Abstract
Background
To quantitatively evaluate the effect of coenzyme Q10 (CoQ10) pretreatment on outcomes of IVF or ICSI in women with diminished ovarian reserve (DOR) based on the existing randomized controlled trials (RCTs).
Methods
Nine databases were comprehensively searched from database inception to November 01, 2023, to identify eligible RCTs. Reproductive outcomes of interest consisted of three primary outcomes and six secondary outcomes. The sensitivity analysis was adopted to verify the robustness of pooled results.
Results
There were six RCTs in total, which collectively involved 1529 participants with DOR receiving infertility treatment with IVF/ICSI. The review of available evidence suggested that CoQ10 pretreatment was significantly correlated with elevated clinical pregnancy rate (OR = 1.84, 95%CI [1.33, 2.53], p = 0.0002), number of optimal embryos (OR = 0.59, 95%CI [0.21, 0.96], p = 0.002), number of oocytes retrieved (MD = 1.30, 95%CI [1.21, 1.40], p < 0.00001), and E2 levels on the day of hCG (SMD = 0.37, 95%CI [0.07, 0.66], p = 0.01), along with a reduction in cycle cancellation rate (OR = 0.60, 95%CI [0.44, 0.83], p = 0.002), miscarriage rate (OR = 0.38, 95%CI [0.15, 0.98], p = 0.05), total days of Gn applied (MD = −0.89, 95%CI [–1.37, −0.41], p = 0.0003), and total dose of Gn used (MD = −330.44, 95%CI [–373.93, −286.96], p < 0.00001). The sensitivity analysis indicated that our pooled results were robust.
Conclusions
These findings suggested that CoQ10 pretreatment is an effective intervention in improving IVF/ICSI outcomes for women with DOR. Still, this meta-analysis included relatively limited sample sizes with poor descriptions of their methodologies. Rigorously conducted trials are needed in the future.
Keywords: Coenzyme Q10, diminished ovarian reserve, IVF, ICSI, meta-analysis
Introduction
Infertility is a severe health concern affecting one in eight reproductive age women, translating to an estimated 12.7% of the women population in the US [1]. The incidence of infertility was 25% in China, and the number of infertility cases increased substantially with age [2]. The causes of female infertility are highly complex, including diminished ovarian reserve (DOR), ovulation disorders, tubal diseases, endometriosis, and so forth [1, 3]. Notably, DOR, one of the highest frequency of known factors contributing to female infertility, is characterized by a decrease in the quantity and quality of oocytes, accompanied by an increase in follicle stimulation hormone (FSH) as well as a decrease in antral follicle count (AFC) and anti-müllerian hormone (AMH) [4]. Indeed, 19% to 26% of women undergoing assisted reproductive technology (ART) were diagnosed with DOR [5]. Moreover, there was considerable evidence demonstrating that women with DOR have poor reproductive outcomes, such as reduced euploid rates, lower probability of pregnancy, higher incidence of cycle cancellation rate and poor ovarian response during in-vitro fertilization (IVF) [4, 6–8]. Thereby, DOR is one of the serious clinical challenging problems for IVF specialists.
However, although diverse ovarian stimulation protocols have been explored to ameliorate the outcomes of IVF for women with DOR, including progestin-primed ovarian stimulation (PPOS) protocols, minimal stimulation protocols, gonadotropin-releasing hormone (GnRH) antagonist protocols, short GnRH agonist protocols, and long GnRH agonist protocols, most of them often generate unsatisfying results. For example, the PPOS protocols introduced to clinical practice several years ago had been proved that it lacked sufficient effects on increasing the number of oocytes retrieved, live birth rate, cleavage rate, fertilization rate, and E2 levels on the day of hCG, but also rose the total dose of gonadotropin (Gn) applied, which was considered as a potential threat of ovarian hyperstimulation syndrome (OHSS) [9]. Besides, GnRH antagonist protocols in IVF cycles might be correlated with thinner endometrium, fewer oocytes retrieved, and lower E2 levels compared to GnRH agonist protocols [10]. Simultaneously, GnRH antagonist protocol had negative effects on endometrial receptivity since it could increase uterine tumour necrosis factor α (TNF-α) as well as natural killer cells in IVF cycles [11]. Moreover, a meta-analysis involving 3872 women suggested that 9% of women receiving short GnRH agonist protocols experienced cycle cancellation, while 5.5% and 14% of female also suffered from that with the use of long protocol [12]. Hence, over the past few years, pretreatment approaches before ovarian stimulation have attracted lots of clinicians.
Coenzyme Q10 (CoQ10), the third most consumed nutritional supplement, has strong antioxidant and pro-oxidant activity [13]. Therefore, insufficient CoQ10 levels are associated with increased oxidative stress, mitochondrial damage, lower reactive oxygen species (ROS) counteraction, less adenosine triphosphate (ATP) synthesis, and subsequent mitochondrial dysfunction [14]. In infertility treatments, CoQ10 supplementation may significantly ameliorate women’s oocyte quality, and alter ovarian environment, along with promote oocyte development by enhancing mitochondrial function and decreasing oxidative stress [14]. Recently, a prospective, randomized controlled trial (RCT) reported that women with DOR undergoing IVF-intracytoplasmic sperm injection (ICSI) achieved more high-quality embryos, higher fertilization rate, and increased number of retrieved oocytes with CoQ10 pretreatment [15]. In addition, a retrospective study enrolling 797 cycles indicated that the combined pretreatment of CoQ10 and dehydroepiandrosterone (DHEA) in women with DOR resulted in more follicles and lower dose of Gn applied, but it didn’t improve the total number of embryos developed, the number of fertilization and retrieved oocytes compared with DHEA alone [16]. Given the fact that the inconsistent conclusions from previous studies might be evaluated insufficiently since their results were derived from a single center study, and their sample sizes were relatively small. Therefore, we conducted this meta-analysis to quantitatively summarize the available evidence and provide an evidence-based reference. The study’s specific concern was as follows: Does CoQ10 pretreatment ameliorate the reproductive outcomes in women with DOR undergoing IVF or ICSI?
