Abstract
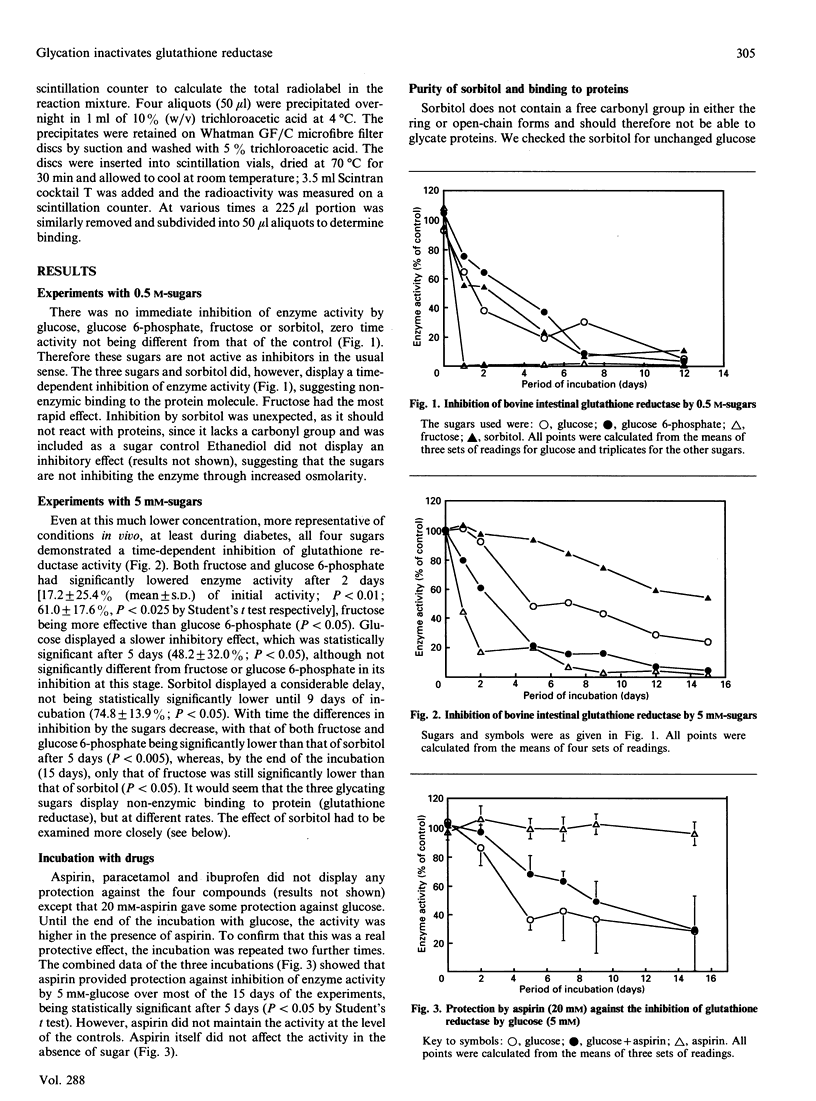
Non-enzymic binding of sugars to proteins (glycation) is a common biological phenomenon that is increased in diabetes. Most work has been directed towards structural proteins which may be present for many years and would continue to accumulate sugar residues. As glycation is a non-specific reaction, other proteins such as enzymes will also be susceptible to glycation and could well display altered activity. We investigated the effect of various sugars whose concentrations increase in diabetes in insulin-independent tissues on glutathione reductase, an enzyme that maintains the GSH level in cells. Glucose, glucose 6-phosphate and fructose all displayed a time-dependent inhibition of glutathione reductase activity, suggesting that these sugars glycate this enzyme. Aspirin gave some protection against the loss of activity induced by glucose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham E. C., Taylor J. F., Lang C. A. Influence of mouse age and erythrocyte age on glutathione metabolism. Biochem J. 1978 Sep 15;174(3):819–825. doi: 10.1042/bj1740819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi T., Ohta H., Hirano K., Hayashi K., Marklund S. L. Non-enzymic glycation of human extracellular superoxide dismutase. Biochem J. 1991 Oct 1;279(Pt 1):263–267. doi: 10.1042/bj2790263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajiboye R., Harding J. J. The non-enzymic glycosylation of bovine lens proteins by glucosamine and its inhibition by aspirin, ibuprofen and glutathione. Exp Eye Res. 1989 Jul;49(1):31–41. doi: 10.1016/0014-4835(89)90073-0. [DOI] [PubMed] [Google Scholar]
- Al-Turk W. A., Stohs S. J., el-Rashidy F. H., Othman S. Changes in glutathione and its metabolizing enzymes in human erythrocytes and lymphocytes with age. J Pharm Pharmacol. 1987 Jan;39(1):13–16. doi: 10.1111/j.2042-7158.1987.tb07154.x. [DOI] [PubMed] [Google Scholar]
- Beswick H. T., Harding J. J. Conformational changes induced in lens alpha- and gamma-crystallins by modification with glucose 6-phosphate. Implications for cataract. Biochem J. 1987 Sep 15;246(3):761–769. doi: 10.1042/bj2460761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakytny R., Harding J. J. Prevention of cataract in diabetic rats by aspirin, paracetamol (acetaminophen) and ibuprofen. Exp Eye Res. 1992 Apr;54(4):509–518. doi: 10.1016/0014-4835(92)90129-g. [DOI] [PubMed] [Google Scholar]
- Bookchin R. M., Gallop P. M. Structure of hemoglobin AIc: nature of the N-terminal beta chain blocking group. Biochem Biophys Res Commun. 1968 Jul 11;32(1):86–93. doi: 10.1016/0006-291x(68)90430-0. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Gabbay K. H., Gallop P. M. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978 Apr 7;200(4337):21–27. doi: 10.1126/science.635569. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Higgins P. J. Reaction of monosaccharides with proteins: possible evolutionary significance. Science. 1981 Jul 10;213(4504):222–224. doi: 10.1126/science.12192669. [DOI] [PubMed] [Google Scholar]
- Ditzel J., Jaeger P., Standl E. An adverse effect of insulin on the oxygen-release capacity of red blood cells in nonacidotic diabetics. Metabolism. 1978 Aug;27(8):927–934. doi: 10.1016/0026-0495(78)90136-1. [DOI] [PubMed] [Google Scholar]
- Eckerskorn U., Hockwin O., Müller-Breitenkamp R., Chen T. T., Knowles W., Dobbs R. E. Evaluation of cataract-related risk factors using detailed classification systems and multivariate statistical methods. Dev Ophthalmol. 1987;15:82–91. doi: 10.1159/000414697. [DOI] [PubMed] [Google Scholar]
