVA was performed with Bonferroni post-hoc test, (B) p < 0.0001, F(5,127) = 89.28; (D) p < 0.0001, F(5,143) = 61.33; (F) p < 0.0001, F(5, 42) = 21.16. Scale bar, 20 μm. The data were presented as means ± SEM; ***p < 0.001 compared to the control; ##p < 0.01; ###p < 0.001 compared to NMDA.
To further investigate the effects of Lico-A on glial cell responses related to neurotoxicity, we performed double labeling immunocytochemistry for the pro-inflammatory cytokine TNF-α and GFAP (Figure 3E). TNF-α expression levels significantly increased in the NMDA-treated group (2.69 ± 0.3%, n = 8, p<0.001) compared to the control (0.47 ± 0.1%, n = 8). This increase was significantly reduced upon treatment with Lico-A at both concentrations (1.00 ± 0.2%, n = 8, p<0.001 for 1.25 μg/ml and 0.93 ± 0.14%, n = 8, p<0.001 for 2.5 μg/ml).
Efficacy of Lico-A in reducing hippocampal necroptosis
To determine Lico-A's role in regulating necroptosis in hippocampal neurons, we analyzed the expression of P-MLKL and P-RIP3, critical markers of necroptosis, following NMDA exposure (Figure 4). Western blot analysis displayed a significant elevation in P-MLKL levels in the NMDA-treated neurons (2.06±0.25, n = 4, p<0.01) compared to the control group (1±0, n = 4). Lico-A treatments at both 1.25 and 2.5 μg/ml concentrations led to marked reductions in P-MLKL expression, with the higher dose effectively normalizing levels to control group values (1.66±0.03%, n = 3 for 1.25 μg/ml and 1.00±0.20%, n = 4, p<0.01 for 2.5 μg/ml) (Figure 4B). Furthermore, P-RIP3 expression also showed an increasing trend in the NMDA-treated group (1.33±0.05%, n = 3) compared to the control group (1±0, n = 4) (Figure 4D). This increase was dose-dependently reduced by Lico-A treatment (1.11±0.02%, n = 2 for 1.25 μg/ml and 0.90±0.21%, n = 4 for 2.5 μg/ml). This indicates that Lico-A is effective in reducing NMDA-induced necroptosis in hippocampal neurons by inhibiting the phosphorylation of MLKL and RIP3.
Figure 4.
Effects of Lico-A on reducing hippocampal necroptosis. A and C, Western blot analysis depicts the expression levels of phospho-MLKL(P-MLKL) and phospho-RIP3 (P-RIP3) in the hippocampal neurons, using β-actin as a loading control. B and D, the graph displays the quantification of P-MLKL and P-RIP3 normalized to β-actin. Notably, the NMDA NMDA + Lico-A (2.5 µg/ml) treated groups exhibited significantly lower levels of P-MLKL compared to the NMDA group. One-way ANOVA was performed with Bonferroni post-hoc test, (B) p = 0.0025, F(3,11) = 9.195; (D) p = 0.1069, F(3,8) = 2.823. Data were presented as means ± SEM; **p < 0.01 compared to the control; ##p < 0.01 compared to the NMDA group.
Discussion
This study delved into the neuroprotective role of Lico-A against NMDA-induced neurotoxicity in hippocampal neurons. Our findings highlighted a significant decrease in neuronal viability upon NMDA treatment, which was notably improved with Lico-A treatment (Figure 1). A key finding was the reduction in synaptic puncta, specifically targeting the postsynaptic protein PSD-95 and the presynaptic protein synaptophysin, following NMDA exposure. This reduction was effectively reversed by Lico-A treatment (Figure 2). Additionally, NMDA-induced neurodegenerative changes in astrocytes and microglia, such as glial activation and increased production of proinflammatory cytokine, were alleviated by Lico-A (Figure 3). Moreover, Lico-A inhibited the phosphorylation of MLKL and RIP3, which are key markers of NMDA-induced necroptosis in hippocampal neurons (Figure 4). These results highlight Lico-A’s efficacy in alleviating NMDA-induced neurodegenerative damage and morphological changes in hippocampal neurons, reducing glial cell activation, and ultimately promoting cell survival (Figure 5).
Figure 5.
Schematic diagram of the effects of Lico-A in cultured rat hippocampal neurons.
Lico-A, a flavonoid from licorice roots and utilized in traditional Chinese medicine, has demonstrated neuroprotective effects against Aβ toxicity, linked to Alzheimer's disease, by modulating NRF2-related antioxidative pathways and activating CREB-dependent survival pathways (Lee et al. 2018). Lico-A has also been shown to lower reactive oxygen species (ROS) and malondialdehyde (MDA) levels and inhibit pro-inflammatory cytokines like TNF-α and IL-6, exhibiting antioxidant and anti-inflammatory properties (Liu et al. 2018). Lico-A’s neuroprotective potential in disorders such as Alzheimer's and Parkinson's has been increasingly validated (Busquets et al. 2018; Olloquequi et al. 2023) suggesting its beneficial role in hippocampal neurons.