Materials and methods
This study (PROSPERO registration No. CRD42023468370) was conducted following the preferred reporting program of the systematic review and meta-analysis (PRISMA) [17].
Search strategy
A thorough search of the following nine databases from their inception to November 01, 2023, was performed: Scopus, PubMed, Cochrane Library, Web of Science, Sinomed, EBSCO, Wanfang, VIP Information, and China National Knowledge Infrastructure (CNKI). The search strategy consisted of three components: clinical condition (decreased ovarian reserve, declined ovarian reserve, low ovarian reserve, and diminished ovarian reserve); intervention (IVF, ICSI, and assisted reproduction technology), and study type (randomized clinical trial). All records were limited to Chinese and English languages. Two independent researchers (G.Y.L and X.L.L) checked all retrieved documents for eligibility by examining the titles, abstracts, and full texts of all relevant articles. In addition, reference lists of included records were also evaluated manually to identify additional studies as much as possible. Any discrepancies were addressed by the corresponding author (L.W.X) if necessary.
Inclusion and exclusion criteria
Inclusion criteria were as follows: I, women diagnosed with DOR(AFC < 5 ∼ 7 or FSH ≥ 10 IU/L or AMH < 1.1 ng/mL) [18]; II, participants used CoQ10 pretreatment as intervention regardless of treatment duration; III, women underwent IVF or ICSI; IV, study was an RCT; V, study provided the diagnostic criteria for DOR and reported reproductive outcomes with sufficient data. If CoQ10 was utilized as a pretreatment in IVF or ICSI and the same concomitant treatment (e.g. Vitamin E, and Femoston) as the trial group was adopted by the control group, the records would be included as well.
The exclusion criteria were: I, participants incorporated with abnormal endometrium, reproductive tumors, intrauterine adhesion, polycystic ovary syndrome (PCOS), chromosomal abnormalities, and uterine malformation; II, meta-analyses, duplicate publications, reviews, animal experiments, and case reports; III, study not reported in English or Chinese.
Data extraction and quality assessment
Data were extracted from included articles by two independent researchers (G.Y.L. and X.L.L.) using standardized forms. The characteristics of these records that potentially correlated with the outcomes were recorded as follows: methodological features, first author, publication year, population baseline characteristics, details of the pretreatment strategy, protocol of control ovarian stimulation, and outcomes in each group. Two independent reviewers (G.Y.L. and X.L.L.) stringently evaluated the risk of bias in the included studies with the help of the Cochrane Collaboration Handbook (http://handbook.cochrane.org). Each study was defined as “unclear”, “high”, or “low” risk of bias within each domain. Disagreements were resolved by seeking an opinion from the corresponding author (L.W.X).
The primary outcome measures were clinical pregnancy rate (definitive clinical signs of pregnancy or a pregnancy examined by ultrasonography visualization of one or more gestational sacs [19]), the number of optimal embryos (the number of blastomeres > 6, even symmetry in shape, and the rate of fragmentation < 20% in day 3 [20]), and cycle cancellation rate.
The secondary outcomes were miscarriage rate (the spontaneous loss of a pregnancy before 20 weeks of gestation), the number of oocytes retrieved, E2 levels on the day of hCG, endometrial thickness on the day of hCG, total days of Gn applied, and total dose of Gn applied.
Statistical analysis
Statistical analyses were carried out using Revman software 5.3. Dichotomous variables were estimated with odds ratios (ORs) and corresponding 95% confidence intervals (CIs). Continuous results were assessed with mean difference (MD) or standardized mean differences (SMDs) with 95% CIs. Heterogeneity among studies was measured with the I2 statistic. I2 = 0% revealed no heterogeneity and the fixed effect model was carried out. Otherwise, the appropriate random effect model was performed [21]. p value ≤ 0.05 illustrated a beneficial effect of CoQ10 pretreatment on reproductive outcomes compared to the control group. Further, the sensitivity analysis was also implemented to verify the robustness of the pooled results via omitting individual studies.
Results
Included articles
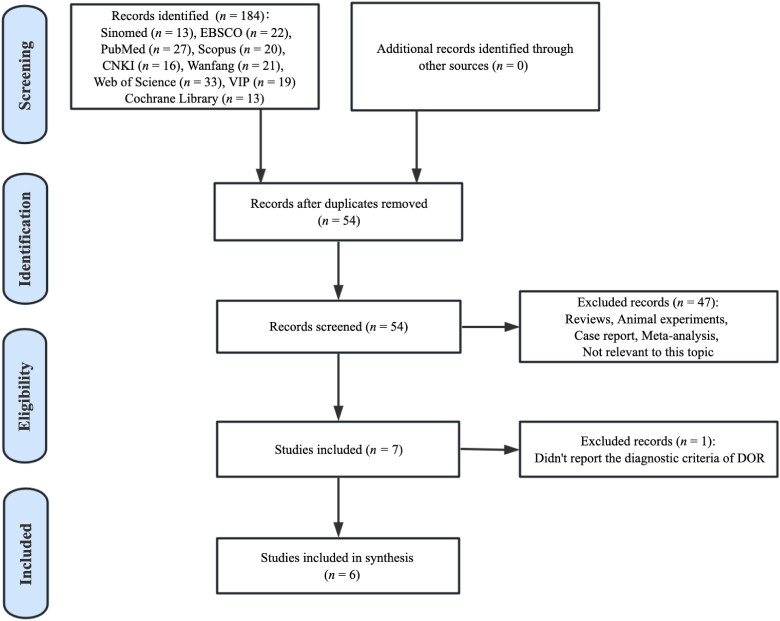
This meta-analysis retrieved 184 potentially relevant records. By removing duplicates, 54 articles were included. After screening the title and abstract, 47 were excluded since they did not meet the inclusion criteria. Then, after full-text assessment, one publication was removed because it did not state the diagnostic criteria of DOR [16]. Eventually, six RCTs were included in the meta-analysis for quantitative synthesis (Figure 1).
