Abstract
Background
Hypersensitivity reactions (HSRs) can occur unexpectedly and be life-threatening when gadolinium-based contrast agents (GBCAs) are used. Gadolinium deposition disease (GDD) and symptoms associated with gadolinium exposure (SAGE) have been controversial for a long time. However, similar studies are currently incomplete or outdated. Therefore, comparing the safety of different GBCAs in terms of HSRs and GDD/SAGE using the latest post-marketing safety data should yield further insights into safely using GBCAs.
Methods
The safety differences between all GBCAs to GDD and the spectrum of GBCA-related HSRs were all compared and analyzed by using the World Health Organization database VigiBase and the FDA Adverse Event Reporting System (FAERS) database in this study. A further analysis of SAGE was also conducted using FAERS data. The lower limit of the reporting odds ratio (ROR) 95% confidence interval was used for signal detection. Moreover, the frequency of HSRs was calculated by dividing the number of reports in VigiBase by the total sales volume (measured in millions) from 2008 to 2022 in the IQVIA Multinational Integrated Data Analysis System. All adverse events were standardized using the Medical Dictionary for Drug Regulatory Activities (MedDRA) 26.0.
Results
This study shows that all GBCAs have the potential to induce HSRs, with nonionic linear GBCAs exhibiting a comparatively lower signal. According to standardized MedDRA query stratification analysis, gadobutrol had a greater ROR025 for angioedema. The ROR025 of gadobenate dimeglumine and gadoteridol is larger for anaphylactic/anaphylactoid shock conditions. Regarding severe cutaneous adverse reactions, only gadoversetamide and gadodiamide showed signals in FAERS and VigiBase. There were also differences in the frequency of HSRs between regions. Regarding GDD, gadoterate meglumine, and gadoteridol had a lower ROR025. An analysis of the 29 preferred terms linked to SAGE indicated that special consideration should be given to the risk of skin induration associated with gadoversetamide, gadopentetate dimeglumine, gadobenate dimeglumine, gadodiamide, and gadoteridol. Additionally, gadodiamide and gadoteridol pose a greater risk of skin tightness compared to other GBCAs.
Conclusions
The risk differences among GBCAs using data from several sources were compared in this study. However, as a hypothesis-generating method, a clear causal relationship would require further research and validation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03537-2.
Keywords: Gadolinium-based contrast agents, VigiBase, FAERS, IQVIA-MIDAS, Hypersensitivity reactions, Gadolinium deposition disease, Symptoms associated with gadolinium exposure
Background
Gadolinium-based contrast agents (GBCAs) are chelates of gadolinium (Gd), a paramagnetic metal that induces a magnetic field to enhance tissue contrast to characterize lesions and assess perfusion and flow-related abnormalities [1, 2]. From 1988, when the first GBCA was introduced to the market, to date, there are nine GBCAs widely used in clinical practice. GBCAs can be classified into linear and macrocyclic chelates according to their chemical structure and ionic and nonionic according to the Gd ionic state [3].
As the use of GBCAs increases, understanding more specific symptomatic differences in the occurrence of HSRs across GBCAs can provide guidance for clinical use. According to an 8-year cohort study [4], the incidence of HSR was 0.4%, which is lower than iodinated contrast media, but concerns remain. However, it is mainly clinical studies [5–7] and case reports [8–10] that focus on HSRs to GBCAs. There are fewer studies of more specific responses to HSRs due to some limitations; only one pharmacovigilance study analyzed data on anaphylaxis from 1988 to 2012 [11]. In addition, it has been shown that in patients who have been treated with GBCAs, Gd may be retained sites, mainly in the bones and skin, with only a small amount present in the brain [12, 13]. The term “gadolinium deposition disease” (GDD) was first proposed by Semelka and colleagues in 2016 to describe symptoms reported by patients with normal renal function after exposure to GBCAs [14]. Although there is still much controversy regarding GDD, the Uppsala Monitoring Centre (UMC) and the FDA have been using GDD as a preferred term (PT) for the collection of reports since the term GDD emerged. However, members of the American College of Radiology’s Committee on Drugs and Contrast Agents in 2022 proposed a new term to describe symptoms reported after intravascular exposure to GBCAs—symptoms associated with gadolinium exposure (SAGE) [15]. This term was used in place of other nomenclatures that assumed causation but had not been scientifically proven, whereas according to the current study, SAGE is a combination that contains multiple PTs. Therefore, combining GDD and SAGE data may provide a more complete picture of the symptoms involved. Currently, similar studies are not comprehensive enough or out of date [16–18], so analyzing GBCA-associated adverse events (AEs) using the latest post-marketing data may provide the most up-to-date data to inform the safe clinical use of GBCAs.
This study aimed to compare the risk of HSRs, and GDD/SAGE of all GBCAs using disproportionality analysis, namely gadopentetate dimeglumine, gadobenate dimeglumine, gadoxetate disodium, gadoterate meglumine, gadodiamide, gadoversetamide, gadoteridol, and gadobutrol (gadofosveset was excluded because of too little data) in clinical practice by using two of the world’s largest databases of AEs, the World Health Organization database VigiBase or the FDA Adverse Event Reporting System (FAERS). However, it is important to emphasize that disproportionality analysis is a hypothesis-generating activity and represents potential statistical associations alone without causality [19]; explicit causality requires further research and verification. We additionally approximated the frequency of HSRs from 2008 to 2022 based on the VigiBase and the IQVIA Multinational Integrated Data Analysis System (IQVIA-MIDAS) to compensate for the two safety databases’ incapacity to do so without having the whole number of uses.
Methods
Data sources
This study extracted data from VigiBase (from inception to July 3, 2023) and FAERS (from inception to March 31, 2023) for GBCAs. VigiBase is the unique WHO global database of reported potential side effects of medicinal products, developed and maintained by UMC. With more than 30 million reports of suspected AEs of medications filed by the WHO Programme for International Drug Monitoring member nations since 1968, it is the largest database of its kind in the world. The FAERS is a database that contains AE reports, medication error reports and product quality complaints resulting in AEs that were submitted to the FDA. The FDA’s post-marketing safety surveillance program for drugs and therapeutic biologic products is intended to be supported by the database. We mined and cleaned pharmacovigilance data from FAERS using the open tool OpenVigil 2.1 [20]. In addition, we extracted the total sales volume of each GBCA from the IQVIA-MIDAS [21] from 2008 to 2022, with sales volume in standardized units. IQVIA-MIDAS is internally validated through other sales data sources and shows a high level of data accuracy [22].
Study design
This study included all HSR-related AEs categorized by group queries according to the Medical Dictionary for Drug Regulatory Activities (MedDRA) 26.0. The narrow version of the standardized MedDRA query (SMQ) was used to define HSRs because it provides better predictability while retaining comparable sensitivity to the broad version [23]. Based on allergic symptoms, we defined HSRs in the following three SMQ levels: angioedema, severe cutaneous adverse reactions (SCARs), and anaphylactic/anaphylactoid shock conditions (ASCs). PTs in hypersensitivity or anaphylactic reactions but not included in the three SMQ levels were regarded as anaphylactic reactions, others. In addition, GDD was retrieved at the PT level in the FAERS and VigiBase. Based on a study [24], we categorized the symptoms included in SAGE into 29 PTs under 6 system organ classes and further analyzed SAGE using the FAERS database.
