Abstract
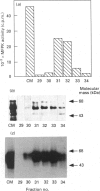
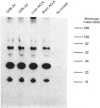
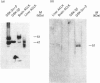
Multifunctional ATP-citrate lyase kinase (ACLK) exhibits several properties that are similar to glycogen-synthase kinase-3 (GSK-3). The molecular cloning of two distinct mammalian GSK-3 cDNAs and a Drosophila melanogaster (fruitfly) homologue, zeste-white3sgg, has established the existence of a GSK-3 subfamily. A multifunctional protein kinase first identified as an ACLK has recently been shown to exhibit several similarities to the alpha- and beta-forms of GSK-3. Here we have used immunological and biochemical analyses to directly compare these enzymes. Thus purified preparations of ACLK isolated from brain and liver preferentially cross-react with anti-GSK-3 alpha antisera and phosphorylate previously defined substrates of GSK-3 at identical sites. Conversely, both alpha- and beta-forms of GSK-3 phosphorylated ATP-citrate lyase at the same site(s) targeted by ACLK. These, and other similarities, demonstrate ACLK to be identical with, or highly related to, GSK-3 alpha, the implications of which are discussed.
Full text
PDF
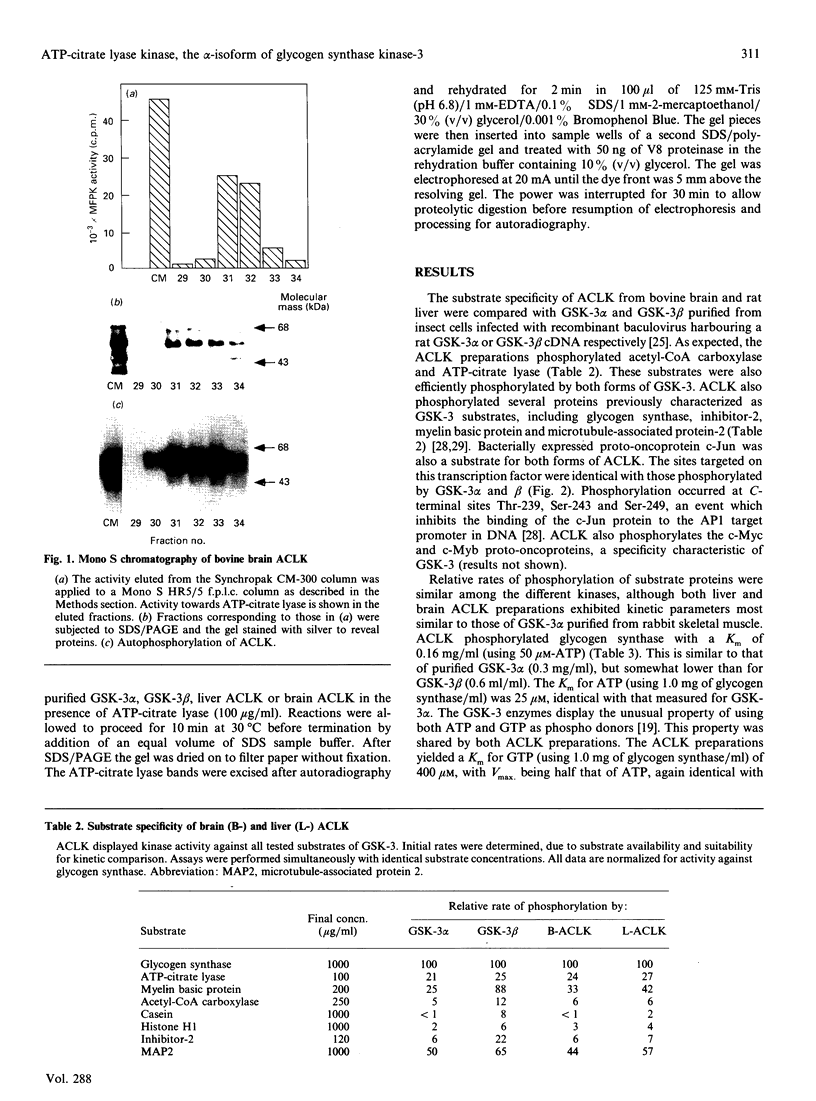
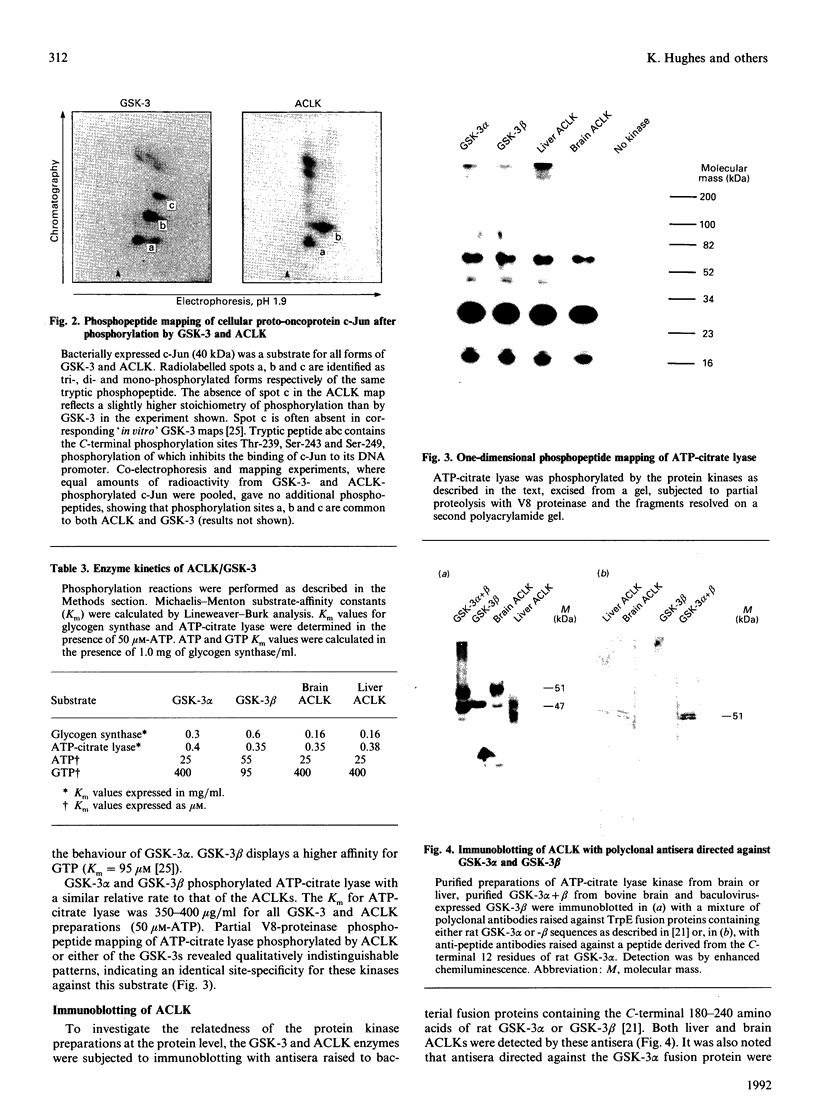


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. C., Kowaloff E. M., Witters L. A., Dennihy D. T., Avruch J. Purification of a hepatic 123,000-dalton hormone-stimulated 32P-peptide and its identification as ATP-citrate lyase. J Biol Chem. 1979 Aug 25;254(16):8052–8056. [PubMed] [Google Scholar]
- Bourouis M., Moore P., Ruel L., Grau Y., Heitzler P., Simpson P. An early embryonic product of the gene shaggy encodes a serine/threonine protein kinase related to the CDC28/cdc2+ subfamily. EMBO J. 1990 Sep;9(9):2877–2884. doi: 10.1002/j.1460-2075.1990.tb07477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- DePaoli-Roach A. A. Synergistic phosphorylation and activation of ATP-Mg-dependent phosphoprotein phosphatase by F A/GSK-3 and casein kinase II (PC0.7). J Biol Chem. 1984 Oct 10;259(19):12144–12152. [PubMed] [Google Scholar]
