Abstract
We describe a case of a young patient with a recurrent pleomorphic xanthoastrocytoma (PXA) showing unusual cell-in-cell (CiC) phenomena. We observed mostly viable but also necrotic neutrophils engulfed within tumor cells. The recurrent tumor was immunopositive for BRAFV600E mutant protein and showed CDKN2 homozygous deletions typical of PXA. Both genetic alterations were also reported in the original primary tumor. Unlike the original tumor that was GFAP and Olig-2 immunopositive, the recurrent neoplasm was largely negative for GFAP and Olig-2 suggesting dedifferentiation. The large malignant cells that contained the neutrophils were negative for histiocytic and lymphohematopoietic markers. Whereas CDKN2 homozygous deletion is common in PXA, its presence is rare in histiocytic neoplasms. Both reactive astrocytes and glial neoplasms very rarely may engulf neutrophils in a process resembling emperipolesis or cellular cannibalism. Future work may clarify which type of CiC pathway is involved.
Keywords: Glioma, Brain tumor, Pleomorphic xanthoastrocytoma, Phagocytosis, Case report, Emperipolesis, Cell-in-cell
Introduction
Pleomorphic xanthoastrocytomas (PXAs) are astrocytic tumors presenting in children or young adults, and generally have a good prognosis, although CNS WHO grade 3 PXAs with elevated mitotic activity and necrosis have a 5 year survival rate of 65 %1. Histologically, they are characterized by pleomorphic cells including spindle cells, mononucleated and multinucleated giant cells, eosinophilic granules, reticulin deposition, and large amounts of cytoplasmic lipids2, and are typically positive for GFAP and S1003. Common molecular alterations include homozygous CDKN2A/B deletion in 60–90 % and BRAF V600E mutation in 60–80 % of cases4,5. Standard treatment includes maximal resection for all grades with postoperative radiation for grade 3 PXAs, and BRAF V600E mutant tumors can be treated with a combination of dabrafenib and trametinib to inhibit BRAF and MAPK pathways6. Cell-in-cell phenomena (CiC), or the internalization of one cell within another (Figure 1), has only been reported in one previous case of malignant glioma7. Here, we present a case of an aggressive, recurrent CNS WHO grade 3 PXA with CDKN2 deletion and BRAFV600E mutation with a rare histologic finding of CiC in tumor cells with engulfed neutrophils, many of which appeared viable.
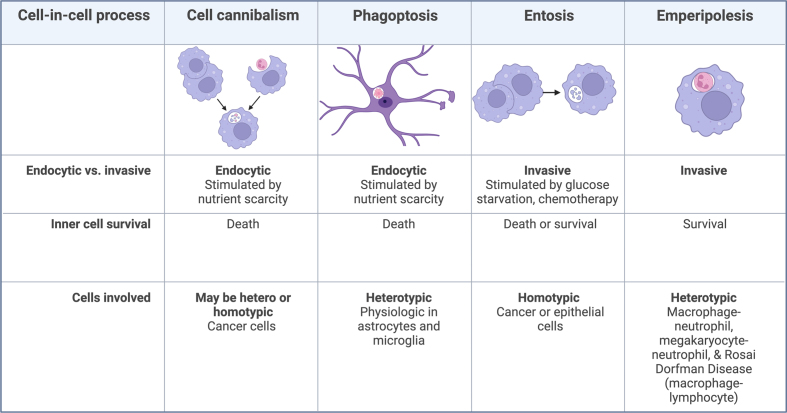
Figure 1. General Characteristics of Cell-in-cell processes.
In endocytic processes host cells internalize a target cell. Invasive processes are driven by activity of the internalized cells. “Cell types” refers to the host cells. Homotypic refers to the engulfing cell and the internalized cell being of the same cell type. Heterotypic means that the engulfing and internalized cell types are different. Emperipolesis historically involves survival of the internalized cell, but some authors extend its usage to cases wherein there may be death of the internalized cells. Cell-in-cell processes have been previously reviewed13,17. Previous literature is available on cannibalism18-20, phagoptosis21, phagocytosis12 and emperipolesis22.