Figure 1.
Flow diagram of study selection.
Study characteristics
In total, 1529 women with DOR undergoing IVF/ICSI from six studies were randomized, of which 761 were allocated to the CoQ10 pretreatment group and 768 to the control group. All included studies were conducted in China and published between 2017 and 2023. The sample sizes in each study ranged from 50 to 900. Four studies reported full baseline characteristics (e.g. age, duration of infertility, and BMI) of the participants in both groups [15, 22–24]. Two studies compared the value of CoQ10 plus DHEA with DHEA [23, 25]; one study compared the effects of CoQ10 plus femoston with femoston [26]; two studies compared the effects of CoQ10 plus Vitamin E with Vitamin E [22, 24]; and one study compared the effects of CoQ10 with no treatment [15]. Besides, in women with DOR, the ovarian stimulation protocol varied between GnRH antagonist protocol and PPOS protocol. With respect to the duration of pretreatment, five records [22–25] applied CoQ10 combined with Vitamin E, DHEA or femoston for three months before receiving ovarian stimulation, and one study [15] used CoQ10 alone for 60 days (Table 1).
Table 1.
Study characteristics.
| Study | Year | Sample size (n) | Age (year) |
Duration of infertility (year) |
BMI (kg/m2) |
Pretreatment regimen |
Stimulation protocol | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T/C | T | C | T | C | T | C | T | C | ||||
| Xia [23] | 2023 | 65/65 | 37.32 ± 5.51 | 36.69 ± 5.91 | 5.42 ± 4.91 | 4.92 ± 4.83 | 22.90 ± 3.46 | 23.59 ± 3.42 | Q10 + DHEA | DHEA | GnRH antagonist protocol | ①③④⑤⑥⑦⑧⑨ |
| Xu [22] | 2023 | 450/450 | 40.84 ± 3.07 | 40.63 ± 3.62 | 4.59 ± 1.03 | 4.63 ± 0.95 | 22.73 ± 2.61 | 22.80 ± 2.24 | Q10 + Vitamin E | VitaminE | PPOS | ①②③④⑧⑨ |
| Tang [25] | 2022 | 102/92 | 38.2 ± 0.7 | 37.9 ± 0.8 | 8.0 ± 0.5 | 8.3 ± 0.4 | NA | NA | Q10 + DHEA | DHEA | PPOS | ①②④⑤⑧⑨ |
| Liang [26] | 2019 | 43/43 | 35.42 ± 1.93 | 35.78 ± 1.86 | 5.81 ± 1.23 | 5.69 ± 1.18 | NA | NA | Q10 + Femoston | Femoston | GnRH antagonist protocol | ①②③⑧⑨ |
| Xu [15] | 2018 | 76/93 | 32.50 ± 3.30 | 31.92 ± 3.68 | 3 (2, 4) | 3 (2, 3) | 21.85 ± 2.51 | 22.24 ± 3.07 | Q10 | None | GnRH antagonist protocol | ①②③④⑤ ⑥⑦⑧⑨ |
| Zou [24] | 2017 | 25/25 | 35-40 | 35-40 | 4.6 ± 3.8 | 4.7 ± 2.8 | 21.6 ± 3.2 | 21.0 ± 3.8 | Q10 + Vitamin E | VitaminE | PPOS | ①②③⑥⑦⑧⑨ |
T: trial group; C: control group; BMI: body mass index; NA: not available; Q10: coenzyme Q10; DHEA: dehydroepiandrosterone; GnRH: gonadotropin-releasing hormone; PPOS: progestin-primed ovarian stimulation; ① Clinical pregnancy rate; ② The number of oocytes retrieved; ③ The number of optimal embryos; ④ Cycle cancellation rate; ⑤ Miscarriage rate; ⑥ E2 levels on the day of hCG; ⑦ Endometrial thickness on the day of hCG; ⑧ Total days of Gn applied; ⑨ Total dose of Gn applied.
Risk of bias
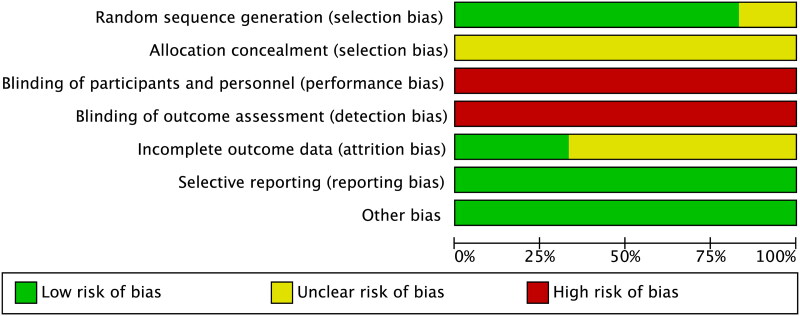
In evaluating the risk of bias of the evidence, we completely followed the instructions of the Cochrane Collaboration Handbook. First, five articles clearly stated how participants were randomized, thus they were rated as a low risk in the domain of random sequence generation. Still, one study was judged as an unclear risk in this field since they didn’t report how random sequence was generated. Second, all included studies failed to provide the details on the allocation concealment, which was considered as an unclear risk in relation to this field. Third, six studies didn’t adopt blinding methods thoroughout their studies, thereby leading to a high risk rating. Fourth, selective reporting and other potential biases were not detected across studies, and thus it was rated as low risk (Figure 2).