Four patterns of drugs were identified: primary suspect drug, secondary suspect drug, interacting drug, and concomitant drug. According to the procedure used by the UMC, concomitant-related records were eliminated to improve signal strength [25]. Since GDD and SAGE are rare and still controversial, we only estimate the frequency of HSRs of GBCAs from 2008 to 2022 based on VigiBase and IQVIA-MIDAS. The United Nations list of geographical divisions was used for the division of regions or countries. Active ingredient names were used when searching in the VigiBase, FAERS, and IQVIA-MIDAS databases.
Statistical analysis
Reporting odds ratio (ROR) was employed to show whether risk signals were present. The signal of disproportionate reporting was defined as ROR025 greater than one and at least three cases. We used Microsoft Office Excel 2016 (Los Angeles, CA, USA) to calculate demographic information and disproportionality analysis results. Calculations of ROR and 95%CI were based on 2 × 2 contingency tables. The formulas are:
| Drug category | Event of interest | All other events |
|---|---|---|
| Target drugs | a | b |
| All other drugs in the database | c | d |
The frequency of HSRs was obtained by dividing the number of reports in VigiBase by the total sales volume (measured in millions) over 2008–2022 in IQVIA-MIDAS.
Results
Descriptive analysis of HSRs in VigiBase
In VigiBase, regarding HSRs, the number of cases of different GBCAs in descending order was gadobutrol (n = 9454), gadoterate meglumine (n = 6489), gadopentetate dimeglumine (n = 5160), gadobenate dimeglumine (n = 3653), gadoteridol (n = 2997), gadoxetate disodium (n = 1250), gadodiamide (n = 1064), and gadoversetamide (n = 323). Except for gadoxetate disodium, all GBCAs were reported more by females than males. The reports were mainly concentrated among patients aged 18–64 years. The primary reporting regions for the different GBCAs varied, with gadopentetate dimeglumine, gadobenate dimeglumine, gadodiamide, and gadoversetamide reported predominantly from the Americas; gadoxetate disodium and gadobutrol reported mainly from Asia; and gadoterate meglumine and gadoteridol reported primarily from Europe. The annual reporting rates for individual GBCAs were unstable from 2008 to 2022. The lowest reported rate of serious events (including death, life-threatening, disability, and hospitalization) was gadoxetate disodium (4.0%), while the highest was gadobenate dimeglumine (22.91%) (Table 1).
Table 1.
Characteristics of reports with suspected HSRs sourced from VigiBase
| Gadopentetate dimeglumine | Gadobenate dimeglumine | Gadoxetate disodium | Gadoterate meglumine | Gadodiamide | Gadoversetamide | Gadoteridol | Gadobutrol | ||
|---|---|---|---|---|---|---|---|---|---|
| Total | 5160 | 3653 | 1250 | 6489 | 1064 | 323 | 2997 | 9454 | |
| Gender | F | 3005 (58.24%) | 2202 (60.28%) | 521 (41.68%) | 4123 (63.54%) | 579 (54.42%) | 120 (37.15%) | 1842 (61.46%) | 5737 (60.68%) |
| M | 1800 (34.88%) | 1275 (34.90%) | 551 (44.08%) | 2188 (33.72%) | 336 (31.58%) | 70 (21.67%) | 1046 (34.90%) | 3202 (33.87%) | |
| Unknown | 3556.88%) | 176 (4.82%) | 178 (14.24%) | 178 (2.74%) | 149 (14.00%) | 133 (41.18%) | 109 (3.64%) | 515 (5.45%) | |
| Age | 0–27 days | 1 (0.02%) | 3 (0.08%) | 0 (0%) | 5 (0.08%) | 0 (0%) | 0 (0%) | 3 (0.10%) | 13 (0.14%) |
| 28 days–23 months | 8 (0.16%) | 1 (0.03%) | 0 (0%) | 11 (0.17%) | 2 (0.19%) | 0 (0%) | 1 (0.03%) | 7 (0.07%) | |
| 2–11 years | 85 (1.65%) | 12 (0·33%) | 2 (0.16%) | 130 (2.00%) | 7 (0.66%) | 1 (0.31%) | 23 (0.77%) | 65 (0.69%) | |
| 12–17 years | 138 (2.67%) | 60 (1.64%) | 2 (0.16%) | 199 (3.07%) | 27 (2.54%) | 1 (0.31%) | 58 (1.94%) | 205 (2.17%) | |
| 18–44 years | 1852 (35.89%) | 1097 (30.03%) | 198 (15.84%) | 2251 (34.69%) | 319 (29.98%) | 55 (17.03%) | 991 (33.07%) | 2903 (30.71%) | |
| 45–64 years | 1680 (32.56%) | 1331 (36.44%) | 584 (46.72%) | 2386 (36.77%) | 323 (30.36%) | 57 (17.65%) | 1144 (38.17%) | 3725 (39.40%) | |
| 65–74 years | 461 (8.93%) | 452 (12.37%) | 188 (15.04%) | 638 (9.83%) | 91 (8.55%) | 11 (3.41%) | 348 (11.61%) | 1119 (11.84%) | |
| ≥ 75 years | 214 (4.15%) | 207 (5.67%) | 66 (5.28%) | 248 (3.82%) | 39 (3.67%) | 6 (1.86%) | 155 (5.17%) | 409 (4.33%) | |
| Unknown | 721 (13.97%) | 490 (13.41%) | 210 (16.80%) | 621 (9.57%) | 256 (24.06%) | 192 (59.44%) | 274 (9.14%) | 1008 (10.66%) | |
| UN Continent | Americas | 3404 (65.97%) | 1678 (45.93%) | 227 (18.16%) | 787 (12.13%) | 590 (55.45%) | 312 (96.59%) | 674 (22.49%) | 2321 (24.55%) |
| Asia | 761 (14.75%) | 466 (12.76%) | 870 (69.60%) | 2217 (34.17%) | 149 (14.00%) | 8 (2.48%) | 820 (27.36%) | 4152 (43.92%) | |