Our study induced neurotoxicity through the activation of NMDA receptors (Simões et al. 2018), associated with an increase in intracellular calcium ion concentrations (Ndountse and Chan 2009). The activation of calcium-dependent enzymes, like proteases and phospholipases, leads to morphological alterations in the cytoskeleton and breaches in cellular integrity, ultimately resulting in apoptosis and necroptosis (Fan and Raymond 2007; Cheng et al. 2018). This phenomenon, known as excitotoxicity, is implicated in the development of various central nervous system neurodegenerative disorders (Sims and Zaidan 1995)
Furthermore, this study examined the synapto-protective effects of Lico-A against NMDA-induced synaptic impairment. Building on previous studies focusing on neurodegenerative diseases, particularly epilepsy (Busquets et al. 2018; Olloquequi et al. 2023), we employed NMDA as an excitotoxic agent (Thom et al. 2010) and observed Lico-A’s protective impact on neuronal cells. Moreover, the study quantitatively examined changes in synaptic puncta targeting PSD-95, crucial for the stability of excitatory synapses. PSD-95 is vital for synaptic plasticity (Béïque and Andrade 2003) and its diminished expression is associated with neurodegenerative conditions(Catts et al. 2015). The observed reduction in PSD-95 positive synaptic puncta due to NMDA and their subsequent restoration by Lico-A treatment suggest that Lico-A can mitigate synaptic damage and modulate functional impairment in hippocampal neurons (Figure 2). Given the limited regenerative capacity of the central nervous system compared to the peripheral nervous system (Huebner and Strittmatter 2009), the influence of Lico-A on PSD-95 in hippocampal neurons implies its potential to suppress the processes leading to synaptic damage caused by NMDA-induced neurotoxicity, thereby controlling functional impairment of neurons at an early stage.
NMDA-induced necroptosis (Liu et al. 2014) represents a critical mechanism often utilized in models of epilepsy and seizures, where excitotoxicity plays a predominant role (Choi et al. 2021). Excitotoxicity and necroptosis, though distinct processes, may converge under certain conditions, particularly in neuronal disorders and brain damage. Overactivation of glutamate receptors and resulting cellular stress can activate necroptosis-related intracellular signaling pathways. The core receptors of necroptosis, RIPK1 and RIPK3, can be triggered by cell stress due to excitotoxicity, leading to membrane rupture and release of cellular contents. This may result in the discharge of damage-associated molecular patterns (DAMPs) and pro-inflammatory cytokines, creating an inflammatory milieu that amplifies cellular damage. Thus, both processes are related to cell death, and their interplay can contribute to the progression of various pathological conditions.
Furthermore, our study explored the processes of necroptosis and glial activation in hippocampal neurons. Glial activation, marked by increased activity of glial cell in response to central nervous system damage, often triggers the release of inflammatory cytokines, such as TNF-α. This can initiate necroptotic pathways involving RIPK1 and RIPK3. This increased glial activation and subsequent necroptosis contribute to chronic neuroinflammation, which further damages neural tissues due to continuous inflammatory signaling and cell death.
Our findings underscore Lico-A's efficacy in mitigating these processes in hippocampal neurons exposed to NMDA-induced neurotoxicity. By reducing glial activation and the release of TNF-α, Lico-A disrupts the necroptotic signaling pathway, thereby preserving neuronal viability. Utilizing GFAP and IBA-1 as markers, we noted that Lico-A mitigated the cellular activation and increased intensity caused by NMDA (Figure 3). Furthermore, the ability of Lico-A to decrease phosphorylated MLKL and RIP3 levels (Figure 4) highlights its potential to inhibit necroptosis. These results underscore Lico-A's potential as a natural substance in aiding the viability of hippocampal neurons in various neurodegenerative `diseases. This suggests that Lico-A might serve as a promising therapeutic agent in neurodegenerative diseases, highlighting the need for further research in this area.
Acknowledgements
The authors would like to thank Geum Pyo Hong for his technical support.