Figure 2.
Risk of bias assessment.
Primary outcome measurements
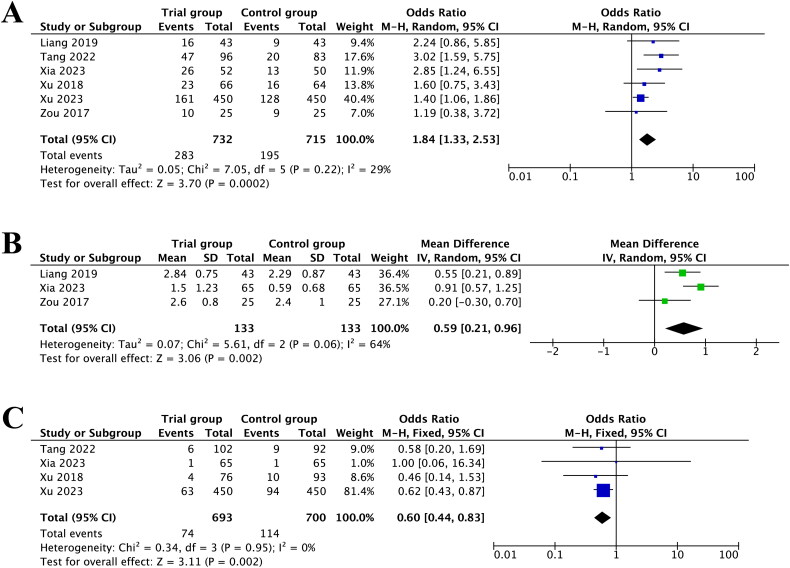
The forest plot of intervention effects on IVF/ICSI outcomes suggested that CoQ10 pretreatment was efficacious in improving clinical pregnancy rate (OR = 1.84, 95%CI [1.33, 2.53], p = 0.0002, I2 = 29%) (Figure 3A). The sensitivity analysis suggested that the results were robust.
Figure 3.
Forest plot of studies evaluating clinical pregnancy rate (A); the number of optimal embryos (B); cycle cancellation rate (C).
Furthermore, with respect to the number of optimal embryos, after removing one study [22] via sensitivity analysis, the heterogeneity reduced from 91% to 64%. The pooled results demonstrated that pretreatment with CoQ10 was significantly associated with higher number of optimal embryos compared to control group (MD = 0.59, 95%CI [0.21, 0.96], p = 0.002, I2 = 64%) (Figure 3B).
In addition, four studies reported the cycle cancellation rate. The pooled results proved that participants with DOR undergoing IVF/ICSI with CoQ10 pretreatment had lower cycle cancellation rate (OR = 0.60, 95%CI [0.44, 0.83], p = 0.002, I2 = 0%) (Figure 3C) than women in the control group. The sensitivity analyses illustrated that there was no individual study impacting the pooled estimates.
The results above are generalized in Table 2.
Table 2.
The pooled results of forest plot for clinical outcomes.
| Clinical outcomes | Participants (n) | OR/SMD/MD 95% CI | p | I2 (%) | Model |
|---|---|---|---|---|---|
| Clinical pregnancy rate | 1447 | 1.84 [1.33, 2.53] | 0.0002 | 29 | Random |
| The number optimal embryos | 266 | 0.59 [0.21, 0.96] | 0.002 | 64 | Random |
| Cycle cancellation rate | 1393 | 0.60 [0.44, 0.83] | 0.002 | 0 | Fixed |
| Miscarriage rate | 145 | 0.38 [0.15, 0.98] | 0.05 | 0 | Fixed |
| The number of oocytes retrieved | 330 | 1.30 [1.21, 1.40] | < 0.00001 | 0 | Fixed |
| E2 levels on the day of hCG | 180 | 0.37 [0.07, 0.66] | 0.01 | 0 | Fixed |
| Endometrial thickness on the day of hCG | 349 | 0.53 [-0.23, 1.29] | 0.17 | 91 | Random |
| Total days of Gn used | 1166 | −0.89 [-1.37, −0.41] | 0.0003 | 47 | Random |
| Total dose of Gn used | 1360 | −330.44 [-373.93, −286.96] | < 0.00001 | 28 | Random |
Secondary outcome measurements
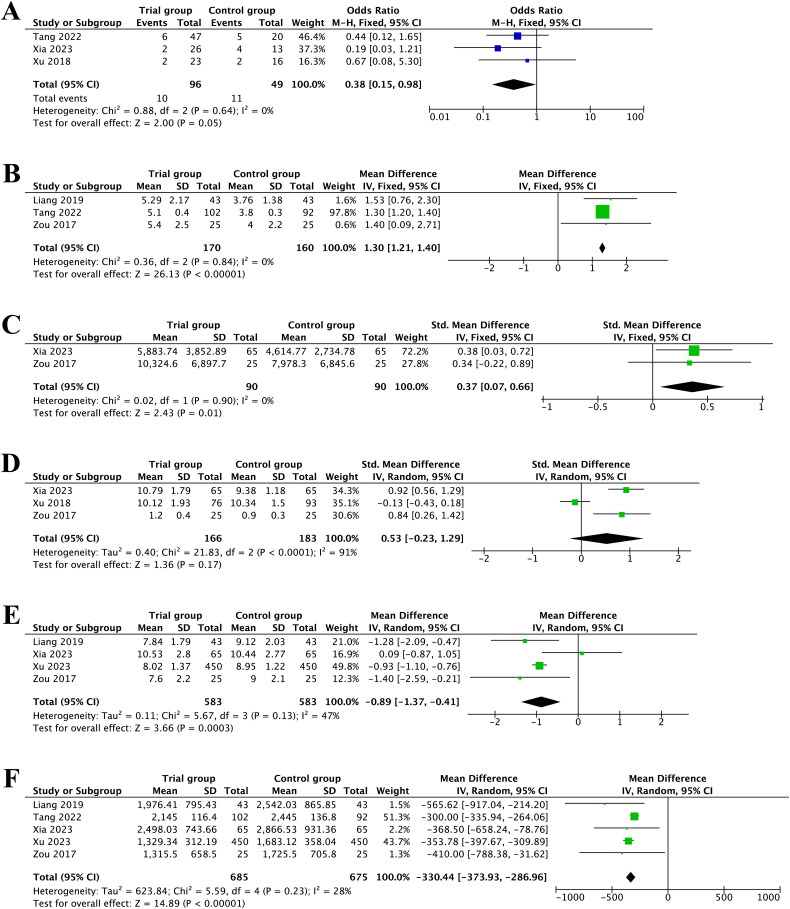
Three studies recorded miscarriage rate, and the pooled results implied that CoQ10 pretreatment had lower miscarriage rate (OR = 0.38, 95%CI [0.15, 0.98], p = 0.05, I2 = 0%) (Figure 4A).