Clinical History
A 10-year-old male presented to outside hospital with a right parietal tumor involving the petrous and mastoid bones with extension into the middle and posterior fossa. The patient had a near total resection followed by radiotherapy and temozolomide. The patient underwent a second resection five months later and was started on daily dabrafenib and trametinib. Available records state that pathology on first resection was right parietal anaplastic pleomorphic xanthoastrocytoma WHO grade III, BRAF mutant. Ten months later, the patient presented with bleeding and swelling of the right ear, then developed right face weakness, difficulty swallowing, dyspnea, and aphonia. Fifteen months after the initial diagnosis, he presented with culture negative febrile leukocytosis (white blood count (WBC) 39 preoperatively). He was started on Vancomycin and Ceftriaxone empirically. CT and MRI revealed increased size of PXA, extending into temporal bone, right cerebellum, right temporal lobe, and the cerebellopontine angle, involving cranial nerves VII and VIII (Figure 2A). Angiogram revealed that the sigmoid sinus and the internal carotid artery were occluded, although collateral circulation was intact.
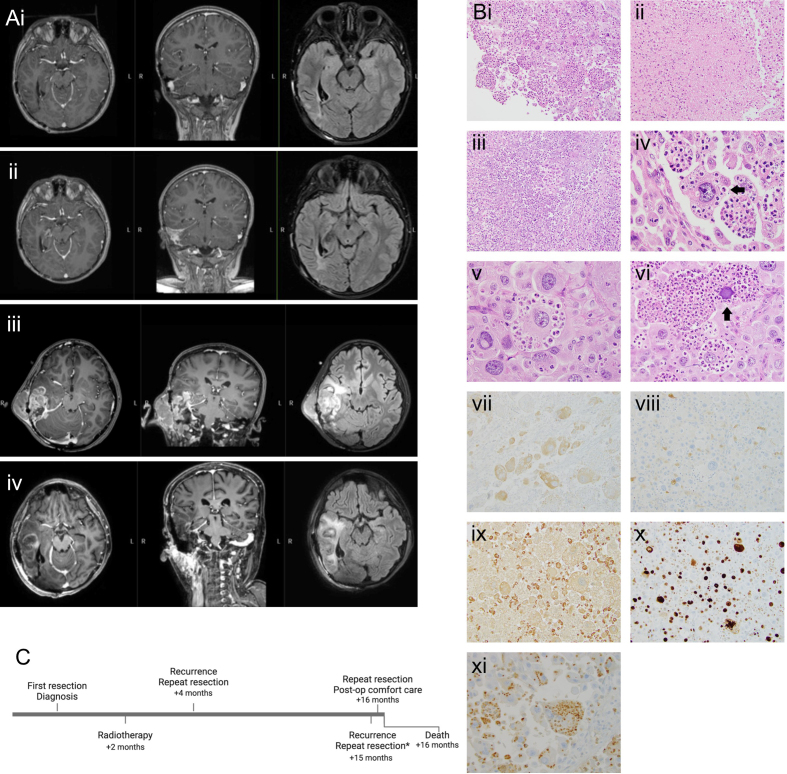
Figure 2.
(A) MRI of a heterogeneously enhancing mass with perilesional edema and progression over time including T1 post-contrast and T2 flair. (i) 12 months post-diagnosis. (ii) 14 months post-diagnosis. (iii) 15 months post-diagnosis, pre-operatively. (iv) 15 months post-diagnosis, post-operatively. (B) Histology. (i) Epithelioid tumor cells sometimes have a “ball of neutrophils” appearance - 200X. (ii) Bland necrosis - 200X. (iii) Necrosis with abundant neutrophils - 200X. (iv) Mostly viable neutrophils within intracytoplasmic vacuolar spaces of cell (arrow) - 600X. (v) Varying degrees of engulfed neutrophil degeneration - 600X. (vi) Degenerate basophilic nucleus in tumor cell (arrow) - 400X. (vii) BRAFV600E immunopositive tumor cells - 200X. (viii) S100 immunonegative tumor cells; S100 immunopositive small histiocytes - 200X. (ix) CD68 immunonegative tumor cells; CD68 immunopositive small histiocytes - 200X. (x) Ki-67 immunopositivity in many tumor nuclei - 200X. (xi) Myeloperoxidase immunostaining highlights the predominantly intracytoplasmic localization of neutrophils - 400X. (C) Timeline of the patient’s clinical course. Histology in (B) is from the patient’s resection at 15 months post-diagnosis.