- Farooqui M. Y., Day W. W., Zamorano D. M. Glutathione and lipid peroxidation in the aging rat. Comp Biochem Physiol B. 1987;88(1):177–180. doi: 10.1016/0305-0491(87)90097-6. [DOI] [PubMed] [Google Scholar]
- Flückiger R., Winterhalter K. H. In vitro synthesis of hemoglobin AIc. FEBS Lett. 1976 Dec 1;71(2):356–360. doi: 10.1016/0014-5793(76)80969-6. [DOI] [PubMed] [Google Scholar]
- Friedburg D., Manthey K. F. Glutathione and NADP linked enzymes in human senile cataract. Exp Eye Res. 1973 Feb;15(2):173–177. doi: 10.1016/0014-4835(73)90116-4. [DOI] [PubMed] [Google Scholar]
- Garner M. H., Spector A. ATP hydrolysis kinetics of Na,K-ATPase in cataract. Exp Eye Res. 1986 Apr;42(4):339–348. doi: 10.1016/0014-4835(86)90027-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez A. M., Sochor M., McLean P. Effect of experimental diabetes on glycolytic intermediates and regulation of phosphofructokinase in rat lens. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1173–1179. doi: 10.1016/0006-291x(80)91596-x. [DOI] [PubMed] [Google Scholar]
- Gundberg C. M., Anderson M., Dickson I., Gallop P. M. "Glycated" osteocalcin in human and bovine bone. The effect of age. J Biol Chem. 1986 Nov 5;261(31):14557–14561. [PubMed] [Google Scholar]
- Harding J. J. Disulphide cross-linked protein of high molecular weight in human cataractous lens. Exp Eye Res. 1973 Nov 25;17(4):377–383. doi: 10.1016/0014-4835(73)90247-9. [DOI] [PubMed] [Google Scholar]
- Harding J. J., Egerton M., Harding R. S. Protection against cataract by aspirin, paracetamol and ibuprofen. Acta Ophthalmol (Copenh) 1989 Oct;67(5):518–524. doi: 10.1111/j.1755-3768.1989.tb04102.x. [DOI] [PubMed] [Google Scholar]
- Harding J. J. Free and protein-bound glutathione in normal and cataractous human lenses. Biochem J. 1970 May;117(5):957–960. doi: 10.1042/bj1170957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding J. J., van Heyningen R. Drugs, including alcohol, that act as risk factors for cataract, and possible protection against cataract by aspirin-like analgesics and cyclopenthiazide. Br J Ophthalmol. 1988 Nov;72(11):809–814. doi: 10.1136/bjo.72.11.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelton G. A., Lang C. A. Glutathione contents of tissues in the aging mouse. Biochem J. 1980 Apr 15;188(1):25–30. doi: 10.1042/bj1880025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huby R., Harding J. J. Non-enzymic glycosylation (glycation) of lens proteins by galactose and protection by aspirin and reduced glutathione. Exp Eye Res. 1988 Jul;47(1):53–59. doi: 10.1016/0014-4835(88)90023-1. [DOI] [PubMed] [Google Scholar]
- Iberg N., Flückiger R. Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J Biol Chem. 1986 Oct 15;261(29):13542–13545. [PubMed] [Google Scholar]
- Isaac N. E., Walker A. M., Jick H., Gorman M. Exposure to phenothiazine drugs and risk of cataract. Arch Ophthalmol. 1991 Feb;109(2):256–260. doi: 10.1001/archopht.1991.01080020102053. [DOI] [PubMed] [Google Scholar]
- Jedziniak J. A., Chylack L. T., Jr, Cheng H. M., Gillis M. K., Kalustian A. A., Tung W. H. The sorbitol pathway in the human lens: aldose reductase and polyol dehydrogenase. Invest Ophthalmol Vis Sci. 1981 Mar;20(3):314–326. [PubMed] [Google Scholar]