Figure 4.
Forest plot of studies evaluating miscarriage rate (A); the number of oocytes retrieved (B); E2 levels on the day of hCG (C); endometrial thickness on the day of hCG (D); total days of Gn used (E); total dose of Gn used (F).
Besides, four studies investigated the number of oocytes retrieved. After omitting one study [22] by sensitivity analysis, the heterogeneity diminished from 96% to 0%. Subsequently, the pooled result from three studies comprising 330 participants revealed a remarkable increase in the number of oocytes retrieved with CoQ10 pretreatment (MD = 1.30, 95%CI [1.21, 1.40], p < 0.00001, I2 = 0%) (Figure 4B).
Two studies measured the effects of CoQ10 pretreatment on the E2 levels on the day of hCG. A statistically significant effect of pretreatment with CoQ10 on the E2 levels on the day of hCG was observed (SMD = 0.37, 95%CI [0.07, 0.66], p = 0.01, I2 = 0%) (Figure 4C).
A total of three studies involving 349 participants examined the impact of CoQ10 pretreatment on endometrial thickness on the day of hCG. The results of this analysis illustrated that the endometrial thickness wasn’t improved in the CoQ10 group relative to the control group (SMD = 0.53, 95%CI [–0.23, 1.29], p = 0.17, I2 = 91%) (Figure 4D).
Moreover, five studies evaluated the total days of Gn applied. After excluding one study [25] according to sensitivity analysis, the heterogeneity reduced from 77% to 47%. It was noteworthy that CoQ10 pretreatment had a beneficial effect on shortening the total days of Gn application (MD = −0.89, 95%CI [–1.37, −0.41], p = 0.0003, I2 = 47%) (Figure 4E).
Additionally, the results based on five RCTs revealed that CoQ10 pretreatment was closely correlated with a reduction in the total dose of Gn applied (MD = −330.44, 95%CI [–373.93, −286.96], p < 0.00001, I2 = 28%) (Figure 4F).
The overall results above are summarized in Table 2.
Discussion
CoQ10 pretreatment before ovarian stimulation has been recognized as a beneficial strategy for ameliorating the outcomes of IVF/ICSI in infertile women [27, 28]. The therapeutic mechanisms of CoQ10 in the reproductive field are rather sophisticated. A growing body of studies proved that CoQ10 could promote embryo development and increase the rates of fertilization by improving the mRNA expression of FSHR and PCNA and reducing ROS levels in the ovary [29]. Besides, the expression of apoptosis-related genes Caspase 3, Bax, HSD11B1, and FKBP5 in oocytes could be significantly decreased with the help of CoQ10 supplementation, which was closely associated with the developmental competence of an embryo [30, 31]. A recent study based on network pharmacology indicated that CoQ10 might exert its oocyte quality improving influence through multiple mechanisms, including FoxO, IL-1, MAPK, and Jak-STAT signaling pathways [32]. Furthermore, another study also found that CoQ10 contributed to improving fertility and oocyte aging resistance by restoring chromosome alignment, mitochondrial distribution, and spindle formation, together with increasing the expression of Sod1, Smarca2, Sdha, and Nduf3 in oocytes [33]. Moreover, some investigations also found that CoQ10 could significantly reduce spontaneous abortion via declining the levels of ROS, IFN-γ, TNF-α, and altering T cell subpopulation ratios in peripheral blood [34]. Notably, a recent study suggested that chromosome segregation defects at early embryogenesis and chromosome morphology during diakinesis could be rescued with CoQ10 supplementation via modifying the levels of DSB formation and improving DNA damage in oocytes and embryos [35]. Further elucidation is needed to clarify the underlying mechanisms by which CoQ10 contributes to infertility correlated with DOR.