- Dent P., Lavoinne A., Nakielny S., Caudwell F. B., Watt P., Cohen P. The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature. 1990 Nov 22;348(6299):302–308. doi: 10.1038/348302a0. [DOI] [PubMed] [Google Scholar]
- Denton R. M. Early events in insulin actions. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:293–341. [PubMed] [Google Scholar]
- Embi N., Rylatt D. B., Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980 Jun;107(2):519–527. [PubMed] [Google Scholar]
- Hemmings B. A., Aitken A., Cohen P., Rymond M., Hofmann F. Phosphorylation of the type-II regulatory subunit of cyclic-AMP-dependent protein kinase by glycogen synthase kinase 3 and glycogen synthase kinase 5. Eur J Biochem. 1982 Oct;127(3):473–481. doi: 10.1111/j.1432-1033.1982.tb06896.x. [DOI] [PubMed] [Google Scholar]
- Hemmings B. A., Yellowlees D., Kernohan J. C., Cohen P. Purification of glycogen synthase kinase 3 from rabbit skeletal muscle. Copurification with the activating factor (FA) of the (Mg-ATP) dependent protein phosphatase. Eur J Biochem. 1981 Oct;119(3):443–451. doi: 10.1111/j.1432-1033.1981.tb05628.x. [DOI] [PubMed] [Google Scholar]
- Hughes K., Pulverer B. J., Theocharous P., Woodgett J. R. Baculovirus-mediated expression and characterisation of rat glycogen synthase kinase-3 beta, the mammalian homologue of the Drosophila melanogaster zeste-white 3sgg homeotic gene product. Eur J Biochem. 1992 Jan 15;203(1-2):305–311. doi: 10.1111/j.1432-1033.1992.tb19860.x. [DOI] [PubMed] [Google Scholar]
- Lastick S. M., McConkey E. H. HeLa ribosomal protein S6. Insulin and dibutyryl cyclic AMP affect different phosphopeptides. J Biol Chem. 1981 Jan 25;256(2):583–585. [PubMed] [Google Scholar]
- Parker P. J., Caudwell F. B., Cohen P. Glycogen synthase from rabbit skeletal muscle; effect of insulin on the state of phosphorylation of the seven phosphoserine residues in vivo. Eur J Biochem. 1983 Jan 17;130(1):227–234. doi: 10.1111/j.1432-1033.1983.tb07140.x. [DOI] [PubMed] [Google Scholar]
- Picton C., Woodgett J., Hemmings B., Cohen P. Multisite phosphorylation of glycogen synthase from rabbit skeletal muscle. Phosphorylation of site 5 by glycogen synthase kinase-5 (casein kinase-II) is a prerequisite for phosphorylation of sites 3 by glycogen synthase kinase-3. FEBS Lett. 1982 Dec 13;150(1):191–196. doi: 10.1016/0014-5793(82)81332-x. [DOI] [PubMed] [Google Scholar]