The patient was taken to the operating room for mass resection. The patient’s prior craniotomy was extended in a translabyrinthine approach, and epidural and intradural tumor was debulked. A mastoidectomy and petrosectomy were performed, and the facial nerve was transected. The semicircular canals, cochlea, and vestibular aqueduct were opened and obliterated. Tumor within the sigmoid sinus was removed en bloc. A frozen specimen collected during the case was consistent with patient's known pleomorphic xanthoastrocytoma. Tissue was sent for cultures (aerobic, anaerobic, fungal) but these ultimately did not grow out any organisms. Following the procedure, the patient was taken to the postoperative recovery unit in stable condition. Post-operatively the patient failed a swallow test and required percutaneous endoscopic gastrostomy placement. Repeat blood cultures were negative, and WBC diminished to 30. The patient was transferred nine days after admission to a children’s hospital. At discharge, he had unilateral facial swelling, decreased facial sensation, face asymmetry, and absent hearing. An MRI subsequently demonstrated residual tumor regrowth and he underwent further resection the following month. His post-operative course was complicated by pseudo-meningocele for which a subdural drain was placed. The drain subsequently clogged and began to leak, and neuroimaging demonstrated further tumor progression. The patient deteriorated clinically, became unresponsive on exam and required supplemental oxygen. The patient’s family elected for comfort-focused care and the patient passed away 16 months after his initial diagnosis.
Pathology
The final diagnosis was recurrent pleomorphic xanthoastrocytoma (PXA) CNS WHO grade 3 with BRAFV600E mutation and homozygous CDKN2 deletion. The recurrent tumor showed large, markedly pleomorphic epithelioid cells with frequent mitotic activity, necrosis and intracellular neutrophils (Figure 2B). Associated tumor necrosis was bland (Figure 2Bii) or contained abundant neutrophils (Figure 2Biii). Intracellular, often viable though sometimes degenerating or necrotic, neutrophils in vacuolar spaces were present within the tumor cytoplasm (Figure 2Biv–vi). The tumor cells were often intact with well-preserved nuclei but some cells showed degenerated nuclei (Figure 2Bvi). In many areas, extracellular neutrophils were sparse or inconspicuous despite the presence of abundant intracellular neutrophils (Figure 2Biv–v). Unlike the original tumor that expressed both GFAP and Olig2, the recurrent tumor was largely immunonegative for GFAP and Olig2. The large tumor cells were BRAFV600E immunopositive (Figure 2Bvii) and FISH analysis demonstrated a CDKN2A homozygous deletion identical to what was reported in the original tumor. Testing for microorganisms including gram, GMS, PAS, and AFB stains as well as spirochete and TB/mycobacterium immunostains was negative. Histiocytic and lymphohematopoietic markers were negative or largely negative in the large tumor cells, including S100, CD163, CD68, CD1a, Langerin, Factor XIIIa, CD123, and CD35. There was also a population of small histiocytes that were immunopositive for S100, CD68 and CD163 but immunonegative for BRAFV600E, CD1a and Langerin compatible with a reactive non-neoplastic infiltrate. The contrasting BRAFV600E, S100 and CD68 immunoprofiles of the large tumor cells (BRAFV600E+, S100-, CD68-) and the small histiocytes (BRAFV600E-, S100+, CD68+) are seen in Figures 2Bviii–ix. The Ki-67 proliferation index of the tumor was high (Figure 2Bx). There was negative immunostaining of the large tumor cells for MAP2, SOX10, EMA, Keratin AE1/3, Melan A, SMA, CD21, CD15, CD45, CD23, CD33, Desmin, MPO, Lysozyme, HMB-45, CD20, CD3, CD43, and CD42b. INI-1 nuclear staining was retained. Neutrophils within tumor cells were immunopositive for MPO (Figure 2Bxi).
