Abstract
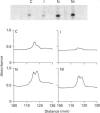
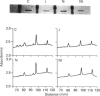
NG108-15 neuroblastoma x glioma somatic hybrid cells were permeabilized in the presence of [32P]NAD+ and then cultured for 18 h. Resolution of the cell proteins on polyacrylamide gels revealed [32P]ADP-ribosylation of five major protein species with molecular mass values of 52 kDa, 44 kDa, 35 kDa, 30 kDa and 25 kDa. A similar pattern of labelling was also seen when NG108-15 cell membranes were incubated with [32P]NAD+ and hydrolysis of the product revealed mono(ADP-ribosyl)ation. Immunoprecipitation of these products with anti-Gs alpha antiserum revealed a single band identical to cholera toxin substrate. Culture of [32P]NAD(+)-loaded cells for 18 h in the presence of 50 mM-nicotinamide inhibited the eukaryotic mono(ADP-ribosyl)transferase activity. Inhibition of the eukaryotic enzyme was also accompanied by an increase in the abundance of Gs alpha, whether measured by Western blotting with anti-Gs alpha antibody (two separate antisera) or by cholera toxin-dependent [32P]ADP-ribosylation. There was no accompanying change in the abundance of G beta. The increase in Gs alpha abundance in nicotinamide-treated NG108-15 cells was accompanied by a 2-fold increase in basal adenylate cyclase activity (measured in the presence of GTP), and by a smaller but significant increase in iloprost-dependent activation of adenylate cyclase. Receptor number or affinity was not affected by nicotinamide, since this treatment did not alter the binding parameters of [3H]iloprost to NG108-15 cell membranes. Short-term exposure of cells to nicotinamide for 1 h revealed no significant difference in either basal or agonist-stimulated adenylate cyclase activity. These results reveal that mono(ADP-ribosyl)ation of Gs alpha by eukaryotic ADP-ribosyltransferase modifies the abundance and activity of Gs alpha in NG108-15 cells, and hence may play a role in the hormonal regulation of cell function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Chang F. H., Bourne H. R. Cholera toxin induces cAMP-independent degradation of Gs. J Biol Chem. 1989 Apr 5;264(10):5352–5357. [PubMed] [Google Scholar]
- Edwards R. J., MacDermot J., Wilkins A. J. Prostacyclin analogues reduce ADP-ribosylation of the alpha-subunit of the regulatory Gs-protein and diminish adenosine (A2) responsiveness of platelets. Br J Pharmacol. 1987 Mar;90(3):501–510. doi: 10.1111/j.1476-5381.1987.tb11199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G. S., Cohn J. N. Heart failure: mechanisms of cardiac and vascular dysfunction and the rationale for pharmacologic intervention. FASEB J. 1990 Oct;4(13):3068–3075. doi: 10.1096/fasebj.4.13.2210153. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Inageda K., Nishina H., Tanuma S. Mono-ADP-ribosylation of Gs by an eukaryotic arginine-specific ADP-ribosyltransferase stimulates the adenylate cyclase system. Biochem Biophys Res Commun. 1991 May 15;176(3):1014–1019. doi: 10.1016/0006-291x(91)90383-i. [DOI] [PubMed] [Google Scholar]
- Kelly E., Keen M., Nobbs P., MacDermot J. Segregation of discrete GS alpha-mediated responses that accompany homologous or heterologous desensitization in two related somatic hybrids. Br J Pharmacol. 1990 Feb;99(2):309–316. doi: 10.1111/j.1476-5381.1990.tb14700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenimer J. G. Desensitization of PGE1 receptors in neuroblastoma-glioma hybrid cells. Prostaglandins. 1982 Mar;23(3):311–318. doi: 10.1016/0090-6980(82)90076-4. [DOI] [PubMed] [Google Scholar]
- Klinz F. J., Costa T. Cholera toxin differentially decreases membrane levels of alpha and beta subunits of G proteins in NG108-15 cells. Eur J Biochem. 1990 Mar 30;188(3):567–576. doi: 10.1111/j.1432-1033.1990.tb15437.x. [DOI] [PubMed] [Google Scholar]
- Leigh P. J., Cramp W. A., MacDermot J. Identification of the prostacyclin receptor by radiation inactivation. J Biol Chem. 1984 Oct 25;259(20):12431–12436. [PubMed] [Google Scholar]