Funding Statement
This study was supported by the National Research Foundation of Korea (NRF) (NRF-2019M3C7A1031455 and NRF-2022R1F1A1075083).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Anand KS, Dhikav V.. 2012. Hippocampus in health and disease: An overview. Ann Indian Acad Neurol. 15:239–246. doi: 10.4103/0972-2327.104323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béïque JC, Andrade R.. 2003. PSD-95 regulates synaptic transmission and plasticity in rat cerebral cortex. J Physiol. 546:859–867. doi: 10.1113/jphysiol.2002.031369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilge S. 2022. Neurotoxicity, types, clinical manifestations, diagnosis and treatment. In Neurotoxicity-New Advances; IntechOpen: London, UK. doi: 10.5772/intechopen.101737. [DOI] [Google Scholar]
- Busquets O, Ettcheto M, Verdaguer E, Castro-Torres RD, Auladell C, Beas-Zarate C, Folch J, Camins A.. 2018. JNK1 inhibition by Licochalcone A leads to neuronal protection against excitotoxic insults derived of kainic acid. Neuropharmacology. 131:440–452. doi: 10.1016/j.neuropharm.2017.10.030. [DOI] [PubMed] [Google Scholar]
- Catts VS, Derminio DS, Hahn C-G, Weickert CS.. 2015. Postsynaptic density levels of the NMDA receptor NR1 subunit and PSD-95 protein in prefrontal cortex from people with schizophrenia. NPJ Schizophr. 1:1–8. doi: 10.1038/npjschz.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Liu Z, Meng R, Shi C, Guo N.. 2017. Antioxidative and anticancer properties of Licochalcone A from licorice. J Ethnopharmacol. 198:331–337. doi: 10.1016/j.jep.2017.01.028. [DOI] [PubMed] [Google Scholar]
- Cheng S-y, Wang S-c, Lei M, Wang Z, Xiong K.. 2018. Regulatory role of calpain in neuronal death. Neural Regen Res. 13:556. doi: 10.4103/1673-5374.228762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I-Y, Shim JH, Kim M-H, Yu WD, Kim YJ, Choi G, Lee JH, Kim HJ, Cho K-O.. 2021. Truncated Neogenin promotes hippocampal neuronal death after acute seizure. Neuroscience. 470:78–87. doi: 10.1016/j.neuroscience.2021.06.039. [DOI] [PubMed] [Google Scholar]
- Coley AA, Gao W-J.. 2018. PSD95: A synaptic protein implicated in schizophrenia or autism? Prog Neuro-Psychopharmacolog Biol Psychiatry. 82:187–194. doi: 10.1016/j.pnpbp.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X-x, Wang Y, Qin Z-h.. 2009. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 30:379–387. doi: 10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Tang H-B, Shan L-Q, Liu S-C, Huang D-G, Chen X, Chen Z, Yang M, Yin X-H, Yang H.. 2019. Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats. J Neuroinflammation. 16:1–15. doi: 10.1186/s12974-018-1391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan MM, Raymond LA.. 2007. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington's disease. Prog Neurobiol. 81:272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Chan FK-M, Kroemer G.. 2017. Necroptosis: mechanisms and relevance to disease. Annu Rev Pathol: Mech Dis. 12:103–130. doi: 10.1146/annurev-pathol-052016-100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong N, Park J-S, Kim HJ.. 2022. Synapto-protective effect of lithium on HIV-1 Tat-induced synapse loss in rat hippocampal cultures. Animal Cells Syst. 26:1–9. doi: 10.1080/19768354.2021.2018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Pan H, Peng J, He J, Tang M, Yan S, Rong J, Li J, Zheng Z, Wang H.. 2021. Resveratrol inhibits necroptosis by mediating the TNF-α/RIP1/RIP3/MLKL pathway in myocardial hypoxia/reoxygenation injury. Acta Biochim Biophys Sin. 53:430–437. doi: 10.1093/abbs/gmab012. [DOI] [PubMed] [Google Scholar]
- Huebner EA, Strittmatter SM.. 2009. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ. 48:339–351. doi: 10.1007/400_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Thayer SA.. 2009. Lithium increases synapse formation between hippocampal neurons by depleting phosphoinositides. Mol Pharmacol. 75:1021–1030. doi: 10.1124/mol.108.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-J, Lee SH, Do Quang B, Tran T-T, Kim Y-G, Ko J, Choi W-Y, SY L, Ryu J-H.. 2023. Avenanthramide-C shows potential to Alleviate Gingival Inflammation and Alveolar Bone Loss in experimental periodontitis. Mol Cells. 46:627–636. doi: 10.14348/molcells.2023.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau H-C, Schenker LT, Kennedy MB, Seeburg PH.. 1995. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Lee SY, Chiu YJ, Yang SM, Chen CM, Huang CC, Lee-Chen GJ, Lin W, Chang KH.. 2018. Novel synthetic chalcone-coumarin hybrid for Aβ aggregation reduction, antioxidation, and neuroprotection. CNS Neurosci Ther. 24:1286–1298. doi: 10.1111/cns.