- Karplus P. A., Schulz G. E. Refined structure of glutathione reductase at 1.54 A resolution. J Mol Biol. 1987 Jun 5;195(3):701–729. doi: 10.1016/0022-2836(87)90191-4. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Cerami A. Synthesis of hemoglobin AIc in normal and diabetic mice: potential model of basement membrane thickening. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3687–3691. doi: 10.1073/pnas.72.9.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara L. S., Machado P. E. Age-related changes of glutathione content, glutathione reductase and glutathione peroxidase activity of human erythrocytes. Braz J Med Biol Res. 1991;24(5):449–454. [PubMed] [Google Scholar]
- Mayhew J. A., Gillon K. R., Hawthorne J. N. Free and lipid inositol, sorbitol and sugars in sciatic nerve obtained post-mortem from diabetic patients and control subjects. Diabetologia. 1983 Jan;24(1):13–15. doi: 10.1007/BF00275940. [DOI] [PubMed] [Google Scholar]
- Mohan M., Sperduto R. D., Angra S. K., Milton R. C., Mathur R. L., Underwood B. A., Jaffery N., Pandya C. B., Chhabra V. K., Vajpayee R. B. India-US case-control study of age-related cataracts. India-US Case-Control Study Group. Arch Ophthalmol. 1989 May;107(5):670–676. doi: 10.1001/archopht.1989.01070010688028. [DOI] [PubMed] [Google Scholar]
- Murakami K., Kondo T., Ohtsuka Y., Fujiwara Y., Shimada M., Kawakami Y. Impairment of glutathione metabolism in erythrocytes from patients with diabetes mellitus. Metabolism. 1989 Aug;38(8):753–758. doi: 10.1016/0026-0495(89)90061-9. [DOI] [PubMed] [Google Scholar]
- Ohrloff C., Hockwin O., Olson R., Dickman S. Glutathione peroxidase, glutathione reductase and superoxide dismutase in the aging lens. Curr Eye Res. 1984 Jan;3(1):109–115. doi: 10.3109/02713688408997191. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Karplus P. A., Schulz G. E. Crystallographic analysis of the binding of NADPH, NADPH fragments, and NADPH analogues to glutathione reductase. Biochemistry. 1988 Jun 14;27(12):4465–4474. doi: 10.1021/bi00412a038. [DOI] [PubMed] [Google Scholar]
- Perry A. C., Ni Bhriain N., Brown N. L., Rouch D. A. Molecular characterization of the gor gene encoding glutathione reductase from Pseudomonas aeruginosa: determinants of substrate specificity among pyridine nucleotide-disulphide oxidoreductases. Mol Microbiol. 1991 Jan;5(1):163–171. [PubMed] [Google Scholar]
- Perry R. E., Swamy M. S., Abraham E. C. Progressive changes in lens crystallin glycation and high-molecular-weight aggregate formation leading to cataract development in streptozotocin-diabetic rats. Exp Eye Res. 1987 Feb;44(2):269–282. doi: 10.1016/s0014-4835(87)80011-8. [DOI] [PubMed] [Google Scholar]
- Rahbar S., Blumenfeld O., Ranney H. M. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun. 1969 Aug 22;36(5):838–843. doi: 10.1016/0006-291x(69)90685-8. [DOI] [PubMed] [Google Scholar]
- Raza K., Harding J. J. Non-enzymic modification of lens proteins by glucose and fructose: effects of ibuprofen. Exp Eye Res. 1991 Feb;52(2):205–212. doi: 10.1016/0014-4835(91)90260-l. [DOI] [PubMed] [Google Scholar]
- Rendell M., Nierenberg J., Brannan C., Valentine J. L., Stephen P. M., Dodds S., Mercer P., Smith P. K., Walder J. Inhibition of glycation of albumin and hemoglobin by acetylation in vitro and in vivo. J Lab Clin Med. 1986 Oct;108(4):286–293. [PubMed] [Google Scholar]
- Rogers K. M., Augusteyn R. C. Glutathione reductase in normal and cataractous human lenses. Exp Eye Res. 1978 Dec;27(6):719–721. doi: 10.1016/0014-4835(78)90041-6. [DOI] [PubMed] [Google Scholar]
- Ross W. M., Creighton M. O., Trevithick J. R., Stewart-DeHaan P. J., Sanwal M. Modelling cortical cataractogenesis: VI. Induction by glucose in vitro or in diabetic rats: prevention and reversal by glutathione. Exp Eye Res. 1983 Dec;37(6):559–573. doi: 10.1016/0014-4835(83)90132-x. [DOI] [PubMed] [Google Scholar]