Discussion
We present a case of a PXA with several unusual features, including invasion through bone and soft tissue, loss of GFAP and Olig2 immunoreactivity, severe acute inflammatory infiltrate, and unusual CiC with internalized neutrophils. Alternate or concurrent diagnoses of infection or histiocytosis were considered, but cultures and special stains for microorganisms and histiocytic and lymphohematopoietic markers were negative. Rosai Dorfman disease (RDD) can occur in the CNS and is characterized by abundant histiocytes with emperipolesis. However, host tumor cells were immunonegative for histiocyte marker S100, had loss of CDKN2A inconsistent with RDD, and internalized neutrophils rather than lymphocytes, a rare finding for RDD8. Ultimately the diagnosis of recurrent PXA with BRAFV600E mutation and CDKN2A deletion was favored due to the patient’s history of tumor with an identical molecular phenotype.
The loss of GFAP and Olig2 staining, tissue necrosis with invasion of surrounding tissue, and history of multiple recurrences suggests that this patient’s PXA dedifferentiated. Within the central nervous system, CiC has been observed only in highly anaplastic tumors: large cell medulloblastoma9-11, and one prior case of malignant glioma. In this 1986 glioma case, pleomorphic, sparsely GFAP positive “monster cells” with cytoplasmic lipids, reticulin fibers, and multiple nuclei had internalized viable polymorphonuclear neutrophils as well as mononuclear and small tumor cells7, similar to the present case. These observations pose the question for future study of which molecular mechanisms facilitate CiC in these aggressive gliomas. Healthy astrocytes conduct phagocytosis physiologically12, but would typically result in destruction of the internalized material13. Neutrophils participate in a unique type of emperipolesis in which they invasively enter megakaryocytes and then donate their membranes for the production of platelets; it is unknown whether rapidly dividing cancer cells could use neutrophils for membrane production in a similar manner14,15. CDKN2A mutation is associated with homotypic CiC (entosis)16, but it is unknown whether CDKN2A mutation may contribute to heterotypic CiC as well. As the field evolves, cases of CiC in new contexts may supplement rigorous molecular analyses to characterize the pathways involved and better understand the biology of aggressive gliomas.
Conflicts of Interest Statement
The authors have no conflicts of interest to report.
Funding Statement
The authors acknowledge that they received no funding in support for this work.
References
- Lee C, Byeon Y, Kim GJ, et al. Exploring prognostic factors and treatment strategies for long-term survival in pleomorphic xanthoastrocytoma patients. Sci Rep. 2024;14(1):1-10. DOI: 10.1038/s41598-024-55202-6 [DOI] [PMC free article] [PubMed]
- Kepes J, Rubinstein L, Eng L. Pleomorphic xanthoastrocytoma: a distinctive meningocerebral glioma of young subjects with relatively favorable prognosis. A study of 12 cases. Cancer. 1979;44(5):1839-1852. DOI: [DOI] [PubMed]
- Sharma A, Nand Sharma D, Julka PK, Rath GK. Pleomorphic xanthoastrocytoma – a clinico-pathological review. Neurol Neurochir Pol. 2011;45(4):379-386. DOI: 10.1016/S0028-3843(14)60109-2 [DOI] [PubMed]
- Ebrahimi A, Korshunov A, Reifenberger G, et al. Pleomorphic xanthoastrocytoma is a heterogeneous entity with pTERT mutations prognosticating shorter survival. Acta Neuropathol Commun. 2022;10(1):1-10. DOI: 10.1186/S40478-021-01308-1 [DOI] [PMC free article] [PubMed]