This meta-analysis provides some findings that CoQ10 administration before an ART cycle was associated with an increase in clinical pregnancy rate, the number of oocytes retrieved, and the number of optimal embryos, along with a decrease in cycle cancellation rate, which is the most vital concern for infertile women undergoing ART. Moreover, CoQ10 pretreatment was more beneficial in ameliorating the E2 levels on the day of hCG, which might be an effective therapeutic choice for women with low E2 levels during ovarian stimulation. Besides, mounting research had found that women with DOR have a higher risk of miscarriage, which remains an ongoing clinical challenge in the reproductive field [36, 37]. In this meta-analysis, we found that oral administration of CoQ10 before ART resulted in a significant reduction in miscarriage rate when compared with control or no-treatment groups. Meanwhile, the incidence of OHSS, one of the serious complications during ovarian stimulation, was positively correlated with the dosage of Gn applied [38, 39]. Therefore, clinicians always strive to lower the risk of OHSS as much as possible. Our study demonstrated that the total days and total dose of Gn used in women with DOR undergoing IVF/ICSI could be remarkably reduced with the help of CoQ10 pretreatment. Notably, although the dosage of Gn was reduced, the outcomes of IVF/ICSI weren’t lowered. Briefly, we consider CoQ10 pretreatment to be a valuable option for women with DOR, based on the pooled evidence above. Additionally, other nutraceutical supplementation such as inositols, alpha-lipoic acid, vitamin D, and metformin have also been proved in improving ovarian reserve and infertility via recovering metabolic disturbances as well as modulating endocrine parameters [40–44]. Hence, it definitely throws light on exploring the clinical value between nutraceutical supplementation and the outcomes of ART in the future.
A previous meta-analysis [45] focusing on CoQ10 for women undergoing ART suggested that CoQ10 supplementation is beneficial in ameliorating clinical pregnancy rate for infertile patients with PCOS or poor ovarian response (POR). While there were some differences between their study and this meta-analysis: First, the prior study without a registered protocol solely searched three databases before 2020 and included five studies with 449 participants, while our meta-analysis comprehensively screened nine databases based on our registered protocol and yielded six RCTs involving 1529 women. Second, the previous study only qualitatively evaluated limited outcomes such as live birth rate, clinical pregnancy rate, and miscarriage rate. Whereas we investigated three kinds of primary outcomes (clinical pregnancy rate, the number of optimal embryos, and cycle cancellation rate) and six kinds of secondary outcomes (miscarriage rate, the number of oocytes retrieved, E2 levels on the day of hCG, endometrial thickness on the day of hCG, total days of Gn applied, and total dose of Gn applied). Third, the former meta-analysis seemed hard to reflect the actual effects of CoQ10 in women undergoing ART, as their results drew from a mix of participants of POR and PCOS. Nevertheless, this meta-analysis only included participants with DOR receiving CoQ10 treatment before IVF/ICSI, which might provide a more specific reflection on the effects of CoQ10 pretreatment in this unique population.
Yet, this study has several limitations. First, the six studies were conducted in China, although we didn’t restrict our search scale to one region. For example, a potential study [16] exploring the value of CoQ10 in women with DOR undergoing IVF in Canada was excluded due to the lack of detailed reporting of diagnostic criteria, which met one of the exclusion criteria of this meta-analysis. Second, two included studies with skewed distributions outcomes adopted median (25th - 75th percentiles) for statistical analysis, and therefore we were unable to pool their related variables into this analysis. Third, we failed to estimate the publication bias based on Begg’s and Egger’s tests since there were less than ten studies included. But, all results of this meta-analysis were robust after being verified through sensitivity analysis. Fourth, although two pooled results had high heterogeneity (64% and 91%), we failed to conduct a subgroup analysis to explore the sources of heterogeneity, because only three studies reported the related outcomes, which might also be an inherent deficiency of this study. Fifth, six included RCTs had a poor design in their blinding methods, thus they were rated as high risk of bias in the domain of blinding. Sixth, there are some outcomes such as premature luteinizing hormone (LH) surge and embryo implantation rate which reported in our study protocol, whereas we had insufficient data to pool these outcomes systematically for none of the included articles reported these assessments. To ensure the quality of RCTs, this meta-analysis involved stringent inclusion and exclusion criteria, and a substantial endeavor was adopted to conduct a comprehensive article screening.
Conclusion
The current analysis indicates that CoQ10 pretreatment has positive effects on improving the outcomes among women with DOR undergoing IVF/ICSI, including increasing clinical pregnancy rate, number of oocytes retrieved, number of optimal embryos, and E2 levels on the day of hCG, along with decreasing cycle cancellation rate, miscarriage rate, total days of Gn applied, and total dose of Gn used. However, this meta-analysis included relatively limited sample sizes with poor descriptions of their methodologies. In the future, researchers should perform well-designed studies aimed at diminishing the risk of bias and further verifying the current evidence.
Funding Statement
This work was supported by the Shanghai Science and Technology Commission under Grant 23Y21920300.
Disclosure statement
No potential conflict of interest was reported by the authors.
Author contributions statement
All authors contributed to the study conception and design. GYL: Project conception, Literature search, Data analysis and interpretation, Manuscript writing. XLL: Data analysis, Literature search, Methodological advice. SJYL: Data analysis, Literature search, Methodological advice. LWX: Methodological advice, Manuscript revising. All authors commented on previous versions of the manuscript, and all authors read and approved the final manuscript.