- Poulter L., Ang S. G., Gibson B. W., Williams D. H., Holmes C. F., Caudwell F. B., Pitcher J., Cohen P. Analysis of the in vivo phosphorylation state of rabbit skeletal muscle glycogen synthase by fast-atom-bombardment mass spectrometry. Eur J Biochem. 1988 Aug 15;175(3):497–510. doi: 10.1111/j.1432-1033.1988.tb14222.x. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S., Benjamin W. B. Cyclic nucleotide-independent protein kinase from rat liver. Purification and characterization of a multifunctional protein kinase. J Biol Chem. 1985 Oct 5;260(22):12280–12286. [PubMed] [Google Scholar]
- Ramakrishna S., Benjamin W. B. Fat cell protein phosphorylation. Identification of phosphoprotein-2 as ATP-citrate lyase. J Biol Chem. 1979 Sep 25;254(18):9232–9236. [PubMed] [Google Scholar]
- Ramakrishna S., Benjamin W. B. Insulin action rapidly decreases multifunctional protein kinase activity in rat adipose tissue. J Biol Chem. 1988 Sep 5;263(25):12677–12681. [PubMed] [Google Scholar]
- Ramakrishna S., Benjamin W. B. Phosphorylation of ATP-citrate lyase by a cyclic AMP-independent protein kinase from rat liver. FEBS Lett. 1981 Feb 23;124(2):140–144. doi: 10.1016/0014-5793(81)80122-6. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S., Benjamin W. B. Phosphorylation of different sites of acetyl CoA carboxylase by ATP-citrate lyase kinase and cyclic AMP-dependent protein kinase. Biochem Biophys Res Commun. 1983 Dec 16;117(2):435–443. doi: 10.1016/0006-291x(83)91219-6. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S., D'Angelo G., Benjamin W. B. Sequence of sites on ATP-citrate lyase and phosphatase inhibitor 2 phosphorylated by multifunctional protein kinase (a glycogen synthase kinase 3 like kinase). Biochemistry. 1990 Aug 21;29(33):7617–7624. doi: 10.1021/bi00485a011. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S., Murthy K. S., Benjamin W. B. Effect of insulin on ATP-citrate lyase phosphorylation: regulation of peptide A and peptide B phosphorylations. Biochemistry. 