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-J, Park K-S, Lee S-H.. 2021. Curcumin targets both apoptosis and necroptosis in acidity-tolerant prostate carcinoma cells. BioMed Res Int. 2021:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-J, Park K-S, Nam H-S, Cho M-K, Lee S-H.. 2020. Apigenin causes necroptosis by inducing ROS accumulation, mitochondrial dysfunction, and ATP depletion in malignant mesothelioma cells. The Korean J Physiol Pharmacol: Official J Korean Physiol Soc Korean Soc Pharmacol. 24:493–502. doi: 10.4196/kjpp.2020.24.6.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Wang X, Li Y, Xu L, Yu X, Ge L, Li J, Zhu Y, He S.. 2014. Necroptosis mediates TNF-induced toxicity of hippocampal neurons. BioMed Res Int. 2014:290182. doi: 10.1155/2014/290182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ma Y, Wei X, Fan T.. 2018. Neuroprotective effect of licochalcone A against oxygen-glucose deprivation/reperfusion in rat primary cortical neurons by attenuating oxidative stress injury and inflammatory response via the SIRT1/Nrf2 pathway. J Cell Biochem. 119:3210–3219. doi: 10.1002/jcb.26477. [DOI] [PubMed] [Google Scholar]
- Machin A, Purwanto DA, Sugianto P, Subadi I, Susilo I, Ardianto C, Hidayati AN, Utomo B.. 2020. Camellia sinensis with its active compound EGCG can decrease necroptosis via inhibition of HO-1 expression. EurAsian J BioSciences. 14:1813–1820. [Google Scholar]
- Ndountse LT, Chan HM.. 2009. Role of N-methyl-D-aspartate receptors in polychlorinated biphenyl mediated neurotoxicity. Toxicol Lett. 184:50–55. doi: 10.1016/j.toxlet.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Olloquequi J, Ettcheto M, Cano A, Fortuna A, Bicker J, Sánchez-Lopez E, Paz C, Ureña J, Verdaguer E, Auladell C.. 2023. Licochalcone A: a potential multitarget drug for Alzheimer’s disease treatment. Int J Mol Sci. 24:14177. doi: 10.3390/ijms241814177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DC, Tewari BP, Chaunsali L, Sontheimer H.. 2019. Neuron–glia interactions in the pathophysiology of epilepsy. Nat Rev Neurosci. 20:282–297. doi: 10.1038/s41583-019-0126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Pekna M.. 2016. Reactive gliosis in the pathogenesis of CNS diseases. Biochimica et Biophysica Acta (BBA)-Molecular Basis Dis. 1862:483–491. doi: 10.1016/j.bbadis.2015.11.014. [DOI] [PubMed] [Google Scholar]
- Porter NM, Thibault O, Thibault V, Chen K-C, Landfield PW.. 1997. Calcium channel density and hippocampal cell death with age in long-term culture. J Neurosci. 17:5629–5639. doi: 10.1523/JNEUROSCI.17-14-05629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh Y, Lee SB, Kim M, Kim M-H, Kim HJ, Cho K-O.. 2023. Alleviation of hippocampal necroptosis and neuroinflammation by NecroX-7 treatment after acute seizures. Front Pharmacol. 14:1187819. doi: 10.3389/fphar.2023.1187819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões AP, Silva CG, Marques JM, Pochmann D, Porciúncula LO, Ferreira S, Oses JP, Beleza RO, Real JI, Köfalvi A.. 2018. Glutamate-induced and NMDA receptor-mediated neurodegeneration entails P2Y1 receptor activation. Cell Death Dis. 9:297. doi: 10.1038/s41419-018-0351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims NR, Zaidan E.. 1995. Biochemical changes associated with selective neuronal death following short-term cerebral ischaemia. Int J Biochem Cell Biol. 27:531–550. doi: 10.1016/1357-2725(95)00026-L. [DOI] [PubMed] [Google Scholar]
- Terreros-Roncal J, Moreno-Jiménez E, Flor-García M, Rodríguez-Moreno C, Trinchero MF, Cafini F, Rábano A, Llorens-Martín M.. 2021. Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science. 374:1106–1113. doi: 10.1126/science.abl5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, Mathern GW, Cross JH, Bertram EH.. 2010. Mesial temporal lobe epilepsy: How do we improve surgical outcome? Ann Neurol. 68:424–434. doi: 10.1002/ana.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bosch L, Van Damme P, Bogaert E, Robberecht W.. 2006. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochimica et Biophysica Acta (BBA)-Molecular Basis Dis. 1762:1068–1082. doi: 10.1016/j.bbadis.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Yun E, Kwon BS, Kim J, Lee A.. 2023. Ginsenoside Rg3 attenuates pulmonary fibrosis by inhibiting endothelial to mesenchymal transition. Animal Cells Syst. 27:159–170. doi: 10.1080/19768354.2023.2244549. [DOI] [PMC free article] [PubMed] [Google Scholar]