| Europe | 899 (17.42%) | 1460 (39.97%) | 138 (11.04%) | 3381 (52.09%) | 278 (26.13%) | 2 (0.62%) | 1503 (50.15%) | 2681 (28.36%) | |
| Oceania | 89 (1.72%) | 47 (1.29%) | 14 (1.12%) | 100 (1.54%) | 41 (3.85%) | 1 (0.31%) | 0 (0%) | 289 (3.06%) | |
| Africa | 7 (0.14%) | 2 (0.05%) | 1 (0.08%) | 5 (0.08%) | 6 (0.56%) | 0 (0%) | 0 (0%) | 11 (0.12%) | |
| Years of reporting | 2022 | 93 (1.80%) | 89 (2.44%) | 42 (3.36%) | 523 (8.06%) | 23 (2.16%) | 0 (0%) | 204 (6.81%) | 599 (6.34%) |
| 2021 | 101 (1.96%) | 105 (2.87%) | 95 (7.60%) | 684 (10.54%) | 13 (1.22%) | 0 (0%) | 316 (10.54%) | 882 (9.33%) | |
| 2020 | 93 (1.80%) | 247 (6.76%) | 137 (10.96%) | 632 (9.74%) | 20 (1.88%) | 0 (0%) | 408 (13.61%) | 908 (9.60%) | |
| 2019 | 203 (3.93%) | 286 (7.83%) | 241 (19.28%) | 794 (12.24%) | 148 (13.91%) | 4 (1.24%) | 362 (12.08%) | 1002 (10.60%) | |
| 2018 | 71 (1.38) | 173 (4.74%) | 111 (8.88%) | 880 (13.56%) | 23 (2.16%) | 10 (3.10%) | 349 (11.64%) | 1153 (12.20%) | |
| 2017 | 87 (1.69%) | 242 (6.62%) | 102 (8.16%) | 540 (8.32%) | 29 (2.73%) | 3 (0.93%) | 187 (6.24%) | 888 (9.39%) | |
| 2016 | 93 (1.80%) | 276 (7.56%) | 75 (6.00%) | 373 (5.75%) | 37 (3.48%) | 8 (2.48%) | 162 (5.41%) | 736 (7.79%) | |
| 2015 | 152 (2.95%) | 291 (7.97%) | 97 (7.76%) | 403 (6.21%) | 56 (5.26%) | 12 (3.72%) | 82 (2.74%) | 773 (8.18%) | |
| 2014 | 214 (4.15%) | 473 (12.95%) | 105 (8.40%) | 429 (6.61%) | 41 (3.85%) | 5 (1.55%) | 103 (3.44%) | 648 (6.85%) | |
| 2013 | 99 (1.92%) | 113 (3.09%) | 53 (4.24%) | 140 (2.16%) | 26 (2.44%) | 2 (0.62%) | 70 (2.34%) | 335 (3.54%) | |
| 2012 | 276 (5.35%) | 140 (3.83%) | 48 (3.84%) | 135 (2.08%) | 42 (3.95%) | 11 (3.41%) | 55 (1.84%) | 382 (4.04%) | |
| 2011 | 360 (6.98%) | 194 (5.31%) | 31 (2.48%) | 155 (2.39%) | 86 (8.08%) | 31 (9.60%) | 66 (2.20%) | 232 (2.45%) | |
| 2010 | 385 (7.46%) | 174 (4.76%) | 15 (1.20%) | 79 (1.22%) | 76 (7.14%) | 39 (12.07%) | 93 (3.10%) | 117 (1.24%) | |
| 2009 | 557 (10.79%) | 177 (4.85%) | 8 (0.64%) | 32 (0.49%) | 93 (8.74%) | 59 (18.27%) | 100 (3.34%) | 49 (0.52%) | |
| 2008 | 295 (5.72%) | 475 (13.00%) | 1 (0.08%) | 70 (1.08%) | 58 (5.45%) | 10 (3.10%) | 98 (3.27%) | 42 (0.44%) | |
| Outcome | Death | 55 (1.07%) | 45 (1.23%) | 0 (0.00%) | 14 (0.22%) | 32 (3.01%) | 22 (6.81%) | 30 (1.00%) | 50 (0.53%) |
| Life-threatening | 154 (2.98%) | 268 (7.34%) | 17 (1.36%) | 241 (3.71%) | 30 (2.82%) | 3 (0.93%) | 186 (6.21%) | 257 (2.72%) | |
| Disability | 24 (0.47%) | 15 (0.41%) | 1 (0.08%) | 13 (0.20%) | 28 (2.63%) | 13 (4.02%) | 14 (0.47%) | 27 (0.29%) | |
| Hospitalization | 231 (4.48%) | 509 (13.93%) | 32 (2.56%) | 507 (7.81%) | 50 (4.70%) | 24 (7.43%) | 330 (11.01%) | 589 (6.23%) | |
| Other | 4696 (91.01%) | 2816 (77.09%) | 1200 (96.00%) | 5714 (88.06%) | 924 (86.84%) | 261 (80.80%) | 2437 (81.31%) | 8531 (90.24%) | |
| Reported rate of serious events | 8.99% | 22.91% | 4.00% | 11.94% | 13.16% | 19.2% | 18.69% | 9.26% |
HSR hypersensitivity reactions, UN United Nations
Disproportionality analysis results of suspected AEs in FAERS and VigiBase
As shown in Tables 2 and 3, all GBCAs showed significant signals of HSRs in both FAERS and VigiBase. For nonionic GBCAs, the results of both databases indicated that the two linear GBCAs had the lowest probability of HSRs among all GBCAs, with gadodiamide (ROR025 = 2.24 and 1.44 (in VigiBase and FAERS, respectively)) having a lower ROR025 than gadoversetamide (ROR025 = 3.56 and 1.76). For ionic GBCAs, the FAERS results showed that all linear GBCAs had lower ROR025 than the macrocyclic type, whereas the VigiBase results showed that only the other two linear GBCAs, except for gadobenate dimeglumine, had lower ROR025 than the macrocyclic GBCAs.
Table 2.
Disproportionality analysis results of all suspected HSRs associated with GBCAs in VigiBase
| Target GBCAs | All other drugs | ||||
|---|---|---|---|---|---|
| Event (a) | No event (b) | Event (c) | No event (d) | ROR (95% CI) | |
| Gadopentetate dimeglumine | 6414 | 5280 | 5,618,410 | 29,339,215 | 6.35 (6.12–6.58) |
| Gadobenate dimeglumine | 4270 | 2503 | 5,476,204 | 29,486,344 | 9.19 (8.74–9.65) |
| Gadoxetate disodium | 1371 | 1257 | 5,200,202 | 29,766,491 | 6.24 (5.78–6.74) |
| Gadoterate meglumine | 7540 | 5371 | 5,598,164 | 29,358,246 | 7.36 (7.11–7.62) |
| Gadodiamide | 1240 | 2882 | 5,327,434 | 29,637,765 | 2.39 (2.24–2.56) |
| Gadoversetamide | 385 | 589 | 4,866,805 | 30,101,542 | 4.04 (3.56–4.60) |
| Gadoteridol | 3546 | 2029 | 5,406,117 | 29,557,629 | 9.56 (9.05–10.09) |
| Gadobutrol | 11,507 | 6213 | 5,636,201 | 29,315,400 | 9.63 (9.34–9.94) |
GBCAs gadolinium-based contrast agents, CI confidence interval, ROR reporting odds ratio, HSRs hypersensitivity reactions
Table 3.