- Sasaki J., Arora V., Cottam G. L. Nonenzymatic galactosylation of human LDL decreases its metabolism by human skin fibroblasts. Biochem Biophys Res Commun. 1982 Sep 30;108(2):791–796. doi: 10.1016/0006-291x(82)90898-1. [DOI] [PubMed] [Google Scholar]
- Scrutton N. S., Berry A., Perham R. N. Redesign of the coenzyme specificity of a dehydrogenase by protein engineering. Nature. 1990 Jan 4;343(6253):38–43. doi: 10.1038/343038a0. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Christen W. G., Manson J. E., Buring J. E., Sperduto R. D., Hennekens C. H. Low-dose aspirin and risks of cataract in a randomized trial of US physicians. Arch Ophthalmol. 1991 Feb;109(2):252–255. doi: 10.1001/archopht.1991.01080020098052. [DOI] [PubMed] [Google Scholar]
- Shaklai N., Garlick R. L., Bunn H. F. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J Biol Chem. 1984 Mar 25;259(6):3812–3817. [PubMed] [Google Scholar]
- Shames S. L., Kimmel B. E., Peoples O. P., Agabian N., Walsh C. T. Trypanothione reductase of Trypanosoma congolense: gene isolation, primary sequence determination, and comparison to glutathione reductase. Biochemistry. 1988 Jul 12;27(14):5014–5019. doi: 10.1021/bi00414a010. [DOI] [PubMed] [Google Scholar]
- Standl E., Kolb H. J. 2,3-Diphosphoglycerate fluctuations in erythrocytes reflecting pronounced blood glucose variation. In-vivo and in-vitro studies in normal, diabetic and hypoglycaemic subjects. Diabetologia. 1973 Dec;9(6):461–466. doi: 10.1007/BF00461689. [DOI] [PubMed] [Google Scholar]
- Stevens V. J., Rouzer C. A., Monnier V. M., Cerami A. Diabetic cataract formation: potential role of glycosylation of lens crystallins. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2918–2922. doi: 10.1073/pnas.75.6.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhlberg M. R., Hietanen E. Glutathione and glutathione-metabolizing enzymes in the erythrocytes of healthy children and in children with insulin-dependent diabetes mellitus, juvenile rheumatoid arthritis, coeliac disease and acute lymphoblastic leukaemia. Scand J Clin Lab Invest. 1991 Apr;51(2):125–130. doi: 10.1080/00365519109091097. [DOI] [PubMed] [Google Scholar]
- Swamy M. S., Abraham E. C. Inhibition of lens crystallin glycation and high molecular weight aggregate formation by aspirin in vitro and in vivo. Invest Ophthalmol Vis Sci. 1989 Jun;30(6):1120–1126. [PubMed] [Google Scholar]
- Uitto J., Perejda A. J., Grant G. A., Rowold E. A., Kilo C., Williamson J. R. Glycosylation of human glomerular basement membrane collagen: increased content of hexose in ketoamine linkage and unaltered hydroxylysine-O-glycosides in patients with diabetes. Connect Tissue Res. 1982;10(3-4):287–296. doi: 10.3109/03008208209008054. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. Excessive nonenzymatic glycosylation of peripheral and central nervous system myelin components in diabetic rats. Diabetes. 1983 Jul;32(7):670–674. doi: 10.2337/diab.32.7.670. [DOI] [PubMed] [Google Scholar]
- van Boekel M. A. The role of glycation in aging and diabetes mellitus. Mol Biol Rep. 1991 May;15(2):57–64. doi: 10.1007/BF00364840. [DOI] [PubMed] [Google Scholar]
- van Heyningen R., Harding J. J. Do aspirin-like analgesics protect against cataract? A case-control study. Lancet. 1986 May 17;1(8490):1111–1113. doi: 10.1016/s0140-6736(86)91834-9. [DOI] [PubMed] [Google Scholar]