1989 Jan 24;28(2):856–860. doi: 10.1021/bi00428a067. [DOI] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Rylatt D. B., Aitken A., Bilham T., Condon G. D., Embi N., Cohen P. Glycogen synthase from rabbit skeletal muscle. Amino acid sequence at the sites phosphorylated by glycogen synthase kinase-3, and extension of the N-terminal sequence containing the site phosphorylated by phosphorylase kinase. Eur J Biochem. 1980 Jun;107(2):529–537. [PubMed] [Google Scholar]
- Sheorain V. S., Ramakrishna S., Benjamin W. B., Soderling T. R. Phosphorylation of sites 3 and 2 in rabbit skeletal muscle glycogen synthase by a multifunctional protein kinase (ATP-citrate lyase kinase). J Biol Chem. 1985 Oct 5;260(22):12287–12292. [PubMed] [Google Scholar]
- Siegfried E., Perkins L. A., Capaci T. M., Perrimon N. Putative protein kinase product of the Drosophila segment-polarity gene zeste-white3. Nature. 1990 Jun 28;345(6278):825–829. doi: 10.1038/345825a0. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Rubin C. S., Rosen O. M. Insulin-treated 3T3-L1 adipocytes and cell-free extracts derived from them incorporate 32P into ribosomal protein S6. Proc Natl Acad Sci U S A. 1980 May;77(5):2641–2645. doi: 10.1073/pnas.77.5.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witters L. A., Tipper J. P., Bacon G. W. Stimulation of site-specific phosphorylation of acetyl coenzyme A carboxylase by insulin and epinephrine. J Biol Chem. 1983 May 10;258(9):5643–5648. [PubMed] [Google Scholar]
- Woodgett J. R. A common denominator linking glycogen metabolism, nuclear oncogenes and development. Trends Biochem Sci. 1991 May;16(5):177–181. doi: 10.1016/0968-0004(91)90071-3. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Cohen P. Multisite phosphorylation of glycogen synthase. Molecular basis for the substrate specificity of glycogen synthase kinase-3 and casein kinase-II (glycogen synthase kinase-5). Biochim Biophys Acta. 1984 Aug 14;788(3):339–347. doi: 10.1016/0167-4838(84)90047-5. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990 Aug;9(8):2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]