Disproportionality analysis results of all suspected HSRs associated with GBCAs in FAERS
| Target GBCAs | All other drugs | ||||
|---|---|---|---|---|---|
| Event (a) | No event (b) | Event (c) | No event (d) | ROR (95% CI) | |
| Gadopentetate dimeglumine | 2028 | 2571 | 870,039 | 10,280,468 | 9.32 (8.79–9.88) |
| Gadobenate dimeglumine | 1052 | 1346 | 835,625 | 10,317,083 | 9.65 (8.90–10.46) |
| Gadoxetate disodium | 109 | 151 | 736,957 | 10,417,889 | 10.20 (7.98–13.06) |
| Gadoterate meglumine | 408 | 327 | 860,947 | 10,293,424 | 14.92 (12.90–17.25) |
| Gadodiamide | 194 | 1556 | 773,622 | 10,379,734 | 1.67 (1.44–1.94) |
| Gadoversetamide | 96 | 646 | 712,441 | 10,441,923 | 2.18 (1.76–2.70) |
| Gadoteridol | 361 | 562 | 799,682 | 10,354,501 | 8.32 (7.29–9.49) |
| Gadobutrol | 1443 | 1267 | 877,486 | 10,274,910 | 13.34 (12.37–14.38) |
GBCAs gadolinium-based contrast agents, CI confidence interval, ROR reporting odds ratio, HSRs hypersensitivity reactions
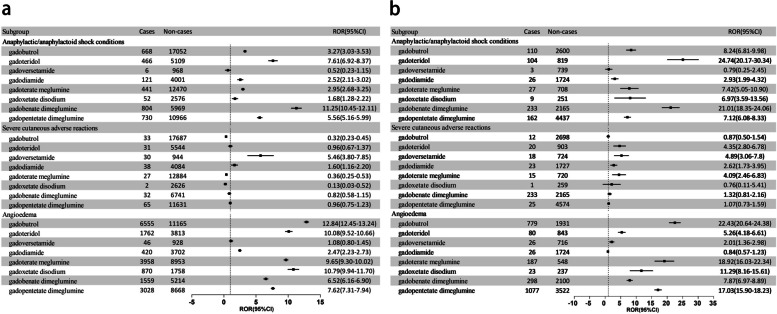
Figure 1a and 1b display the results of disproportionality analysis for three SMQ levels in VigiBase and FAERS. For ASCs, in both databases, it was shown that the ROR025 was greater for gadobenate dimeglumine (ROR025 = 10.45 and 18.35 (in VigiBase and FAERS, respectively)) and gadoteridol (ROR025 = 6.92 and 20.17), with no significant signal for gadoversetamide. For SCARs, both databases indicated that gadoversetamide (ROR025 = 3.80 and 3.06) had the most significant signal; gadopentetate dimeglumine, gadobenate dimeglumine, gadoxetate disodium, and gadobutrol had no significant signals. For angioedema, gadobutrol (ROR025 = 12.45 and 20.64) had the largest ROR025, gadodiamide had the smallest ROR025 in VigiBase (ROR025 = 2.23), and no significant signal in FAERS, while gadoversetamide had no significant signal in VigiBase and the smallest ROR025 (ROR025 = 1.36) in FAERS. Overall, the risk of angioedema with nonionic linear GBCAs was minimal.
Fig. 1.
a Disproportionality analysis results of hypersensitivity-associated SMQ levels in VigiBase. b Disproportionality analysis results of hypersensitivity-associated SMQ levels in FAERS. SMQ, standardized MedDRA query
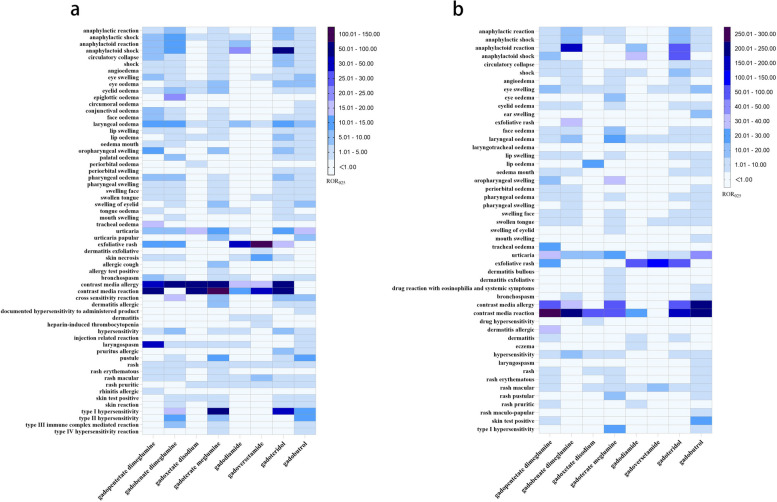
We further analyzed the PTs under SMQ levels comparatively, and the differences are shown in Fig. 2a and 2b, with specific values shown in the additional file (Additional file 1: Table S1-S2). Based on the results of the two databases, the following results can be summarized: gadopentetate dimeglumine had the highest likelihood of eye swelling (ROR025 = 6.06 and 11.16 (in VigiBase and FAERS, respectively)), swollen tongue (ROR025 = 3.93 and 6.35), and tracheal oedema (ROR025 = 18.94 and 27.70); gadobenate dimeglumine had the strongest signal regarding anaphylactic reaction (ROR025 = 9.56 and 16.53) and anaphylactoid reaction (ROR025 = 10.63 and 163.81); for gadoterate meglumine, swelling of eyelid (ROR025 = 6.92 and 7.26) and type I hypersensitivity (ROR025 = 77.72 and 24.82) were the highest signal; regarding gadoversetamide, the ROR025 of exfoliative rash (ROR025 = 110.98 and 125.14) and rash macular (ROR025 = 9.3 and 10.95) were the most significant; for gadoteridol, anaphylactoid shock (ROR025 = 56.22 and 58.05), circulatory collapse (ROR025 = 5.99 and 3.86), and shock (ROR025 = 7.40 and 18.55) were the strongest signal; gadobutrol had the highest signal about lip swelling (ROR025 = 3.59 and 5.94), mouth swelling (ROR025 = 1.84 and 1.52), urticaria (ROR025 = 18.91 and 41.76), and skin test positive (ROR025 = 2.79 and 23.07).
Fig. 2.
a Disproportionality analysis results of hypersensitivity-associated PTs in VigiBase. b Disproportionality analysis results of hypersensitivity-associated PTs in FAERS. PT, preferred term
The disproportionality analysis results of GDD are shown in Table 4. According to the results of VigiBase, linear GBCAs had a higher risk than macrocyclic GBCAs. Gadobutrol and gadobenate dimeglumine had higher ROR025 based on FAERS results. For SAGE, no GBCAs showed signals in psychiatric disorders; all GBCAs prompted signals in ≥ 10 PTs except gadoxetate disodium, which did not prompt signals at any of the PTs; gadopentetate dimeglumine had a minimum number of signals at the PT level of only 10; gadodiamide and gadoversetamide prompted signals in 15 PTs, both prompted the highest number of signals, and gadoversetamide generated the strongest signal in 14 PTs. What is more, except for gadoversetamide, the risk of gadopentetate dimeglumine, gadobenate dimeglumine, gadodiamide, and gadoteridol regarding skin induration requires extra attention. Gadodiamide and gadoteridol have a higher risk of skin tightness than other GBCAs. The specific values of the signals are shown in Table 5.
Table 4.