Ethics statement
Ethical approval was not required for this meta-analysis because all the data used in this study were obtained from published articles.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
References
- 1.Carson SA, Kallen AN.. Diagnosis and management of infertility: a review. JAMA. 2021;326(1):65–76. doi: 10.1001/jama.2021.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Z, Zheng D, Wu H, et al. . Epidemiology of infertility in China: a population-based study. BJOG. 2018;125(4):432–441. doi: 10.1111/1471-0528.14966. [DOI] [PubMed] [Google Scholar]
- 3.Smith S, Pfeifer SM, Collins JA.. Diagnosis and management of female infertility. JAMA. 2003;290(13):1767–1770. doi: 10.1001/jama.290.13.1767. [DOI] [PubMed] [Google Scholar]
- 4.Jaswa EG, McCulloch CE, Simbulan R, et al. . Diminished ovarian reserve is associated with reduced euploid rates via preimplantation genetic testing for aneuploidy independently from age: evidence for concomitant reduction in oocyte quality with quantity. Fertil Steril. 2021;115(4):966–973. doi: 10.1016/j.fertnstert.2020.10.051. [DOI] [PubMed] [Google Scholar]
- 5.Devine K, Mumford SL, Wu M, et al. . Diminished ovarian reserve in the United States assisted reproductive technology population: diagnostic trends among 181,536 cycles from the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System. Fertil Steril. 2015;104(3):612–619.e3. doi: 10.1016/j.fertnstert.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumbak B, Oral E, Kahraman S, et al. . Young patients with diminished ovarian reserve undergoing assisted reproductive treatments: a preliminary report. Reprod Biomed Online. 2005;11(3):294–299. doi: 10.1016/s1472-6483(10)60836-x. [DOI] [PubMed] [Google Scholar]
- 7.Kawwass JF, Hipp HS, Session DR, et al. . Severity of diminished ovarian reserve and chance of success with assisted reproductive technology. J Reprod Med. 2017;62(3–4):153–160. [PMC free article] [PubMed] [Google Scholar]
- 8.Cedars MI. Managing poor ovarian response in the patient with diminished ovarian reserve. Fertil Steril. 2022;117(4):655–656. doi: 10.1016/j.fertnstert.2022.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Lin G, Zhong X, Li S, et al. . The clinical value of progestin-primed ovarian stimulation protocol for women with diminished ovarian reserve undergoing IVF/ICSI: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14:1232935. doi: 10.3389/fendo.2023.1232935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao J, Chang S, Chen S.. The effectiveness of gonadotropin-releasing hormone antagonist in poor ovarian responders undergoing in vitro fertilization: a systematic review and meta-analysis. Fertil Steril. 2013;100(6):1594. doi: 10.1016/j.fertnstert.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Xu B, Wang J, Xia L, et al. . Increased uterine NK cell numbers and perforin expression during the implantation phase in IVF Cycles with GnRH antagonist protocol. Sci Rep. 2017;7(1):39912. doi: 10.1038/srep39912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siristatidis CS, Gibreel A, Basios G, et al. . Gonadotrophin-releasing hormone agonist protocols for pituitary suppression in assisted reproduction. Cochrane Database Syst Rev. 2015;2015(11):CD006919. doi: 10.1002/14651858.CD006919.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arenas-Jal M, Suñé-Negre JM, García-Montoya E.. Coenzyme Q10 supplementation: efficacy, safety, and formulation challenges. Compr Rev Food Sci Food Saf. 2020;19(2):574–594. doi: 10.1111/1541-4337.12539. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez-Varela C, Labarta E.. Does coenzyme Q10 supplementation improve human oocyte quality? Int J Mol Sci. 2021;22(17):9541. doi: 10.3390/ijms22179541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Nisenblat V, Lu C, et al. . Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reprod Biol Endocrinol. 2018;16(1):29. doi: 10.1186/s12958-018-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gat I, Blanco Mejia S, Balakier H, et al. . The use of coenzyme Q10 and DHEA during IUI and IVF cycles in patients with decreased ovarian reserve. Gynecol Endocrinol. 2016;32(7):534–537. doi: 10.3109/09513590.2015.1137095. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535–b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Expert Group of Consensus on Clinical Diagnosis & Management of Diminished Ovarian Reserve and Reproductive Endocrinology & Fertility Preservation Section of Chinese Society on Fertility Preservation under Chinese Preventive Medicine Association. Consensus on clinical diagnosis and management of diminished ovarian reserve. J Reproductive Med. 2022;31(4):425–434. doi: 10.3969/j.issn.1004-3845.2022.04.001. [DOI] [Google Scholar]
- 19.Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. . International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520–1524. doi: 10.1016/j.fertnstert.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology . The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 21.Riley RD, Higgins JP, Deeks JJ.. Interpretation of random effects meta-analyses. BMJ. 2011;342(feb10 2):d549–d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Liu W, Sun R.. Effects of coenzyme Q10 for advanced age patients with diminished ovarian reserve undergoing IVF-ET. Shenzhen J Integr Traditional Chinese and Western Med. 2023;33(1):105–108. doi: 10.16458/j.cnki.1007-0893.2023.01.033. [DOI] [Google Scholar]
- 23.Xia L, Dong Q.. Effect of coenzyme Q10 combined with dehydroepiandrosterone on ovarian function and in vitro artificial fertilization in infertile women with hypoovarian reserve. Maternal & Child Health Care of China. 2023;38(11):2017–2021. doi: 10.19829/j.zgfybj.issn.1001-4411.2023.11.021. [DOI] [Google Scholar]