- Vaubel R, Zschernack V, Tran QT, et al. Biology and grading of pleomorphic xanthoastrocytoma-what have we learned about it? Brain Pathol. 2021;31(1):20-32. DOI: 10.1111/BPA.12874 [DOI] [PMC free article] [PubMed]
- Shaikh N, Brahmbhatt N, Kruser TJ, et al. Pleomorphic xanthoastrocytoma: a brief review. CNS Oncology. 2019;8(3). DOI: 10.2217/CNS-2019-0009 [DOI] [PMC free article] [PubMed]
- Gherardi R, Baudrimont M, Nguyen JP, et al. Acta Neuropathol (Berl) Monstrocellular Heavily Lipidized Malignant Glioma. Acta Neuropathol. 1986;69:28-32. DOI: 10.1007/BF00687035 [DOI] [PubMed]
- Rajyalakshmi R, Akhtar M, Swathi Y, Chakravarthi R, Reddy JB, Priscilla MB. Cytological Diagnosis of Rosai–Dorfman Disease: A Study of Twelve Cases with Emphasis on Diagnostic Challenges. J Cytol. 2020;37(1):46. DOI: 10.4103/JOC.JOC_4_19 [DOI] [PMC free article] [PubMed]
- Fais S, Overholtzer M. Cell-in-cell phenomena in cancer. Nat Rev Cancer. 2018;18:758-766. DOI: 10.1038/s41568-018-0073-9 [DOI] [PubMed]
- Youness E, Barlogie B, Ahearn M, Trujillo J. Tumor cell phagocytosis. Its occurrence in a patient with medulloblastoma. Arch Pathol Lab Med. 1980;104(12):651-653. [PubMed]
- Escamilla V, Franco-Macías E, Calderón-Cabrera C, et al. Bone marrow cellular cannibalism by medulloblastoma. Am J Hematol. 2015;90(5):466-467. DOI: 10.1002/AJH.23892 [DOI] [PubMed]
- Konishi H, Koizumi S, Kiyama H. Phagocytic astrocytes: Emerging from the shadows of microglia. Glia. 2022;70(6):1009-1026. DOI: 10.1002/GLIA.24145 [DOI] [PMC free article] [PubMed]
- Brown GC. Cell death by phagocytosis. Nat Rev Immunol 2023 242. 2023;24(2):91-102. DOI: 10.1038/s41577-023-00921-6 [DOI] [PubMed]
- Cunin P, Bouslama R, Machlus KR, et al. Megakaryocyte emperipolesis mediates membrane transfer from intracytoplasmic neutrophils to platelets. Elife. 2019;8. DOI: 10.7554/ELIFE.44031 [DOI] [PMC free article] [PubMed]
- Yener Y, Dikmenli M. The effects of acrylamide on the frequency of megakaryocytic emperipolesis and the mitotic activity of rat bone marrow cells. J Sci Food Agric. 2011;91(10):1810-1813. DOI: 10.1002/JSFA.4388 [DOI] [PubMed]
- Mackay HL, Muller PAJ. Biological relevance of cell-in-cell in cancers. Biochem Soc Trans. 2019;47(2):725-732. DOI: 10.1042/BST20180618 [DOI] [PMC free article] [PubMed]
- Borensztejn K, Tyrna P, Gaweł AM, et al. Classification of Cell-in-Cell Structures: Different Phenomena with Similar Appearance. Cells. 2021;10(10):2569. DOI: 10.3390/CELLS10102569 [DOI] [PMC free article] [PubMed]
- Fu P, Chen F, Pan Q, et al. The different functions and clinical significances of caveolin-1 in human adenocarcinoma and squamous cell carcinoma. Onco Targets Ther. 2017;10:819. DOI: 10.2147/OTT.S123912 [DOI] [PMC free article] [PubMed]
- Gupta N, Jadhav K, Shah V. Emperipolesis, entosis and cell cannibalism: Demystifying the cloud. J Oral Maxillofac Pathol. 2017;21(1):92. DOI: 10.4103/0973-029X.203763 [DOI] [PMC free article] [PubMed]
- Caruso RA, Fedele F, Finocchiaro G, Arena G, Venuti A. Neutrophil-tumor cell phagocytosis (cannibalism) in human tumors: An update and literature review. Exp Oncol. 2012;34(3):306-311. https://exp-oncology.com.ua/pdf/2012/34_3/1175.pdf [PubMed]
- Brown GC, Neher JJ. Eaten alive! Cell death by primary phagocytosis: “phagoptosis.” Trends Biochem Sci. 2012;37(8):325-332. DOI: 10.1016/J.TIBS.2012.05.002 [DOI] [PubMed]
- Müller W, Dahmen HG. Lymphocytes within glial cells (“emperipolesis”) in a case of a granular cell tumor. Acta Neuropathol. 1978;44(2):163-165. DOI: 10.1007/BF00691486 [DOI] [PubMed]