Disproportionality analysis results for suspected GDD cases of GBCAs in FAERS and VigiBase
| Subgroup | FAERS | VigiBase | ||
|---|---|---|---|---|
| n | ROR025 | n | ROR025 | |
| Gadopentetate dimeglumine | 38 | 425.13 | 168 | 1748.36 |
| Gadobenate dimeglumine | 48 | 1145.87 | 174 | 3246.28 |
| Gadoxetate disodium | 1 | 31.65 | 127 | 5123.18 |
| Gadoterate meglumine | 17 | 775.42 | 20 | 89.38 |
| Gadodiamide | 27 | 707.39 | 157 | 4525.03 |
| Gadoversetamide | 18 | 981.19 | 144 | 18,618.41 |
| Gadoteridol | 5 | 134.05 | 11 | 95.83 |
| Gadobutrol | 74 | 1995.04 | 71 | 323.81 |
ROR reporting odds ratio, GBCAs gadolinium-based contrast agents, GDD gadolinium deposition disease
Table 5.
ROR025 of suspected SAGE-related PTs
| Gadopentetate dimeglumine | Gadobenate dimeglumine | Gadoxetate disodium | Gadoterate meglumine | Gadodiamide | Gadoversetamide | Gadoteridol | Gadobutrol | |
|---|---|---|---|---|---|---|---|---|
| Skin and subcutaneous tissue disorders | ||||||||
| Pain of skin | 10.904 | 14.933 | / | 2.518 | 29.934 | 49.283 | 28.921 | / |
| Skin burning sensation | 2.628 | 3.766 | / | 4.539 | 2.668 | 5.396 | 2.247 | 4.471 |
| Skin discoloration | / | / | / | 2.074 | 10.778 | 17.82 | 13.686 | 2.045 |
| Skin induration | 235.878 | 195.828 | / | / | 488.827 | 655.195 | 400.833 | 7.906 |
| Skin tightness | / | / | / | 18.855 | 233.292 | / | 271.484 | 6.609 |
| Musculoskeletal and connective tissue disorders | ||||||||
| Arthralgia | 1.12 | 1.093 | / | / | 2.31 | 2.19 | 1.049 | / |
| Bone pain | / | / | / | 4.21 | 3.953 | 6.304 | 3.016 | 2.177 |
| Joint stiffness | / | / | / | / | / | 40.735 | 24.8 | / |
| Muscle fatigue | / | / | / | / | / | 1.169 | / | / |
| Muscle tightness | 7.548 | 4.041 | / | 1.867 | 7.371 | 13.167 | 10.56 | 2.819 |
| Muscle twitching | / | / | 6.986 | / | / | / | 5.372 | |
| Muscular weakness | 2.138 | 2.069 | / | 1.602 | 4.929 | 6.085 | 4.456 | / |
| Musculoskeletal chest pain | / | 1.903 | / | / | / | / | / | / |
| Pain in extremity | 2.057 | 1.268 | / | / | 2.823 | 2.455 | 1.884 | / |
| General disorders and administration site conditions | ||||||||
| Asthenia | / | / | / | / | 1.195 | 1.319 | 1.175 | / |
| Pain | 4.519 | 6.109 | / | / | 11.639 | 37.736 | 18.065 | / |
| Nervous system disorders | ||||||||
| Cognitive disorder | / | 1.215 | / | 1.757 | 1.471 | 2.588 | / | 2.283 |
| Headache | / | / | / | 2.154 | / | / | / | 1.112 |
| Paraesthesia | 1.932 | 2.201 | / | 6.937 | 1.367 | / | / | 2.502 |
| Neuralgia | / | / | / | 1.415 | / | / | / | / |
| Investigations | ||||||||
| Quality of life decreased | 9.895 | 6.255 | / | / | 17.876 | 38.002 | 16.395 | 1.739 |
| Number of signals at PT level | 10 | 12 | 0 | 12 | 15 | 15 | 14 | 11 |
ROR reporting odds ratio, SAGE symptoms associated with gadolinium exposure, PTs preferred terms
Time to onset analysis of suspected HSRs
Excluding reports with unknown onset times, the probability of HSRs within 1 day for individual GBCAs ranged from 93.73 to 98.89%, with those greater than 1 day being predominantly associated with SCARs.
Estimation of the frequency of suspected HSRs
Based on data from the IQVIA-MIDAS database from 2008 to 2022, the usage frequency of the different GBCAs varied by region, with gadobutrol (24.92%) having the highest rate of use in the Americas, gadopentetate dimeglumine (48.01%) in Asia, and gadoterate meglumine (48.98%) in Europe (Additional file 1: Fig. S1).
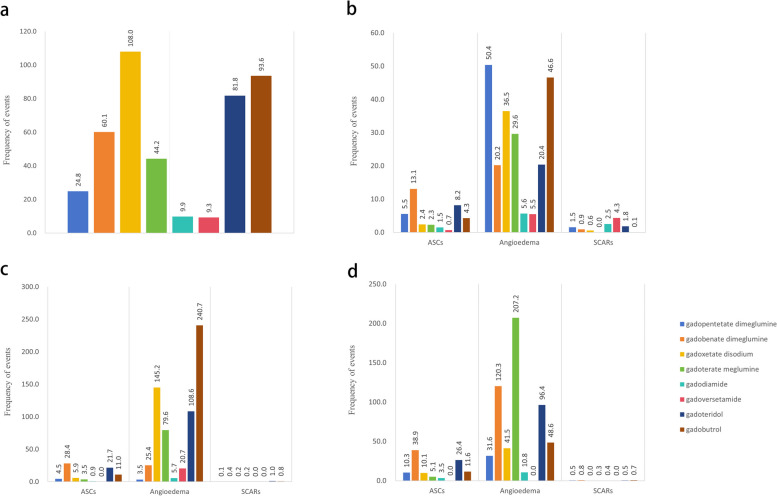
The total sales volumes for 2008–2022 are shown in the additional file (Additional file 1: Table S3), and the highest total frequency of the three SMQs was gadoxetate disodium (Fig. 3a). In addition, we estimated the frequency of each SMQ level for the Americas, Asia, and Europe, and the results are shown in Fig. 3b–d, with sales in the three regions for the years 2008–2022 also displayed (Additional file 1: Table S4). Concerning ASCs, in all three continents, the frequency of ASCs was highest for gadobenate dimeglumine and lowest for gadoversetamide. In the Americas, Asia, and Europe, the highest frequency of angioedema was gadopentetate dimeglumine, gadobutrol, and gadoterate meglumine, respectively; for SCARs, the highest frequency was gadoversetamide, gadoteridol, and gadobenate dimeglumine, respectively.
Fig. 3.
a Total frequency of the three SMQ levels in three regions from 2008 to 2022. b Total frequency of the three SMQ levels in the Americas from 2008 to 2022. c Total frequency of the three SMQ levels in Asia from 2008 to 2022. d Total frequency of the three SMQ levels in Europe from 2008 to 2022. SMQ, standardized MedDRA query; ASCs, anaphylactic/anaphylactoid shock conditions; SCARs, severe cutaneous adverse reactions
Discussion
GBCAs have been used internationally for more than 35 years in hundreds of millions of patients. Although GBCAs are relatively safe, several studies have reported some AEs associated with GBCAs, including HSRs and GDD/SAGE. This study compared the safety of all GBCAs regarding HSRs and GDD/SAGE by analyzing the global post-marketing data, providing data to support a more rational clinical management of GBCA-associated AEs.