- 24.Zou Y, Yin T, Li J, et al. . Effect of co-enzyme Q10 combined with vitamin E on ovary reserve and IVF outcome in women with advanced age. J Reproductive Med. 2017;26(10):1028–1034. doi: 10.3969/j.issn.1004-3845.2017.10.014. [DOI] [Google Scholar]
- 25.Tang T, Liu C, Wu X.. The value of dehydroepiandrosterone combined with coenzyme Q10 for infertile patients with diminished ovarian reserve undergoing IVF-ET. J Clin Res. 2022;39(8):1254–1256. doi: 10.3969/j.issn.1671-7171.2022.08.037. [DOI] [Google Scholar]
- 26.Liang J. Coenzyme Q10 in combination with femoston for patients with diminished ovarian reserve undergoing IVF-ET. Henan Med Res. 2019;28(16):2973–2974. doi: 10.3969/j.issn.1004-437X.2019.16.046. [DOI] [Google Scholar]
- 27.Zhu F, Yin S, Yang B, et al. . TEAS, DHEA, CoQ10, and GH for poor ovarian response undergoing IVF-ET: a systematic review and network meta-analysis. Reprod Biol Endocrinol. 2023;21(1):64. doi: 10.1186/s12958-023-01119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giannubilo SR, Orlando P, Silvestri S, et al. . CoQ10 supplementation in patients undergoing IVF-ET: the relationship with follicular fluid content and oocyte maturity. Antioxidants (Basel). 2018;7(10):141. doi: 10.3390/antiox7100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delkhosh A, Delashoub M, Tehrani AA, et al. . Upregulation of FSHR and PCNA by administration of coenzyme Q10 on cyclophosphamide-induced premature ovarian failure in a mouse model. J Biochem Mol Toxicol. 2019;33(11):e22398. doi: 10.1002/jbt.22398. [DOI] [PubMed] [Google Scholar]
- 30.Heydarnejad A, Ostadhosseini S, Varnosfaderani SR, et al. . Supplementation of maturation medium with CoQ10 enhances developmental competence of ovine oocytes through improvement of mitochondrial function. Mol Reprod Dev. 2019;86(7):812–824. doi: 10.1002/mrd.23159. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Conca M, Gardela J, Mogas T, et al. . Apoptosis and glucocorticoid-related genes mRNA expression is modulated by coenzyme Q10 supplementation during in vitro maturation and vitrification of bovine oocytes and cumulus cells. Theriogenology. 2022;192:62–72. doi: 10.1016/j.theriogenology.2022.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Wang H, Song S, et al. . Systematic understanding of anti-aging effect of coenzyme Q10 on oocyte through a network pharmacology approach. Front Endocrinol (Lausanne). 2022;13:813772. doi: 10.3389/fendo.2022.813772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai X, Lu Y, Zhang M, et al. . Melatonin improves the fertilization ability of post-ovulatory aged mouse oocytes by stabilizing ovastacin and Juno to promote sperm binding and fusion. Hum Reprod. 2017;32(3):598–606. doi: 10.1093/humrep/dew362. [DOI] [PubMed] [Google Scholar]
- 34.Nie X, Dong X, Hu Y, et al. . Coenzyme Q10 stimulate reproductive vatality. Drug Des Devel Ther. 2023;17:2623–2637. doi: 10.2147/DDDT.S386974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hornos Carneiro MF, Shin N, Karthikraj R, et al. . Antioxidant CoQ10 restores fertility by rescuing bisphenol A-induced oxidative DNA damage in the caenorhabditis elegans germline. Genetics. 2020;214(2):381–395. doi: 10.1534/genetics.119.302939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atasever M, Soyman Z, Demirel E, et al. . Diminished ovarian reserve: is it a neglected cause in the assessment of recurrent miscarriage? A cohort study. Fertil Steril. 2016;105(5):1236–1240. doi: 10.1016/j.fertnstert.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Bunnewell SJ, Honess ER, Karia AM, et al. . Diminished ovarian reserve in recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2020;113(4):818–827.e3. doi: 10.1016/j.fertnstert.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Zeng R, Chen H, Zeng X, et al. . The essential role of body weight in adjusting Gn dosage to prevent high ovarian response for women with PCOS during IVF: A retrospective study. Front Endocrinol (Lausanne). 2022;13:922044. doi: 10.3389/fendo.2022.922044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li F, Chen Y, Niu A, et al. . Nomogram model to predict the probability of ovarian hyperstimulation syndrome in the treatment of patients with polycystic ovary syndrome. Front Endocrinol (Lausanne). 2021;12:619059. doi: 10.3389/fendo.2021.619059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coldebella D, Buzzaccarini G, Ferrari J, et al. . Inositols administration: further insights on their biological role. It Journ Gyn Obs. 2023;35(1):30–36. doi: 10.36129/jog.2022.40. [DOI] [Google Scholar]
- 41.Laganà AS, Monti N, Fedeli V, et al. . Does alpha-lipoic acid improve effects on polycystic ovary syndrome? Eur Rev Med Pharmacol Sci. 2022;26(4):1241–1247. doi: 10.26355/eurrev_202202_28116. [DOI] [PubMed] [Google Scholar]
- 42.Menichini D, Forte G, Orrù B, et al. . The role of vitamin D in metabolic and reproductive disturbances of polycystic ovary syndrome: A narrative mini-review. Int J Vitam Nutr Res. 2022;92(2):126–133. doi: 10.1024/0300-9831/a000691. [DOI] [PubMed] [Google Scholar]
- 43.Gullo G, Perino A, Cucinella G.. Open vs. closed vitrification system: which one is safer? Eur Rev Med Pharmacol Sci. 2022;26(4):1065–1067. doi: 10.26355/eurrev_202202_28092. [DOI] [PubMed] [Google Scholar]
- 44.Gullo G, Vassiliadis A, Unfer V, et al. . PCOS and infertility. Metformin administration and ovulation induction in patients with reproductive failures. Preliminary data. giog. 2014;36(4–5):443–447. doi: 10.11138/giog/2014.36.4.443. [DOI] [Google Scholar]
- 45.Florou P, Anagnostis P, Theocharis P, et al. . Does coenzyme Q10 supplementation improve fertility outcomes in women undergoing assisted reproductive technology procedures? A systematic review and meta-analysis of randomized-controlled trials. J Assist Reprod Genet. 2020;37(10):2377–2387. doi: 10.1007/s10815-020-01906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.