- MacDermot J., Higashida H., Wilson S. P., Matsuzawa H., Minna J., Nirenberg M. Adenylate cyclase and acetylcholine release regulated by separate serotonin receptors of somatic cell hybrids. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1135–1139. doi: 10.1073/pnas.76.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod K. G., Milligan G. Biphasic regulation of adenylate cyclase by cholera toxin in neuroblastoma x glioma hybrid cells is due to the activation and subsequent loss of the alpha subunit of the stimulatory GTP binding protein (GS). Cell Signal. 1990;2(2):139–151. doi: 10.1016/0898-6568(90)90017-5. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Tsuyama S. Mono-ADP-ribosylation in brain: purification and characterization of ADP-ribosyltransferases affecting actin from rat brain. J Neurochem. 1991 Oct;57(4):1380–1387. doi: 10.1111/j.1471-4159.1991.tb08304.x. [DOI] [PubMed] [Google Scholar]
- McKenzie F. R., Milligan G. Prostaglandin E1-mediated, cyclic AMP-independent, down-regulation of Gs alpha in neuroblastoma x glioma hybrid cells. J Biol Chem. 1990 Oct 5;265(28):17084–17093. [PubMed] [Google Scholar]
- Milligan G., Unson C. G., Wakelam M. J. Cholera toxin treatment produces down-regulation of the alpha-subunit of the stimulatory guanine-nucleotide-binding protein (Gs). Biochem J. 1989 Sep 1;262(2):643–649. doi: 10.1042/bj2620643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina y Vedia L., Nolan R. D., Lapetina E. G. The effect of iloprost on the ADP-ribosylation of Gs alpha (the alpha-subunit of Gs). Biochem J. 1989 Aug 1;261(3):841–845. doi: 10.1042/bj2610841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Stanley S. J. Histone-dependent and histone-independent forms of an ADP-ribosyltransferase from human and turkey erythrocytes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4809–4812. doi: 10.1073/pnas.78.8.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Isolation of an avian erythrocyte protein possessing ADP-ribosyltransferase activity and capable of activating adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3621–3624. doi: 10.1073/pnas.75.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara S., Yamada K., Yoshimura Y., Shimoyama M. Evidence for the endogenous GTP-dependent ADP-ribosylation of the alpha-subunit of the stimulatory guanyl-nucleotide-binding protein concomitant with an increase in basal adenylyl cyclase activity in chicken spleen cell membrane. Eur J Biochem. 1991 Aug 15;200(1):75–80. doi: 10.1111/j.1432-1033.1991.tb21050.x. [DOI] [PubMed] [Google Scholar]
- Piron K. J., McMahon K. K. Localization and partial characterization of ADP-ribosylation products in hearts from adult and neonatal rats. Biochem J. 1990 Sep 15;270(3):591–597. doi: 10.1042/bj2700591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin P. W., Jacobson E. L., Benjamin R. C., Moss J., Jacobson M. K. Quantitative studies of inhibitors of ADP-ribosylation in vitro and in vivo. J Biol Chem. 1989 Mar 15;264(8):4312–4317. [PubMed] [Google Scholar]
- Richter C., Winterhalter K. H., Baumhüter S., Lötscher H. R., Moser B. ADP-ribosylation in inner membrane of rat liver mitochondria. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3188–3192. doi: 10.1073/pnas.80.11.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Soman G., Mickelson J. R., Louis C. F., Graves D. J. NAD: guanidino group specific mono ADP-ribosyltransferase activity in skeletal muscle. Biochem Biophys Res Commun. 1984 May 16;120(3):973–980. doi: 10.1016/s0006-291x(84)80202-8. [DOI] [PubMed] [Google Scholar]
- Tanuma S., Kawashima K., Endo H. Eukaryotic mono(ADP-ribosyl)transferase that ADP-ribosylates GTP-binding regulatory Gi protein. J Biol Chem. 1988 Apr 15;263(11):5485–5489. [PubMed] [Google Scholar]
- Yost D. A., Moss J. Amino acid-specific ADP-ribosylation. Evidence for two distinct NAD:arginine ADP-ribosyltransferases in turkey erythrocytes. J Biol Chem. 1983 Apr 25;258(8):4926–4929. [PubMed] [Google Scholar]