Based on demographic information, AE reports were concentrated in the 18–64 age group, with a predominance of those ≥ 45 age group, which may be because middle-aged and older adults are the main population using GBCAs. AE reports on females make up the majority of HSR reports. This may be related to the existence of genetic differences between the sexes. Regarding regions, the subject countries of reported AEs varied among GBCAs, which in combination with sales data is mainly, but not absolutely, related to the usage preference of each region. For example, Asia, which has the third highest sales of gadobutrol among the three regions, reported more AEs regarding HSRs than the Americas and Europe, which may be related to genetic and ethnic factors. In our study, the frequency was lower than in previous studies, which may be due to the fact that VigiBase and FAERS are spontaneous reporting systems (SRSs) that do not cover all AEs during the course of the study as in the clinical randomized trials.
Allergy is one of the triggers of angioedema [26]. In the general population, the incidence of severe allergic reactions is 0.01–0.03% [27]. However, 46% of allergic reactions are accompanied by angioedema [28]. Angioedema caused by GBCAs can occur in the face, eyes, lips, throat, etc., and has also been reported in the small intestine [29]. The results of this study showed that gadobutrol had a greater ROR025 for angioedema. However, when considering the amount of use, gadobutrol had the highest frequency of angioedema in Asia, but its percentage of use in Asia was only in the fourth place, which may be related to reporting bias or racial differences. The site of occurrence of angioedema differs among drugs; therefore, when using a particular GBCA, special attention needs to be paid to its high-risk site.
AEs associated with ASCs have been reported in the literature more frequently and sometimes fatally [30–32]. According to the results of FAERS and VigiBase, the ROR025 of gadobenate dimeglumine and gadoteridol is larger. Among the three regions, gadobenate dimeglumine had the highest frequency of ASCs, followed by gadoteridol. Shock is a more serious reaction, and patients who experience shock may not have a history of allergy. Therefore, preparation of resuscitation measures is necessary before administering GBCAs. In addition, skin testing may be beneficial in patients with a history of systemic reactions to Gd [33].
SCARs were mainly urticaria, exfoliative rash, etc., and GBCAs were classified as drugs that could cause acute generalized exanthematous pustulosis [8]. Preclinical research indicates that Gd compounds may activate dermal fibroblasts, encourage their proliferation, and aid in the development of myofibroblasts, hence inducing profibrotic reactions in the skin in vitro. Moreover, Gd affects the synthesis of collagen and the amount of collagen in the skin [34]. In FAERS, only gadoversetamide and gadodiamide hinted the signal for SCARs, while in VigiBase, in addition to gadoversetamide and gadodiamide, gadoteridol and gadoterate meglumine also hinted the signal. Although SCARs are rare, similar reactions may occur multiple times in sensitive patients and may be delayed, so they should be evaluated with caution if reused and observed for several days after use. The frequency of SCARs varies by region, which may be related to regional usage preferences or ethnicity.
GDD was proposed in 2016 [35]; there are several hypotheses about GDD but the mechanism of occurrence and pathophysiology is still unclear. However, some studies have suggested that GDD is more likely to occur with linear GBCAs [36], and the results of VigiBase reaffirm this conclusion. SAGE was proposed in 2018 to describe the symptoms associated with exposure to GBCAs and is used to help researchers report the associated symptoms in a more standardized way to allow for a more coherent interpretation of related studies. GDD can also be considered a subset under the symptoms associated with the gadolinium exposure rubric [35]. This study found differences in the risk of different GBCAs occurring in different symptoms by analyzing the data in FAERS, with gadoversetamide having the strongest signal in all 14 PTs, but in terms of sales volume, its use is now very low, and the risk of associated ADEs is considerably lower. In contrast, gadoxetate disodium did not suggest a suspicious signal. In addition, five GBCAs had a higher risk of skin induration; gadodiamide and gadoteridol also need more attention regarding skin tightness. The AEs of GBCAs involve multiple systems and can ultimately affect a patient’s quality of life, so it is important to prevent the associated risks. The National Institutes of Health held a meeting in 2018 to clarify the direction of future research on gadolinium retention [37]. It is hoped that the results of this study will provide data to support subsequent research.
This study, like many studies of pharmacovigilance databases, has certain limitations. Firstly, the FAERS and VigiBase are SRSs with diverse, uncertain data sources, and the number of reports for a given drug may be affected by the extent of the product’s use, publicity, and the nature of the AEs, as well as underreporting or overreporting. Therefore, even if the results of disproportionality analysis are significant, it is not possible to confirm a causal relationship between the drug and the AEs. Secondly, we recognize that the estimation of AE frequency is rough because not all patients use a single standard unit of GBCA. In addition, the extent of reporting in different areas can affect the estimation of frequency. Thirdly, the quality of relevant reports cannot be guaranteed as there is still a lot of controversy and the understanding of GDD/SAGE is still limited, so we did not estimate the frequency of GDD/SAGE, and the results of disproportionality analysis are only a preliminary probe based on the current data; more research needs to be continued. Finally, the site of use of GBCAs also affects the frequency of HSRs, and we were unable to analyze site-specific frequency further due to missing data. However, the inclusion of real-world data was as comprehensive and systematic as possible to ensure the reliability and authenticity of this study and provide data support for further research.
Conclusions
Different GBCAs exhibited diverse profiles regarding HSRs, which could be further affected by geographic area. Our study provides additional data support for the prevention of HSRs. As for the differences of GBCAs in GDD/SAGE, this study only analyzed the currently available post-marketing data as a preliminary exploration, and more unbiased studies are needed.
Supplementary Information
Additional file 1: Table S1. Disproportionality analysis results of all PTs of suspected HSRs in VigiBase. Table S2. Disproportionality analysis results of all PTs of suspected HSRs in FAERS. Table S3. Sales volume of GBCAs from 2008 to 2022. Table S4. Sales volume of GBCAs in Americas, Asia, and Europe from 2008 to 2022. Fig. S1. Sales volume share of each GBCA in different regions from 2008 to 2022. GBCA: gadolinium-based contrast agent.
Acknowledgements
The authors acknowledge the Uppsala Monitoring Centre (UMC) who manages and provides the data used in the present study. The views expressed in this study are the personal views of the authors and may not reflect the position of the WHO.
Abbreviations
- Gd
Gadolinium
- HSRs
Hypersensitivity reactions
- GBCAs
Gadolinium-based contrast agents
- SAGE
Symptoms associated with gadolinium exposure
- GDD
Gadolinium deposition disease
- FAERS
FDA Adverse Event Reporting System
- ASCs
Anaphylactic/anaphylactoid shock conditions
- SCARs
Severe cutaneous adverse reactions
- AEs
Adverse events
- IQVIA-MIDAS
IQVIA Multinational Integrated Data Analysis System
- UMC
Uppsala Monitoring Centre
- MedDRA
Medical Dictionary for Drug Regulatory Activities
- SMQ
Standardized MedDRA query
- PTs
Preferred terms
- ROR
Reporting odds ratio
- SRSs
Spontaneous reporting systems
Authors’ contributions
Conceptualization, Y.F.L., G.R.F., and H.Q.; resources, Y.F.L. and G.R.F.; data curation, H.Q., T.T.F., and W.J.L.; methodology, Y.F.L., G.R.F., and H.Q.; formal analysis, H.Q., and N.Y.L.; data curation, H.Q., W.J.L., C.D.Q., and T.S.W.; writing—original draft, H.Q.; visualization, H.Q. and X.L.; writing—review and editing, Y.F.L., G.R.F., H.Q., Y.Y.W., and Z.H.W.; revision: H.Q., Y.Y.W. and Z.H.W.; supervision, Y.F.L., G.R.F., and Z.H.W.; funding acquisition: Y.F.L., G.R.F., and Z.H.W.; all authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81973289), Shanghai Key Clinical Discipline Construction Project (02. DY11.03.19.03), Scientific and Innovative Action Plan of Shanghai (22S21902000 and 23S21900900), and Bethune Charitable Foundation (Z04JKM2023E040).
Availability of data and materials
Requests for access to VigiBase should be directed to CustomSearches@who-umc.org. The data obtained from OpenVigil 2.1 can be found at https://openvigil.sourceforge.net/. The data of IQVIA-MIDAS are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Since data in FAERS, VigiBase, and IQVIA-MIDAS are anonymized and publicly available, the requirements for obtaining informed consent and institutional review board approval were waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Guorong Fan, Email: guorfan@163.com.
Yuefen Lou, Email: louyuefen@sina.cn.
References
- 1.Bellin MF. MR contrast agents, the old and the new. Eur J Radiol. 2006;60(3):314–23. 10.1016/j.ejrad.2006.06.021 [DOI] [PubMed] [Google Scholar]
- 2.Granata V, Cascella M, Fusco R, dell’Aprovitola N, Catalano O, Filice S, Schiavone V, Izzo F, Cuomo A, Petrillo A. Immediate adverse reactions to gadolinium-based MR contrast media: a retrospective analysis on 10,608 examinations. Biomed Res Int. 2016;2016:3918292. 10.1155/2016/3918292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gracia Bara MT, Gallardo-Higueras A, Moreno EM, Laffond E, Muñoz Bellido FJ, Martin C, Sobrino M, Macias E, Arriba-Méndez S, Castillo R, et al. Hypersensitivity to gadolinium-based contrast media. Front Allergy. 2022;3:813927. 10.3389/falgy.2022.813927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn YH, Kang DY, Park SB, Kim HH, Kim HJ, Park GY, Yoon SH, Choi YH, Lee SY, Kang HR. Allergic-like hypersensitivity reactions to gadolinium-based contrast agents: an 8-year cohort study of 154 539 patients. Radiology. 2022;303(2):329–36. 10.1148/radiol.210545 [DOI] [PubMed] [Google Scholar]
- 5.Jung JW, Kang HR, Kim MH, Lee W, Min KU, Han MH, Cho SH. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology. 2012;264(2):414–22. 10.1148/radiol.12112025 [DOI] [PubMed] [Google Scholar]
- 6.Mankouri F, Gauthier A, Srisuwatchari W, Moutou A, Borushko A, Popa L, Ehrhardt Y, Demoly P, Chiriac AM. Hypersensitivity to gadolinium-based contrast agents: a single-center retrospective analysis over 7 years. J Allergy Clin Immunol Pract. 2021;9(4):1746–1749.e1742. 10.1016/j.jaip.2020.11.023 [DOI] [PubMed] [Google Scholar]
- 7.Behzadi AH, Zhao Y, Farooq Z, Prince MR. Immediate Allergic reactions to gadolinium-based contrast agents: a systematic review and meta-analysis. Radiology. 2018;286(2):731. 10.1148/radiol.2017174037 [DOI] [PubMed] [Google Scholar]
- 8.Bordel Gómez MT, Martín García C, Meseguer Yebra C, Zafra Cobo MI, Cardeñoso Álvarez ME, Sánchez Estella J. First case report of acute generalized exanthematous pustulosis (AGEP) caused by gadolinium confirmed by patch testing. Contact Dermatitis. 2018;78(2):166–8. 10.1111/cod.12878 [DOI] [PubMed] [Google Scholar]
- 9.Bianchi L, Hansel K, Marietti R, Tramontana M, Stingeni L. Anaphylaxis after first exposure to gadoterate meglumine: a case report and literature review. J Allergy Clin Immunol Pract. 2018;6(6):2124–6. 10.1016/j.jaip.2018.02.028 [DOI] [PubMed] [Google Scholar]
- 10.Rahman SL, Harbinson MT, Mohiaddin R, Pennell DJ. Acute allergic reaction upon first exposure to gadolinium-DTPA: a case report. J Cardiovasc Magn Reson. 2005;7(5):849–51. 10.1080/10976640500288206 [DOI] [PubMed] [Google Scholar]
- 11.Raisch DW, Garg V, Arabyat R, Shen X, Edwards BJ, Miller FH, McKoy JM, Nardone B, West DP. Anaphylaxis associated with gadolinium-based contrast agents: data from the Food and Drug Administration’s Adverse Event Reporting System and review of case reports in the literature. Expert Opin Drug Saf. 2014;13(1):15–23. 10.1517/14740338.2013.832752 [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi M, Levendovszky SR, Hippe DS, Hasegawa M, Murata N, Murata K, Marshall DA, Gonzalez-Cuyar LF, Maravilla KR. Comparison of human tissue gadolinium retention and elimination between gadoteridol and gadobenate. Radiology. 2021;300(3):559–69. 10.1148/radiol.2021204320 [DOI] [PubMed] [Google Scholar]
- 13.Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, Maravilla KR. Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol. 2016;51(7):447–53. 10.1097/RLI.0000000000000252 [DOI] [PubMed] [Google Scholar]
- 14.Semelka RC, Ramalho J, Vakharia A, AlObaidy M, Burke LM, Jay M, Ramalho M. Gadolinium deposition disease: Initial description of a disease that has been around for a while. Magn Reson Imaging. 2016;34(10):1383–90. 10.1016/j.mri.2016.07.016 [DOI] [PubMed] [Google Scholar]
- 15.McDonald RJ, Weinreb JC, Davenport MS. Symptoms associated with gadolinium exposure (SAGE): a suggested term. Radiology. 2022;302(2):270–3. 10.1148/radiol.2021211349 [DOI] [PubMed] [Google Scholar]
- 16.Edwards BJ, Laumann AE, Nardone B, Miller FH, Restaino J, Raisch DW, McKoy JM, Hammel JA, Bhatt K, Bauer K, et al. Advancing pharmacovigilance through academic-legal collaboration: the case of gadolinium-based contrast agents and nephrogenic systemic fibrosis-a Research on Adverse Drug Events and Reports (RADAR) report. Br J Radiol. 2014;87(1042):20140307. 10.1259/bjr.20140307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semelka RC, Prybylski JP, Ramalho M. Influence of excess ligand on nephrogenic systemic fibrosis associated with nonionic, linear gadolinium-based contrast agents. Magn Reson Imaging. 2019;58:174–8. 10.1016/j.mri.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Wang Y, Zhao Q. Data mining and analysis of the adverse events derived signals of 4 gadolinium-based contrast agents based on the US Food and drug administration adverse event reporting system. Expert Opin Drug Saf. 2024;23(3):339–52. 10.1080/14740338.2023.2271834 [DOI] [PubMed] [Google Scholar]
- 19.Almenoff JS, Pattishall EN, Gibbs TG, DuMouchel W, Evans SJ, Yuen N. el statistical tools for monitoring the safety of marketed drugs. Clin Pharmacol Ther. 2007;82(2):157–66. 10.1038/sj.clpt.6100258 [DOI] [PubMed] [Google Scholar]
- 20.Böhm R, Bulin C, Waetzig V, Cascorbi I, Klein HJ, Herdegen T. Pharmacovigilance-based drug repurposing: The search for inverse signals via OpenVigil identifies putative drugs against viral respiratory infections. Br J Clin Pharmacol. 2021;87(11):4421–31. 10.1111/bcp.14868 [DOI] [PubMed] [Google Scholar]
- 21.IQVIA MIDAS® [https://www.iqvia.com/solutions/commercialization/brand-strategy-and-management/market-measurement/midas]
- 22.IQVIA. ACTS: IQVIA quality assurance [https://www.iqvia.com/landing/acts]
- 23.Lin X, Yang J, Weng L, Lin W. Differences in hypersensitivity reactions to iodinated contrast media: analysis of the US Food and Drug Administration Adverse Event Reporting System Database. J Allergy Clin Immunol Pract. 2023;11(5):1494–1502.e1496. 10.1016/j.jaip.2023.01.027 [DOI] [PubMed] [Google Scholar]
- 24.Shahid I, Joseph A, Lancelot E. Use of real-life safety data from international pharmacovigilance databases to assess the importance of symptoms associated with gadolinium exposure. Invest Radiol. 2022;57(10):664–73. 10.1097/RLI.0000000000000880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C, Chen T, Liang J, Guo X, Xu J, Zheng Y, Guo Z, Chi L, Wei L, Chen X, et al. Cardiotoxicity induced by immune checkpoint inhibitors: a pharmacovigilance study from 2014 to 2019 based on FAERS. Front Pharmacol. 2021;12:616505. 10.3389/fphar.2021.616505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn J, Hoffmann TK, Bock B, Nordmann-Kleiner M, Trainotti S, Greve J. Angioedema. Dtsch Arztebl Int. 2017;114(29–30):489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moneret-Vautrin DA, Morisset M, Flabbee J, Beaudouin E, Kanny G. Epidemiology of life-threatening and lethal anaphylaxis: a review. Allergy. 2005;60(4):443–51. 10.1111/j.1398-9995.2005.00785.x [DOI] [PubMed] [Google Scholar]
- 28.Worm M, Edenharter G, Ruëff F, Scherer K, Pföhler C, Mahler V, Treudler R, Lang R, Nemat K, Koehli A, et al. Symptom profile and risk factors of anaphylaxis in Central Europe. Allergy. 2012;67(5):691–8. 10.1111/j.1398-9995.2012.02795.x [DOI] [PubMed] [Google Scholar]
- 29.Maarek R, Sellier N, Seror O, Sutter O. Small bowel angioedema due to intravenous administration of gadobenate dimeglumine. Diagn Interv Imaging. 2019;100(7–8):459–60. 10.1016/j.diii.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 30.Simons CW, Benouni S, Gibbon G, Klaustermeyer W. Severe anaphylactoid shock secondary to gadolinium contrast media. Ann Allergy Asthma Immunol. 2009;103(4):359–60. 10.1016/S1081-1206(10)60541-8 [DOI] [PubMed] [Google Scholar]
- 31.Yao FF, Liu FH. Life-threatening allergic-like reaction after intravenous MRI liver-specific contrast media gadoxetate disodium: a case report. J Magn Reson Imaging. 2020;52(3):958–9. 10.1002/jmri.27055 [DOI] [PubMed] [Google Scholar]
- 32.Virtos M, Ruiz S, Mokrane FZ, Rousseau H, Georges B, Fourcade O, Conil JM. Anaphylactic reaction and cardiac arrest due to gadobenate dimeglumine. Anaesth Crit Care Pain Med. 2015;34(4):247–8. 10.1016/j.accpm.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 33.Hasdenteufel F, Luyasu S, Renaudin JM, Paquay JL, Carbutti G, Beaudouin E, Moneret-Vautrin DA, Kanny G. Anaphylactic shock after first exposure to gadoterate meglumine: two case reports documented by positive allergy assessment. J Allergy Clin Immunol. 2008;121(2):527–8. 10.1016/j.jaci.2007.08.027 [DOI] [PubMed] [Google Scholar]
- 34.Parillo M, Mallio CA, Van der Molen AJ, Rovira A, Ramalho J, Ramalho M, Gianolio E, Karst U, Radbruch A, Stroomberg G, et al. Skin toxicity after exposure to gadolinium-based contrast agents in normal renal function, using clinical approved doses: current status of preclinical and clinical studies. Invest Radiol. 2023;58(8):530–8. 10.1097/RLI.0000000000000973 [DOI] [PubMed] [Google Scholar]
- 35.Semelka RC, Ramalho M. Gadolinium deposition disease: current state of knowledge and expert opinion. Invest Radiol. 2023;58(8):523–9. 10.1097/RLI.0000000000000977 [DOI] [PubMed] [Google Scholar]
- 36.Jost G, Frenzel T, Boyken J, Lohrke J, Nischwitz V, Pietsch H. Long-term excretion of gadolinium-based contrast agents: linear versus macrocyclic agents in an experimental rat model. Radiology. 2019;290(2):340–8. 10.1148/radiol.2018180135 [DOI] [PubMed] [Google Scholar]
- 37.McDonald RJ, Levine D, Weinreb J, Kanal E, Davenport MS, Ellis JH, Jacobs PM, Lenkinski RE, Maravilla KR, Prince MR, et al. Gadolinium retention: a research roadmap from the 2018 NIH/ACR/RSNA workshop on gadolinium chelates. Radiology. 2018;289(2):517–34. 10.1148/radiol.2018181151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Disproportionality analysis results of all PTs of suspected HSRs in VigiBase. Table S2. Disproportionality analysis results of all PTs of suspected HSRs in FAERS. Table S3. Sales volume of GBCAs from 2008 to 2022. Table S4. Sales volume of GBCAs in Americas, Asia, and Europe from 2008 to 2022. Fig. S1. Sales volume share of each GBCA in different regions from 2008 to 2022. GBCA: gadolinium-based contrast agent.
Data Availability Statement
Requests for access to VigiBase should be directed to CustomSearches@who-umc.org. The data obtained from OpenVigil 2.1 can be found at https://openvigil.sourceforge.net/. The data of IQVIA-MIDAS are available from the corresponding author upon reasonable request.